Head and Neck Cancer Therapeutics Market
Head and Neck Cancer Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
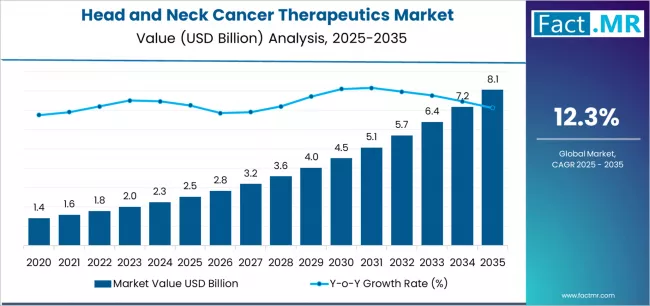
Head and neck cancer therapeutics market is projected to grow from USD 2.5 billion in 2025 to USD 8.1 billion by 2035, at a CAGR of 12.3%. Immunotherapy will dominate with a 60.2% market share, while injectable will lead the route of administration segment with a 90.1% share.
Head and Neck Cancer Therapeutics Market Forecast and Outlook 2025 to 2035
The global head and neck cancer therapeutics market is projected to reach USD 8.05 billion by 2035, recording an absolute increase of USD 5.52 billion over the forecast period. The market is valued at USD 2.53 billion in 2025 and is set to rise at a CAGR of 12.3% during the assessment period.
The overall market size is expected to grow by approximately 3.2 times during the same period, supported by increasing incidence of HPV-associated oropharyngeal cancers among younger patient populations worldwide, driving demand for advanced immunotherapeutic interventions and increasing investments in PD-1/PD-L1 inhibitor development with clinical efficacy across recurrent and metastatic disease applications globally.
Quick Stats for Head and Neck Cancer Therapeutics Market
- Head and Neck Cancer Therapeutics Market Value (2025): USD 2.53 billion
- Head and Neck Cancer Therapeutics Market Forecast Value (2035): USD 8.05 billion
- Head and Neck Cancer Therapeutics Market Forecast CAGR: 12.3%
- Leading Therapy Type in Head and Neck Cancer Therapeutics Market: Immunotherapy (60.2%)
- Key Growth Regions in Head and Neck Cancer Therapeutics Market: Asia Pacific, North America, and Europe
- Top Players in Head and Neck Cancer Therapeutics Market: Merck & Co., Inc., Bristol-Myers Squibb Company, Eli Lilly and Company, AstraZeneca, Sanofi, F. Hoffmann-La Roche Ltd., Takeda Pharmaceutical Company Limited, Teva Pharmaceutical Industries Ltd., Cumberland Pharmaceuticals Inc., Aprea Therapeutics
Patients with head and neck cancers face mounting pressure to manage aggressive tumor progression while addressing treatment-related complications including dysphagia, speech impairment, and quality of life deterioration, with modern immunotherapy products providing documented therapeutic benefits including improved overall survival rates, enhanced disease control, and reduced treatment toxicity compared to conventional chemotherapy interventions alone.
Rising awareness about early detection protocols and expanding precision oncology platforms enabling biomarker-driven treatment selection create substantial opportunities for pharmaceutical manufacturers and oncology care partners. However, limited access to novel biologics in emerging markets and high treatment costs may pose obstacles to widespread patient accessibility.
The immunotherapy segment dominates market activity, driven by extensive clinical evidence supporting immune checkpoint inhibitors' role in improving survival outcomes and response durability across diverse head and neck cancer subtypes worldwide. Oncologists increasingly recognize the therapeutic benefits of PD-1/PD-L1 inhibitors, with typical treatment regimens providing effective tumor control and immune system activation at accessible price points through established oncology distribution networks.
The injectable route of administration demonstrates robust dominance, supported by established infusion center infrastructure and clinical preference for intravenous delivery ensuring optimal drug bioavailability and therapeutic monitoring in oncology settings. Retail and specialty pharmacies emerge as the dominant distribution channel, reflecting specialized handling requirements for high-value biologics and patient support services essential for complex immunotherapy protocols.
Regional dynamics show North America maintaining market leadership, supported by high oncology treatment expenditure and established immunotherapy adoption patterns across cancer care categories. Asia Pacific demonstrates the fastest growth trajectory driven by increasing cancer diagnosis rates and expanding healthcare infrastructure adopting advanced therapeutic approaches, while Europe emphasizes evidence-based treatment protocols and regulatory compliance for biologic quality standards. India leads country-level growth through extensive urban oncology center expansion and growing HPV awareness, followed by China supported by healthcare modernization and domestic immuno-oncology development.
The competitive landscape features moderate concentration with Merck & Co., Inc. maintaining market leadership position, while established pharmaceutical players including Bristol-Myers Squibb Company, Eli Lilly and Company, and AstraZeneca compete through comprehensive oncology portfolios and ongoing clinical development programs across diverse therapeutic applications.
Head and Neck Cancer Therapeutics Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the head and neck cancer therapeutics market is projected to expand from USD 2.53 billion to USD 4.06 billion, resulting in a value increase of USD 1.53 billion, which represents 27.7% of the total forecast growth for the period. This phase of development will be shaped by rising demand for PD-1/PD-L1 inhibitors and combination immunotherapy regimens addressing recurrent disease, product innovation in CAR-T cellular therapies with enhanced tumor-specific targeting capabilities, as well as expanding integration with diagnostic biomarker platforms and molecular profiling services. Companies are establishing competitive positions through investment in clinical trial partnerships, advanced biologics manufacturing facilities, and strategic market expansion across hospital oncology departments, specialty pharmacy channels, and integrated care delivery models.
From 2029 to 2035, the market is forecast to grow from USD 4.06 billion to USD 8.05 billion, adding another USD 3.99 billion, which constitutes 72.3% of the overall expansion. This period is expected to be characterized by the expansion of specialized treatment applications, including tumor-specific vaccine development and next-generation cellular immunotherapies tailored for specific genetic profiles, strategic collaborations between pharmaceutical manufacturers and academic cancer centers, and an enhanced focus on real-world evidence generation and patient-reported outcomes. The growing emphasis on personalized oncology approaches and rising adoption of biomarker-guided treatment selection will drive demand for targeted immunotherapeutic solutions across diverse patient populations.
Head and Neck Cancer Therapeutics Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 2.53 billion |
| Market Forecast Value (2035) | USD 8.05 billion |
| Forecast CAGR (2025-2035) | 12.3% |
Why is the Head and Neck Cancer Therapeutics Market Growing?
The head and neck cancer therapeutics market grows by enabling patients with aggressive malignancies to achieve improved survival outcomes and disease control while managing complex treatment regimens and quality of life preservation without exclusive reliance on conventional chemotherapy approaches.
Patients with head and neck cancers face mounting pressure to control tumor progression and prevent metastatic spread while managing treatment-related toxicities, functional impairments, and psychological distress, with modern immunotherapy formulations typically providing targeted immune activation including enhanced T-cell response for tumor recognition, durable disease control for extended progression-free survival, and favorable safety profiles for reduced treatment burden compared to conventional cytotoxic regimens alone, making immunotherapeutic intervention essential for comprehensive cancer management.
The oncology industry's need for effective and tolerable treatment options creates demand for specialized head and neck cancer therapeutic solutions that can provide superior efficacy, enhance patient quality of life, and support long-term disease control without compromising functional outcomes or causing debilitating side effects.
Healthcare provider endorsements and clinical evidence supporting immunotherapy efficacy drive adoption in oncology hospital environments, radiation therapy centers, and multidisciplinary tumor board settings, where therapeutic outcomes have direct impact on patient survival rates and functional preservation.
The increasing head and neck cancer incidence globally, particularly HPV-associated oropharyngeal cancers affecting younger populations, creates expanding patient populations requiring advanced treatment interventions. Rising awareness about immunotherapy mechanisms and biomarker-driven treatment selection enables informed therapeutic decisions and adherence to evidence-based protocols.
High treatment costs and limited access to specialty oncology care in resource-constrained settings may limit widespread adoption of novel biologics and optimal treatment delivery among diverse patient populations with varying healthcare coverage.
Segmental Analysis
The market is segmented by therapy type, route of administration, distribution channel, and region. By therapy type, the market is divided into immunotherapy, targeted therapy, chemotherapy, and others.
Based on route of administration, the market is categorized into injectable, oral, and others. By distribution channel, the market includes retail and specialty pharmacies, hospital pharmacies, and online pharmacies. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Therapy Type, Which Segment Accounts for the Dominant Market Share?
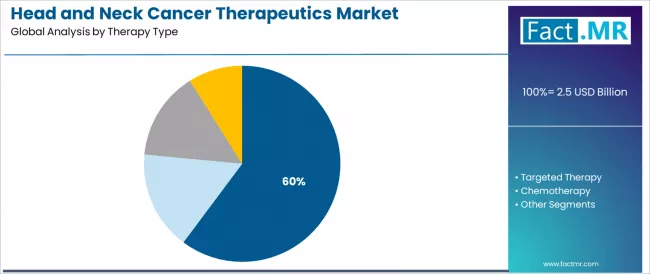
The immunotherapy segment represents the dominant force in the head and neck cancer therapeutics market, capturing 60.2% of the total market share in 2025. This established therapeutic category encompasses solutions featuring clinically validated immune checkpoint inhibition mechanisms and T-cell activation capabilities, including advanced formulations combining PD-1/PD-L1 targeting strategies and combination immunotherapy protocols that enable superior clinical outcomes and patient survival rates across recurrent and metastatic disease applications worldwide.
The immunotherapy segment's market leadership stems from its superior clinical efficacy profile, with solutions capable of addressing fundamental cancer immune evasion mechanisms including checkpoint blockade restoration, tumor microenvironment modulation, and adaptive immune response enhancement while maintaining manageable immune-related adverse event profiles across diverse patient populations.
Within the immunotherapy category, PD-1/PD-L1 inhibitors demonstrate dominant adoption at 36.4% of the therapy type segment, driven by extensive clinical validation in platinum-refractory disease and first-line treatment settings. These checkpoint inhibitors provide breakthrough therapeutic benefits for patients who previously had limited treatment options, establishing new standards of care in recurrent or metastatic head and neck squamous cell carcinoma management.
The immune checkpoint combination therapy sub-segment maintains substantial presence at 13.8%, serving oncologists who require enhanced treatment intensification for aggressive disease presentations. These combination protocols leverage synergistic immune activation mechanisms while managing toxicity profiles through careful patient selection and monitoring strategies.
CAR-T and cellular immunotherapy applications demonstrate emerging adoption at 6.2%, driven by advances in solid tumor targeting technologies and increasing investment in next-generation cellular therapy development. Tumor-specific vaccine research represents 3.8% of the segment, reflecting ongoing clinical investigation in therapeutic vaccination approaches for head and neck malignancies.
Key therapeutic advantages driving the immunotherapy segment include:
- Advanced immune checkpoint modulation mechanisms with clinically demonstrated improvements in overall survival and durable response rates across platinum-resistant disease populations
- Established efficacy in recurrent and metastatic settings allowing improved disease control and quality of life without intensive chemotherapy regimen complexity
- Enhanced safety profile features enabling outpatient treatment delivery and reduced hospitalization requirements while maintaining therapeutic effectiveness
- Superior long-term outcomes providing extended progression-free survival for appropriately selected patients across various disease stages and performance status levels
By Route of Administration, Which Segment Accounts for the Largest Market Share?
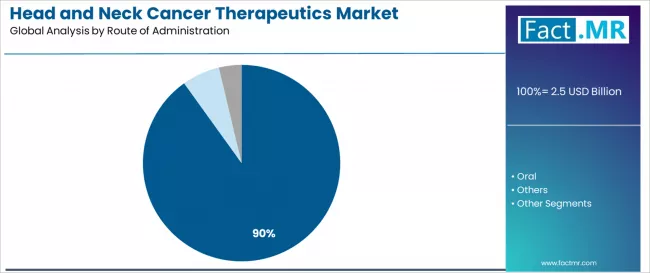
Injectable formulations dominate the head and neck cancer therapeutics route of administration landscape with a 90.1% market share in 2025, reflecting the critical role of parenteral delivery systems in supporting optimal drug bioavailability, precise dosing control, and established oncology infusion protocols across patient populations worldwide. The injectable segment's market leadership is reinforced by clinical preferences for intravenous administration enabling controlled drug delivery, immediate therapeutic monitoring, and compatibility with complex biologic formulations that characterize modern immunotherapy protocols.
Within this segment, intravenous infusion represents the preferred administration method at 56.7%, driven by established infusion center infrastructure, nursing expertise in oncology drug delivery, and optimal pharmacokinetic profiles for monoclonal antibody therapeutics. This sub-segment benefits from comprehensive patient monitoring capabilities and immediate intervention availability for infusion-related reactions.
The subcutaneous injection category maintains meaningful presence at 23.4%, demonstrating growth through specialized requirements for convenient outpatient administration and reduced infusion time demands. This route benefits from patient preference for shorter clinic visits and increasing adoption of home healthcare delivery models in stable patient populations.
Intramuscular administration represents 10.0% of the injectable segment, serving specific therapeutic applications requiring depot formulations or alternative delivery mechanisms for particular patient circumstances.
Key market dynamics supporting route of administration patterns include:
- Intravenous delivery expansion driven by complex biologic formulation requirements and established oncology infusion infrastructure
- Subcutaneous route development trends require specialized formulation technologies for high-concentration antibody delivery and improved patient convenience
- Integration of infusion center capabilities enabling comprehensive patient monitoring and adverse event management during treatment delivery
- Growing emphasis on patient-centric delivery models driving innovation in administration convenience without compromising therapeutic efficacy
By Distribution Channel, Which Segment Accounts for a Significant Market Share?
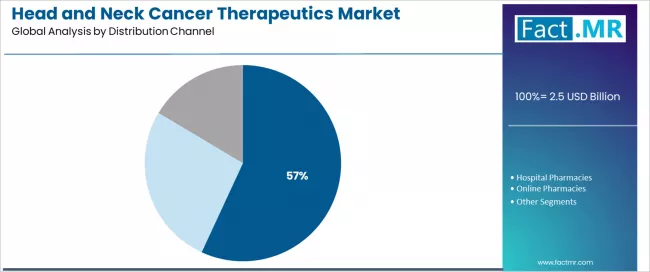
Retail and specialty pharmacies represent a leading distribution channel segment in the head and neck cancer therapeutics market with a 56.9% market share in 2025, reflecting the fundamental role of specialized pharmaceutical distribution in supporting complex biologic handling requirements, patient support services, and reimbursement coordination across oncology care pathways. The retail and specialty pharmacy segment demonstrates consistent demand driven by the need to provide comprehensive medication management, financial assistance navigation, and ongoing patient education throughout extended treatment courses.
Within this segment, specialty oncology pharmacies emerge as the dominant distribution model at 34.8%, driven by expertise in high-value biologic management, prior authorization processing, and integrated patient support programs. These specialized facilities provide critical infrastructure for complex immunotherapy distribution, including cold chain management, restricted distribution compliance, and comprehensive clinical coordination with treating oncologists.
Retail clinical dispensing maintains substantial presence at 16.6%, serving patients requiring accessible pharmacy services integrated with comprehensive oncology care delivery. Remote prescription and authorization platforms represent 5.5% of the segment, reflecting emerging adoption of telepharmacy models and digital health integration in medication access pathways.
Hospital pharmacies demonstrate important presence in the distribution landscape, providing inpatient medication management and acute care setting drug delivery for hospitalized patients receiving intensive treatment regimens. Online pharmacies maintain emerging presence through mail-order specialty pharmacy services and patient convenience optimization in chronic disease management.
Key distribution dynamics include:
- Specialty pharmacy expansion accelerating across integrated healthcare systems with emphasis on comprehensive patient support and adherence programs
- Retail pharmacy partnerships enabling accessible medication distribution while maintaining quality standards for biologic handling
- Technology integration facilitating prior authorization processing and real-time benefit verification supporting patient access
- Growing emphasis on patient financial assistance programs addressing high medication costs and insurance coverage complexities
What are the Drivers, Restraints, and Key Trends of the Head and Neck Cancer Therapeutics Market?
The market is driven by three concrete demand factors tied to oncology treatment outcomes. First, increasing incidence of HPV-associated oropharyngeal cancers creates expanding patient populations requiring effective therapeutic interventions, with immunotherapy representing a critical treatment option for recurrent and metastatic disease in comprehensive care protocols, requiring widespread oncology infrastructure development. Second, growing clinical evidence supporting PD-1/PD-L1 inhibitor efficacy drives oncologist adoption and guideline inclusion, with numerous studies demonstrating significant survival benefits and quality of life improvements through targeted immune activation by 2030. Third, advancing precision medicine capabilities and biomarker identification enable more informed treatment selection approaches that improve response rates while reducing unnecessary treatment exposure and associated healthcare costs.
Market restraints include high treatment costs and limited reimbursement coverage in many healthcare systems that can challenge patient access to novel immunotherapeutic agents, particularly in emerging markets where oncology drug budgets remain constrained and insurance coverage for expensive biologics proves inconsistent. Immune-related adverse events requiring specialized management expertise pose another significant obstacle, as head and neck cancer patients may experience treatment-limiting toxicities including thyroid dysfunction, colitis, and pneumonitis, potentially affecting treatment continuation and requiring multidisciplinary care coordination. Limited predictive biomarkers for treatment response create additional challenges for optimal patient selection, demanding extensive clinical monitoring and potential treatment modifications when initial therapeutic approaches demonstrate insufficient efficacy.
Key trends indicate accelerated combination immunotherapy adoption in developed markets, particularly North America and Europe, where oncologists demonstrate increasing confidence in managing dual checkpoint inhibitor regimens and combination approaches integrating immunotherapy with targeted agents or radiation therapy. Biomarker-driven treatment selection trends toward comprehensive molecular profiling including PD-L1 expression, tumor mutational burden, and microsatellite instability testing enable precision therapeutic decisions that optimize clinical outcomes and healthcare resource utilization. However, the market thesis could face disruption if significant advances in preventive HPV vaccination programs substantially reduce future head and neck cancer incidence or major breakthroughs in curative surgical and radiation techniques reduce reliance on systemic therapeutic interventions.
Analysis of the Head and Neck Cancer Therapeutics Market by Key Countries
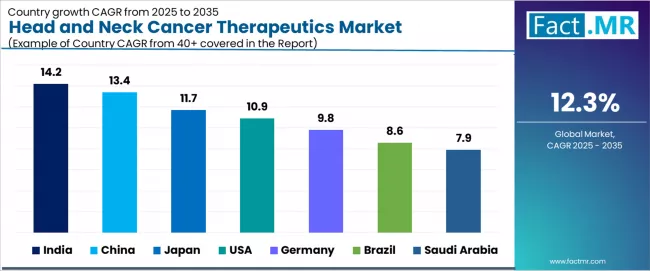
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 14.2% |
| China | 13.4% |
| Japan | 11.7% |
| USA | 10.9% |
| Germany | 9.8% |
| Brazil | 8.6% |
| Saudi Arabia | 7.9% |
The global head and neck cancer therapeutics market is expanding rapidly, with India leading at a 14.2% CAGR through 2035, driven by growing HPV-linked cancer cases, expanding oncology hospital infrastructure, and faster immunotherapy adoption patterns. China follows at 13.4%, supported by healthcare modernization initiatives, local immuno-oncology research and development capabilities, and improving diagnostic rates across urban centers. Japan records 11.7%, reflecting an aging population, standardized cancer treatment protocols, and strong biologics adoption in comprehensive oncology care.
USA advances at 10.9%, leveraging high per-patient spending capacity, wide PD-1 inhibitor utilization, and robust clinical infrastructure. Germany posts 9.8%, focusing on structured treatment guidelines and early diagnostic programs, while Brazil grows steadily at 8.6%, anchored by expansion of cancer treatment centers and increased screening programs. Saudi Arabia demonstrates 7.9% growth, driven by rapid healthcare sector development and introduction of advanced biologics.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the head and neck cancer therapeutics market with a CAGR of 14.2% through 2035. The country's leadership position stems from rapidly increasing HPV-associated cancer diagnosis rates, expanding urban oncology infrastructure, and growing awareness about advanced immunotherapy treatment options driving adoption beyond conventional chemotherapy approaches.
Growth is concentrated in major metropolitan areas and tier-1 cities, including Mumbai, Delhi, Bangalore, Chennai, and Hyderabad, where cancer patients are increasingly accessing evidence-based immunotherapeutic interventions for comprehensive disease management. Distribution channels through specialty oncology hospitals, tertiary care centers, and emerging pharmacy networks expand treatment accessibility across urban populations and educated patient segments. The country's growing integration of international treatment protocols with local healthcare delivery systems provides strong momentum for advanced cancer therapeutics, including comprehensive adoption across income segments seeking improved survival outcomes.
Key market factors:
- Urban cancer patient populations concentrated in metropolitan areas and medical tourism hubs with rising treatment awareness levels
- Healthcare infrastructure expansion through corporate hospital chains and regional cancer centers enabling advanced therapy access
- Comprehensive insurance coverage growth ecosystem, including government schemes and private health insurance plans with proven oncology benefits
- Local pharmaceutical manufacturing development featuring companies establishing biosimilar production capabilities offering competitively priced treatment alternatives
Why is China Emerging as a High-Growth Market?
In major urban centers including Beijing, Shanghai, Guangzhou, and Shenzhen, the adoption of head and neck cancer immunotherapy solutions is accelerating across patient populations, driven by increasing lifestyle-related cancer prevalence and growing emphasis on modern oncology care approaches. The market demonstrates strong growth momentum with a CAGR of 13.4% through 2035, linked to comprehensive healthcare system modernization and increasing investment in domestic immuno-oncology research and development capabilities.
Chinese patients are implementing international standard treatment protocols while benefiting from expanding clinical trial opportunities that enhance access to novel therapeutic agents. The country's expanding middle class creates ongoing demand for premium imported immunotherapy brands, while increasing government support for domestic biopharmaceutical innovation drives development of locally manufactured checkpoint inhibitors and cellular therapies.
Key development areas:
- Urban professional populations leading immunotherapy adoption with emphasis on survival improvement and quality of life preservation
- Distribution expansion through both public tertiary hospitals and private cancer specialty centers providing comprehensive treatment access
- Technology integration enabling molecular diagnostics and precision medicine platforms supporting biomarker-driven treatment selection
- Growing preference for combination treatment protocols alongside domestic manufacturers developing competitive immunotherapy alternatives
What Drives USA Market Resilience?
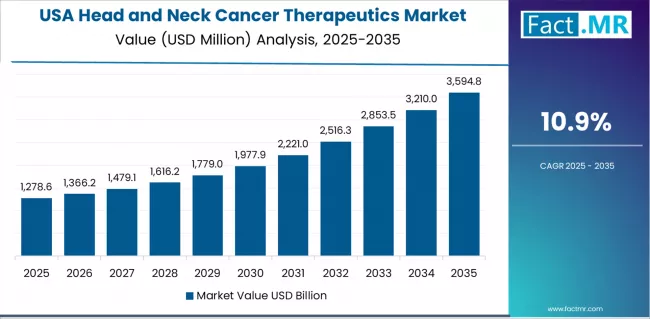
USA market expansion is driven by diverse oncology care delivery models, including comprehensive cancer centers in academic medical environments and community oncology practices across mainstream healthcare networks. The country demonstrates steady growth potential with a CAGR of 10.9% through 2035, supported by continuous innovation from established pharmaceutical manufacturers and specialized biotechnology companies developing next-generation immunotherapeutic agents.
American patients face implementation challenges related to complex insurance authorization processes and high out-of-pocket costs for novel biologics, requiring pharmaceutical companies to provide robust patient assistance programs and demonstrate clear clinical value propositions. However, established specialty pharmacy infrastructure and high oncology treatment awareness create stable baseline demand for head and neck cancer immunotherapies, particularly among patients actively managed in multidisciplinary tumor boards where evidence-based treatment selection drives primary therapeutic decisions.
Market characteristics:
- Oncology patient populations and academic medical centers showing robust demand with substantial annual treatment volumes across therapeutic applications
- Regional practice patterns varying between aggressive combination therapy approaches in comprehensive cancer centers and sequential treatment strategies in community settings
- Future projections indicate continued innovation emphasis with development of novel checkpoint inhibitors and cellular therapy platforms
- Growing adoption of value-based care models and outcomes-based pricing arrangements supporting sustainable market access
How does Germany Demonstrate Clinical Excellence Leadership?
The market in Germany leads in evidence-based head and neck cancer therapeutic adoption based on integration with structured treatment guidelines and pharmaceutical-grade quality standards for enhanced therapeutic reliability. The country shows strong potential with a CAGR of 9.8% through 2035, driven by strict regulatory oversight and physician preferences for clinically validated immunotherapy regimens in major markets, including Bavaria, North Rhine-Westphalia, Baden-Württemberg, and Hesse.
German oncologists are adopting immunotherapy protocols through comprehensive tumor board evaluations and guideline-concordant treatment algorithms for optimal patient outcomes, particularly in university hospital systems and certified cancer centers demanding rigorous evidence standards. Distribution channels through hospital-based oncology pharmacies and specialized cancer treatment facilities expand coverage across urban centers and academically integrated care communities.
Leading market segments:
- Evidence-based treatment adoption in major university medical centers implementing comprehensive clinical validation protocols
- Hospital pharmacy partnerships with oncology departments achieving high prescriber confidence through structured formulary processes
- Strategic collaborations between pharmaceutical manufacturers and clinical research organizations expanding real-world evidence generation
- Focus on health technology assessment and cost-effectiveness evaluation addressing healthcare system sustainability
What Positions Japan for Quality Treatment Leadership?
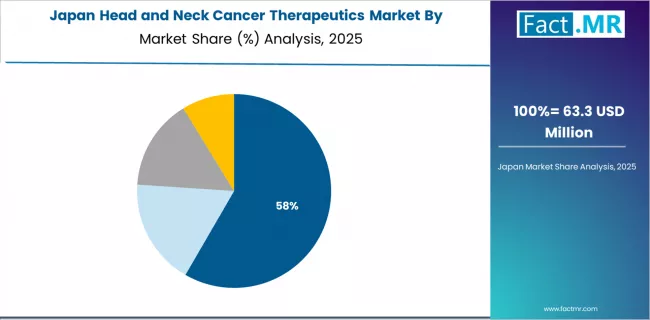
Japan's head and neck cancer therapeutics market demonstrates sophisticated clinical practice patterns focused on treatment quality and comprehensive patient care optimization, with documented integration of advanced immunotherapy protocols achieving substantial improvements in survival outcomes and quality of life measures across oncology applications.
The country maintains steady growth momentum with a CAGR of 11.7% through 2035, driven by aging population demographics emphasizing cancer care quality and continuous refinement of treatment protocols that align with Japanese healthcare standards applied to oncology products. Major metropolitan areas, including Tokyo, Osaka, Nagoya, and Fukuoka, showcase advanced adoption of immunotherapy regimens where pharmaceutical-grade biologics integrate seamlessly with multidisciplinary care coordination and comprehensive supportive care programs.
Key market characteristics:
- Quality-focused oncology practitioners driving demand for proven immunotherapy formulations with emphasis on safety profile and efficacy validation
- Healthcare system partnerships enabling consistent treatment access with comprehensive insurance coverage programs
- Technology collaboration between Japanese pharmaceutical companies and international biologic manufacturers expanding treatment options
- Emphasis on geriatric oncology considerations and age-appropriate treatment protocols addressing elderly patient management
What Characterizes Brazil's Market Development?
In major metropolitan centers including São Paulo, Rio de Janeiro, Brasília, and Belo Horizonte, the adoption of head and neck cancer immunotherapy solutions is expanding across patient populations, driven by increasing cancer awareness and rising healthcare infrastructure investment patterns. The market demonstrates solid growth potential with a CAGR of 8.6% through 2035, linked to comprehensive oncology center expansion and increasing focus on advanced cancer treatment availability through public and private healthcare systems.
Brazilian patients are implementing modern cancer management practices and accessing imported immunotherapy products to enhance survival outcomes while meeting growing expectations in urban oncology environments. The country's expanding network of specialized cancer hospitals creates ongoing demand for evidence-based therapeutic protocols, while increasing clinical trial participation drives early access to investigational agents.
Key development areas:
- Urban cancer patient populations leading immunotherapy adoption with emphasis on survival improvement and treatment modernization
- Distribution expansion through oncology specialty centers and hospital pharmacy networks providing comprehensive medication access
- Local regulatory framework development supporting accelerated approval pathways for innovative cancer therapeutics
- Integration of patient access programs and pharmaceutical assistance initiatives supporting treatment affordability
What drives Healthcare Transformation in Saudi Arabia?
In major cities including Riyadh, Jeddah, Dammam, and Mecca, the adoption of advanced head and neck cancer therapeutics is expanding across patient populations, driven by national healthcare transformation initiatives and growing investment in comprehensive oncology care infrastructure. The market demonstrates meaningful growth potential with a CAGR of 7.9% through 2035, linked to government healthcare vision programs and increasing emphasis on bringing international standard cancer treatments to the domestic healthcare system.
Patients are accessing modern immunotherapy protocols through specialized cancer centers and tertiary hospitals implementing evidence-based treatment guidelines. The country's healthcare system modernization creates ongoing opportunities for pharmaceutical companies introducing novel biologics, while medical tourism development and international hospital partnerships enhance treatment availability.
Key development areas:
- National healthcare initiatives supporting oncology infrastructure development and advanced treatment availability
- Distribution expansion through Ministry of Health facilities and private hospital networks providing comprehensive care access
- Strategic partnerships with international pharmaceutical companies accelerating novel therapy introductions
- Growing emphasis on medical education and oncology specialty training supporting appropriate immunotherapy utilization
Europe Market Split by Country
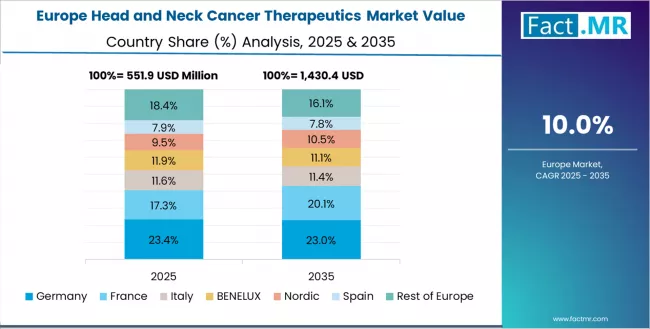
The head and neck cancer therapeutics market in Europe is projected to grow from USD 0.61 billion in 2025 to USD 1.89 billion by 2035, registering a CAGR of 11.9% over the forecast period. Germany is expected to maintain its leadership position with a 29.3% market share in 2025, adjusting to 28.7% by 2035, supported by its extensive university hospital networks, comprehensive cancer center infrastructure, and evidence-based treatment protocol adoption serving major European oncology markets.
UK follows with a 22.1% share in 2025, projected to reach 22.6% by 2035, driven by comprehensive National Health Service oncology programs and specialized cancer treatment centers implementing guideline-concordant immunotherapy protocols. France holds a 19.7% share in 2025, expected to maintain 20.1% by 2035 through ongoing development of academic medical centers and comprehensive cancer care networks.
Italy commands a 14.8% share, while Spain accounts for 10.3% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 3.8% to 4.2% by 2035, attributed to increasing immunotherapy adoption in Nordic countries and emerging Eastern European markets implementing modern oncology treatment standards.
Competitive Landscape of the Head and Neck Cancer Therapeutics Market
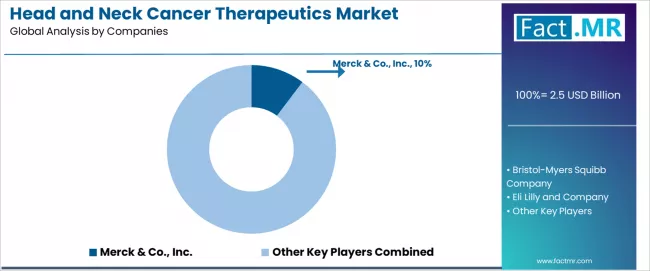
The head and neck cancer therapeutics market features approximately 12-15 meaningful players with moderate concentration, where the top three companies control roughly 35-45% of global market share through established oncology franchises, comprehensive clinical development programs, and extensive specialty pharmacy distribution networks. Competition centers on clinical efficacy demonstration, regulatory approval achievement, and payer reimbursement securing rather than price competition alone.
Market leaders include Merck & Co., Inc., Bristol-Myers Squibb Company, and Eli Lilly and Company, which maintain competitive advantages through comprehensive oncology portfolios, extensive clinical trial infrastructure, and deep expertise in immune-oncology drug development, creating high prescriber confidence among oncologists seeking proven therapeutic solutions. These companies leverage ongoing research collaborations and substantial investment in next-generation immunotherapy platforms to defend market positions while expanding into combination therapy applications and earlier treatment line settings.
Challengers encompass established pharmaceutical companies including AstraZeneca, Sanofi, and F. Hoffmann-La Roche Ltd., which compete through targeted oncology development programs and strategic positioning in specific treatment segments through differentiated mechanisms of action or combination therapy approaches. Specialty pharmaceutical companies, including Takeda Pharmaceutical Company Limited and Teva Pharmaceutical Industries Ltd., focus on specific geographic markets or biosimilar development strategies, offering competitive alternatives in price-sensitive healthcare systems.
Emerging biotechnology companies and specialized oncology developers create competitive pressure through innovative cellular therapy platforms and novel immunotherapy mechanisms, particularly in high-growth markets including USA and China, where clinical trial infrastructure provides advantages in accelerated drug development and early market access.
Market dynamics favor companies that combine robust clinical evidence generation with comprehensive patient support programs that address the complete treatment journey from diagnosis through long-term disease management. Strategic emphasis on biomarker identification, companion diagnostic development, and real-world evidence collection enables differentiation in increasingly competitive immuno-oncology segments across developed and emerging markets.
Global Head and Neck Cancer Therapeutics Market - Stakeholder Contribution Framework
Head and neck cancer therapeutics represent a critical oncology product category that enables patients with aggressive malignancies to achieve improved survival outcomes and disease control while managing complex treatment regimens and quality of life preservation without exclusive pharmaceutical dependency, typically providing targeted immune activation including enhanced tumor recognition, durable response maintenance, and manageable safety profiles compared to conventional chemotherapy alone while ensuring superior clinical outcomes and comprehensive patient support.
With the market projected to grow from USD 2.53 billion in 2025 to USD 8.05 billion by 2035 at a 12.3% CAGR, these solutions offer compelling advantages for immunotherapy applications, specialty pharmacy distribution channels, and diverse patient populations seeking evidence-based cancer treatment options. Scaling market penetration and clinical adoption requires coordinated action across healthcare policy, clinical research standards, pharmaceutical manufacturers, oncology providers, and patient advocacy initiatives.
How Could Governments Spur Local Development and Adoption?
- National Cancer Control Programs: Include head and neck cancer in comprehensive oncology strategies, providing targeted support for early detection campaigns in primary care settings and supporting academic medical centers through research grants and infrastructure funding.
- Reimbursement Policy & Access Support: Implement favorable coverage policies for novel immunotherapies, provide expedited health technology assessment processes for breakthrough cancer treatments, and establish transparent pricing frameworks that encourage manufacturer participation while ensuring patient access.
- Regulatory Framework Development: Create streamlined approval pathways for innovative oncology therapeutics across immunotherapy applications, establish clear biomarker validation requirements for companion diagnostics, and develop international regulatory harmonization protocols that facilitate multinational drug development.
- Healthcare Infrastructure Investment: Fund expansion of oncology treatment centers and infusion facilities, invest in healthcare provider training programs that enhance immuno-oncology expertise, and support specialty pharmacy network development for complex biologic distribution.
- Research & Innovation Support: Establish public-private partnerships for cancer immunotherapy research, support investigator-initiated clinical trials investigating novel treatment combinations, and create regulatory environments that encourage innovation in precision oncology approaches.
How Could Industry Bodies Support Market Development?
- Clinical Standards & Evidence: Define standardized efficacy endpoints for head and neck cancer immunotherapy research across survival, quality of life, and biomarker outcomes, establish universal response criteria and toxicity grading standards, and create evidence repositories that oncologists can rely on for treatment decisions.
- Market Education & Best Practices: Lead messaging that demonstrates immunotherapy benefits, emphasizing appropriate patient selection criteria, evidence-based treatment sequencing, and realistic outcome expectations compared to conventional chemotherapy approaches.
- Quality Assurance Standards: Develop guidelines for biologic manufacturing excellence, cold chain management requirements, and specialty pharmacy handling protocols, ensuring patient safety across production and distribution operations.
- Professional Development: Run certification programs for oncologists, oncology pharmacists, and nursing professionals on optimizing immunotherapy administration, immune-related adverse event management, and multidisciplinary care coordination in diverse practice settings.
How Could Manufacturers and Technology Players Strengthen the Ecosystem?
- Advanced Product Development: Develop next-generation immunotherapy formulations with enhanced efficacy profiles, optimized dosing schedules, and combination regimen characteristics that improve therapeutic effectiveness while reducing treatment burden and healthcare resource utilization.
- Clinical Validation Programs: Provide comprehensive Phase III trial data, long-term survival follow-up studies, and quality of life research that supports oncologist confidence and informed treatment decision-making.
- Patient Support Initiatives: Offer comprehensive copay assistance programs, nursing support services, and educational resources that help patients navigate complex treatment pathways aligned with personal health priorities and financial circumstances.
- Research & Development Networks: Build comprehensive clinical trial infrastructure, collaborative research consortia, and biomarker discovery programs that ensure continuous innovation and treatment optimization across diverse patient populations.
How Could Healthcare Providers and Educators Navigate the Market?
- Evidence-Based Protocol Integration: Incorporate validated immunotherapy protocols into multidisciplinary treatment algorithms, with particular focus on PD-1/PD-L1 inhibitors, combination therapy approaches, and biomarker-guided patient selection for optimal clinical outcomes.
- Patient Counseling Excellence: Establish comprehensive patient education programs addressing treatment expectations, potential side effects, and quality of life considerations through optimized consultation frameworks and shared decision-making protocols.
- Multidisciplinary Care Models: Implement integrated tumor board approaches combining medical oncologists, radiation oncologists, surgeons, and supportive care specialists that provide comprehensive head and neck cancer management addressing medical, functional, and psychosocial aspects.
- Outcomes Monitoring Systems: Develop standardized patient outcome tracking, toxicity surveillance programs, and quality of life assessments that enable continuous protocol refinement and real-world evidence generation.
How Could Investors and Financial Enablers Unlock Value?
- Clinical Research Financing: Provide growth capital for established pharmaceutical companies like Merck & Co., Inc. and Bristol-Myers Squibb Company to fund late-stage clinical trials and post-approval evidence generation supporting label expansion.
- Innovation Investment: Back biotechnology startups developing next-generation cellular therapies, novel checkpoint inhibitor targets, and tumor-specific vaccination platforms that enhance therapeutic options and patient outcomes.
- Market Expansion Funding: Finance geographic expansion strategies for pharmaceutical manufacturers establishing operations in high-growth regions including India and China, supporting local clinical trial infrastructure and regulatory capability development.
- Digital Health Integration: Support companies developing telemedicine oncology platforms, AI-driven treatment optimization algorithms, and patient monitoring applications that enhance treatment adherence and clinical outcomes through technology-enabled care delivery.
Key Players in the Head and Neck Cancer Therapeutics Market
- Merck & Co., Inc.
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- AstraZeneca
- Sanofi
- F. Hoffmann-La Roche Ltd.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
- Cumberland Pharmaceuticals Inc.
- Aprea Therapeutics
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 2.53 Billion |
| Therapy Type | Immunotherapy, Targeted Therapy, Chemotherapy, Others |
| Route of Administration | Injectable, Oral, Others |
| Distribution Channel | Retail & Specialty Pharmacies, Hospital Pharmacies, Online Pharmacies |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | India, China, USA, Germany, UK, Japan, Brazil, Saudi Arabia, and 40+ countries |
| Key Companies Profiled | Merck & Co., Inc., Bristol-Myers Squibb Company, Eli Lilly and Company, AstraZeneca, Sanofi, F. Hoffmann-La Roche Ltd., Takeda Pharmaceutical Company Limited, Teva Pharmaceutical Industries Ltd., Cumberland Pharmaceuticals Inc., Aprea Therapeutics |
| Additional Attributes | Dollar sales by therapy type and route of administration categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with pharmaceutical manufacturers and specialty pharmacy distribution companies, treatment protocol specifications and clinical guideline requirements, integration with biomarker testing platforms and precision medicine approaches, innovations in combination immunotherapy and cellular therapy development, and development of specialized applications with real-world evidence generation and patient outcomes measurement capabilities. |
Head and Neck Cancer Therapeutics Market by Segments
-
Therapy Type :
- Immunotherapy
- Targeted Therapy
- Chemotherapy
- Others
-
Route of Administration :
- Injectable
- Oral
- Others
-
Distribution Channel :
- Retail & Specialty Pharmacies
- Hospital Pharmacies
- Online Pharmacies
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Therapy Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Therapy Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Therapy Type, 2025 to 2035
- Immunotherapy
- Targeted Therapy
- Chemotherapy
- Others
- Y to o to Y Growth Trend Analysis By Therapy Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Therapy Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Route of Administration
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Route of Administration, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Route of Administration, 2025 to 2035
- Injectable
- Oral
- Others
- Y to o to Y Growth Trend Analysis By Route of Administration, 2020 to 2024
- Absolute $ Opportunity Analysis By Route of Administration, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Distribution Channel
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Distribution Channel, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Distribution Channel, 2025 to 2035
- Retail & Specialty Pharmacies
- Hospital Pharmacies
- Online Pharmacies
- Y to o to Y Growth Trend Analysis By Distribution Channel, 2020 to 2024
- Absolute $ Opportunity Analysis By Distribution Channel, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Therapy Type
- By Route of Administration
- By Distribution Channel
- Competition Analysis
- Competition Deep Dive
- Merck & Co., Inc.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- AstraZeneca
- Sanofi
- F. Hoffmann-La Roche Ltd.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
- Cumberland Pharmaceuticals Inc.
- Aprea Therapeutics
- Merck & Co., Inc.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Therapy Type
- Figure 6: Global Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Route of Administration
- Figure 9: Global Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Distribution Channel
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Therapy Type
- Figure 26: North America Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Route of Administration
- Figure 29: North America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Distribution Channel
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Therapy Type
- Figure 36: Latin America Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Route of Administration
- Figure 39: Latin America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Distribution Channel
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Therapy Type
- Figure 46: Western Europe Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Route of Administration
- Figure 49: Western Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by Distribution Channel
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Therapy Type
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Route of Administration
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by Distribution Channel
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Therapy Type
- Figure 66: East Asia Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Route of Administration
- Figure 69: East Asia Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by Distribution Channel
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Therapy Type
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Route of Administration
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by Distribution Channel
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Therapy Type
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Route of Administration
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by Distribution Channel
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the head and neck cancer therapeutics market in 2025?
The global head and neck cancer therapeutics market is estimated to be valued at USD 2.5 billion in 2025.
What will be the size of head and neck cancer therapeutics market in 2035?
The market size for the head and neck cancer therapeutics market is projected to reach USD 8.1 billion by 2035.
How much will be the head and neck cancer therapeutics market growth between 2025 and 2035?
The head and neck cancer therapeutics market is expected to grow at a 12.3% CAGR between 2025 and 2035.
What are the key product types in the head and neck cancer therapeutics market?
The key product types in head and neck cancer therapeutics market are immunotherapy, targeted therapy, chemotherapy and others.
Which route of administration segment to contribute significant share in the head and neck cancer therapeutics market in 2025?
In terms of route of administration, injectable segment to command 90.1% share in the head and neck cancer therapeutics market in 2025.