Demand for Artemisinin Combination Therapy in UK
Demand for Artemisinin Combination Therapy in UK Size and Share Forecast Outlook 2026 to 2036
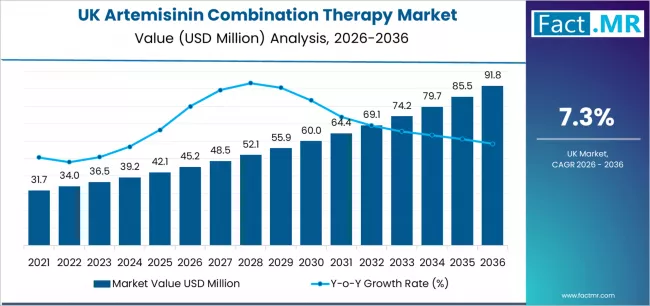
Demand for artemisinin combination therapy in UK is projected to grow from USD 45.2 million in 2026 to USD 91.8 million by 2036, at a CAGR of 7.3%. Artemether + Lumefantrine will dominate with a 42.5% market share, while nhs hospitals and specialist clinics will lead the distribution channel segment with a 48.7% share.
Demand for Artemisinin Combination Therapy in UK 2026 to 2036
Demand for artemisinin combination therapy in the UK is projected to rise from USD 45.2 million in 2026 to USD 91.8 million by 2036, supported by a CAGR of 7.3%. Growth reflects the steady flow of imported malaria cases and the continued prioritization of WHO-aligned treatment pathways across UK healthcare systems.
England remains the primary destination for deployment due to its concentration of specialist infectious disease units and expanding travel medicine networks. Clinical teams are strengthening therapeutic preparedness to address complex case presentations linked to diverse travel patterns and evolving parasite profiles.
Key Takeaways from the UK Artemisinin Combination Therapy Industry
- UK Artemisinin Combination Therapy Sales Value (2026): USD 45.2 million
- UK Artemisinin Combination Therapy Forecast Value (2036): USD 91.8 million
- UK Artemisinin Combination Therapy Forecast CAGR: 7.3%
- Leading Product Type in UK Artemisinin Combination Therapy Industry: Artemether + Lumefantrine (42.5%)
- Key Growth Regions in UK Artemisinin Combination Therapy Industry: England, Scotland, Wales, and Northern Ireland
- Regional Leadership: England holds the leading position in demand
- Key Players in UK Artemisinin Combination Therapy Industry: Novartis AG, Sanofi S.A., Cipla Limited, KPC Pharmaceuticals Incorporated, Fosun Pharmaceutical (Guilin Pharma) Company Limited, Ajanta Pharma Limited, Ipca Laboratories Limited, Desano Incorporated, Hovid Berhad, Mylan N.V.
NHS hospitals and specialist clinics are expected to account for 48.7% of overall demand in 2026. These facilities handle most malaria case management activities and maintain structured diagnostic and treatment workflows. Artemisinin combination therapy supports consistent patient stabilization, dependable parasite clearance, and rapid initiation protocols, which improves treatment continuity across varying case severities.
The artemether + lumefantrine category is projected to hold 42.5% of total demand in 2026. Its prominence stems from predictable pharmacokinetics, broad clinician familiarity, and stronger adherence profiles. The formulation aligns with standardized care pathways and supports resistance mitigation strategies that remain essential for imported malaria management.
Between 2030 and 2036, demand is expected to increase from USD 62.4 million to USD 91.8 million, contributing 63.1% of total ten-year expansion. This period reflects growing reliance on rapid molecular diagnostics, customized dosing protocols for vulnerable patient groups, and greater emphasis on treatment stewardship across regional healthcare clusters. England and Scotland continue to advance specialized clinical capabilities, driving adoption of more refined artemisinin combination therapy platforms. The UK’s landscape shows sustained progress as healthcare providers integrate advanced antimalarial tools, enhance diagnostic accuracy, and reinforce therapeutic consistency to support evolving travel-related disease burdens.
UK Artemisinin Combination Therapy Industry
| Metric | Value |
|---|---|
| UK Artemisinin Combination Therapy Sales Value (2026) | USD 45.2 million |
| UK Artemisinin Combination Therapy Forecast Value (2036) | USD 91.8 million |
| UK Artemisinin Combination Therapy Forecast CAGR (2026-2036) | 7.3% |
Category
| Category | Segment |
|---|---|
| Product | Artemether + Lumefantrine, Artesunate + Amodiaquine, Dihydroartemisinin + Piperaquine, Artesunate + Mefloquine, Pyronaridine + Artesunate, Others |
| Distribution Channel | NHS Hospitals and Specialist Clinics, Private Healthcare Facilities, Travel Medicine Centers, Others |
| Region | England, Scotland, Wales, Northern Ireland |
Segmental Analysis
By Product, Which Segment Holds the Largest Share?
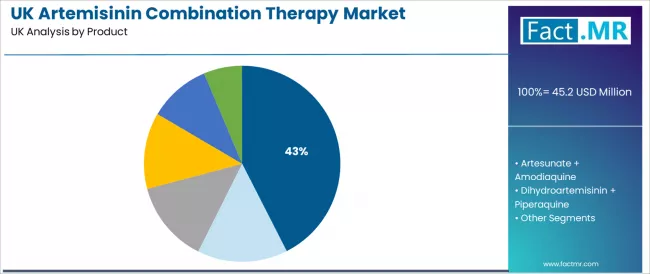
Artemether + lumefantrine is projected to account for 42.5% of artemisinin combination therapy demand in 2026, reflecting its position as the leading product. Clinicians favor this combination for its proven antimalarial effectiveness, consistent therapeutic performance, and compatibility with established treatment pathways. Artemether + lumefantrine adoption in the UK is supported by reliable clinical outcomes, broad practitioner familiarity, and the presence of experienced suppliers that reinforce confidence in large-scale use.
- Compatibility with current treatment protocols supports widespread adoption across healthcare settings.
- Demonstrated clinical reliability strengthens practitioner confidence and long-term utilization.
By Distribution Channel, Which Segment Registers the Highest Share?
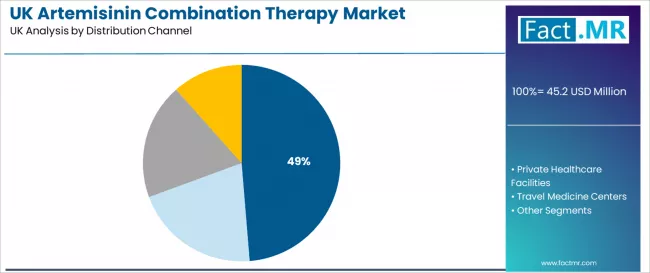
NHS hospitals and specialist clinics are expected to represent 48.7% of demand in 2026, underscoring their role as the primary distribution channel for artemisinin combination therapies. These facilities rely on consistent antimalarial solutions to support infectious disease programs, tropical medicine units, and broader patient-care workflows.The segment’s prominence reflects the UK’s centralized care structure, where treatment accessibility, clinical consistency, and integrated service delivery drive continued utilization across high-volume medical centers.
- Facility-level treatment needs sustain steady demand across NHS hospitals and specialist clinics.
- Integration of artemisinin-based therapies enhances care efficiency and reinforces treatment performance standards.
What are the Drivers, Restraints, and Key Trends in the UK Artemisinin Combination Therapy Industry?
The UK artemisinin combination therapy landscape is expanding as imported malaria cases rise and WHO-aligned treatment protocols gain broader clinical acceptance. England remains the centre of therapeutic development and application, while national guidelines and regional programs in England and Scotland continue to shape treatment selection and adoption cycles. The market still faces restraints, including competition from alternative antimalarial regimens, the need for precise diagnostic validation, and cost and regulatory pressures that influence procurement decisions.
Key trends point toward stronger clinical standards and consolidated validation systems that help suppliers demonstrate treatment reliability without escalating development costs. Healthcare providers are increasingly adopting integrated diagnostic technologies, coordinated quality-management networks, and optimization frameworks that support consistent therapy performance across facilities. These advancements strengthen travel-medicine capabilities, enable more efficient supply networks, while collaborative research models across the UK reduce validation burdens, and accelerate the introduction of next-generation antimalarial solutions.
Analysis of UK Artemisinin Combination Therapy Industry by Key Countries
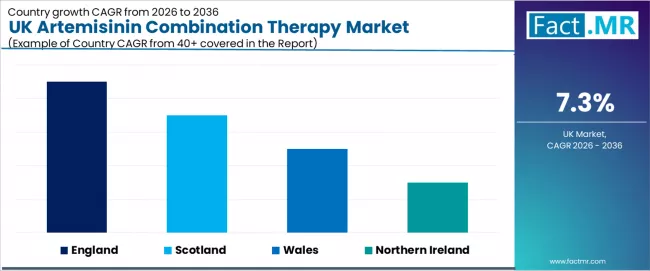
| Region | CAGR (2026-2036) |
|---|---|
| England | 7.5% |
| Scotland | 7.3% |
| Wales | 7.1% |
| Northern Ireland | 6.9% |
Why does England Lead Artemisinin Combination Therapy Demand?
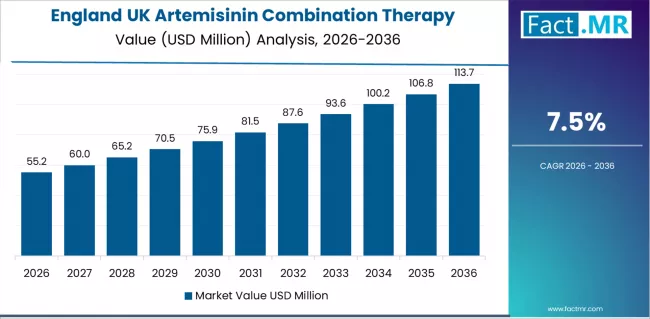
England shows the strongest expansion with a projected CAGR of 7.5% through 2036. Large healthcare networks and strong clinical leadership continue to shape adoption, supported by London’s concentration of medical research and practitioner expertise. Hospitals prioritize therapies with demonstrated reliability, creating sustained demand across treatment programs. Suppliers maintain broad development activities in the region to support ongoing innovation.
- Practitioner expectations drive preference for systems with validated clinical outcomes.
- A strong innovation ecosystem enables deployment of advanced artemisinin combination therapy technologies with long-term commercial feasibility.
Why does Scotland Show Strong Growth?
Scotland is expanding at a CAGR of 7.3%, supported by established healthcare clusters, travel medicine programs, and clinical expertise across Edinburgh, Glasgow, and regional corridors. Providers favor systems that integrate smoothly with existing operations, creating stable adoption patterns. Suppliers continue strengthening their presence to meet increasing therapeutic requirements.
- Patient clusters and favorable operational economics support specialized supplier engagement.
- Growing awareness of therapeutic performance builds Scotland’s competitive clinical position.
Why does Wales Show Consistent Expansion?
Wales is projected to grow at a CAGR of 7.1%, influenced by expanding healthcare services and greater reliance on proven therapeutic pathways. Providers are upgrading treatment capabilities to align with patient expectations and maintain operational competitiveness. Investments in distribution and integration infrastructure support continued adoption.
- Modernization efforts accelerate acceptance of artemisinin combination therapy across varied clinical settings.
- Therapeutic optimization opportunities shape usage patterns across Wales.
What Factors underpin Artemisinin Combination Therapy Demand in Northern Ireland?
Northern Ireland is advancing at a CAGR of 6.9%, supported by expanding clinical capacity and ongoing development across patient-care programs. Providers are evaluating artemisinin combination therapy as part of broader initiatives focused on treatment consistency and improved outcomes. Suppliers are strengthening regional capabilities to support evolving deployment needs.
- Expansion of patient services and diversification of clinical operations reinforce adoption incentives.
- Regional cooperation across facilities supports stable and coordinated deployment environments.
Competitive Landscape of UK Artemisinin Combination Therapy Industry
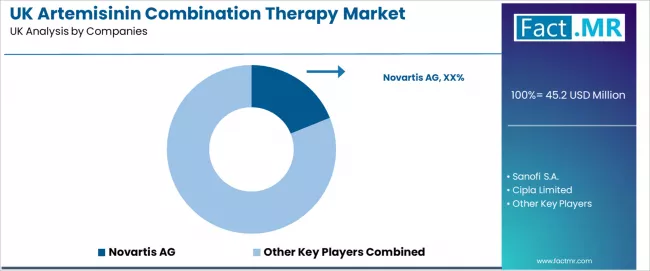
The competitive environment for artemisinin combination therapies in the UK revolves around firms that combine clinical development strength with reliable supply execution and rigorous regulatory compliance. Rivalry is driven by differentiated formulation strategies, evidence packages that support guideline inclusion, and capability in post-launch safety monitoring.
Novartis AG, Sanofi S.A., Cipla Limited and KPC Pharmaceuticals Inc. stand out because they pair global R&D footprints with established distribution channels and technical services that help health systems integrate treatments into clinical pathways. Commercial advantage increasingly depends on demonstrable outcomes, robust pharmacovigilance, and the ability to provide end-to-end support to institutional buyers and procurement consortia.
Public institutions and professional bodies materially influence how therapies reach patients and how industry investments are prioritised. Clearer licensing routes, procurement frameworks that reward supply resilience, and targeted fiscal incentives for domestic production would raise strategic capacity.
Clinical associations can shorten adoption cycles by harmonising treatment standards, certifying competence for deployment in travel-health and migrant-care settings, and facilitating multicentre trials. Shared data platforms for safety, adverse-event reporting, and adherence metrics would reduce duplication, enable faster clinical decision-making, and lower barriers for new entrants with promising formulations.
Operational players and capital providers determine which innovations scale and where capacity grows. Manufacturers can differentiate through batch-control automation, serialization for traceability, and integrated quality systems that reduce lot failures and recall risk. Healthcare providers and logistics partners should prioritise demand aggregation, predictable tender windows, and digital adherence tools to improve real-world effectiveness.
Investors and financiers can accelerate capacity upgrades and consolidation by structuring performance-linked financing, underwriting clinical-infrastructure projects, and supporting pilot programmes that validate new delivery models and commercial partnerships. Together these actions strengthen therapeutic availability while lowering procurement risk for large institutional buyers.
Key Players in UK Artemisinin Combination Therapy Industry
- Novartis AG
- Sanofi S.A.
- Cipla Limited
- KPC Pharmaceuticals Inc.
- Fosun Pharmaceutical (Guilin Pharma) Company Limited
- Ajanta Pharma Limited
- Ipca Laboratories Limited
- Desano Incorporated
- Hovid Berhad
- Mylan N.V.
Scope of the Report
| Item | Value |
|---|---|
| Quantitative Units | USD 91.8 million |
| Product | Artemether + Lumefantrine, Artesunate + Amodiaquine, Dihydroartemisinin + Piperaquine, Artesunate + Mefloquine, Pyronaridine + Artesunate, Others |
| Distribution Channel | NHS Hospitals and Specialist Clinics, Private Healthcare Facilities, Travel Medicine Centers, Others |
| Regions Covered | England, Scotland, Wales, Northern Ireland |
| Key Companies | Novartis AG, Sanofi S.A., Cipla Limited, KPC Pharmaceuticals Incorporated, Fosun Pharmaceutical (Guilin Pharma) Company Limited, Ajanta Pharma Limited, Ipca Laboratories Limited, Desano Incorporated, Hovid Berhad, Mylan N.V., Regional artemisinin combination therapy specialists |
| Additional Attributes | Sales by product and distribution channel segment; regional demand trends across England, Scotland, Wales, and Northern Ireland; competitive landscape with established artemisinin combination therapy suppliers and specialized clinical developers; quality preferences for artemether + lumefantrine versus artesunate + amodiaquine technologies; integration with clinical programs and advanced medical policies, particularly advanced in the England region |
UK Artemisinin Combination Therapy Industry by Segments
-
Product :
- Artemether + Lumefantrine
- Artesunate + Amodiaquine
- Dihydroartemisinin + Piperaquine
- Artesunate + Mefloquine
- Pyronaridine + Artesunate
- Others
-
Distribution Channel :
- NHS Hospitals and Specialist Clinics
- Private Healthcare Facilities
- Travel Medicine Centers
- Others
-
Region :
- England
- Scotland
- Wales
- Northern Ireland
Table of Content
- Executive Summary
- UK Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- UK Market Analysis 2021 to 2025 and Forecast, 2026 to 2036
- Historical Market Size Value (USD Million) Analysis, 2021 to 2025
- Current and Future Market Size Value (USD Million) Projections, 2026 to 2036
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- UK Market Pricing Analysis 2021 to 2025 and Forecast 2026 to 2036
- UK Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2026 to 2036
- Artemether + Lumefantrine
- Artesunate + Amodiaquine
- Dihydroartemisinin + Piperaquine
- Artesunate + Mefloquine
- Pyronaridine + Artesunate
- Others
- Y to o to Y Growth Trend Analysis By Product, 2021 to 2025
- Absolute $ Opportunity Analysis By Product, 2026 to 2036
- UK Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Distribution Channel
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Distribution Channel, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Distribution Channel, 2026 to 2036
- NHS Hospitals and Specialist Clinics
- Private Healthcare Facilities
- Travel Medicine Centers
- Others
- Y to o to Y Growth Trend Analysis By Distribution Channel, 2021 to 2025
- Absolute $ Opportunity Analysis By Distribution Channel, 2026 to 2036
- UK Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2021 to 2025
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2026 to 2036
- UK
- Market Attractiveness Analysis By Region
- UK Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- By Product
- By Distribution Channel
- Market Attractiveness Analysis
- By Country
- By Product
- By Distribution Channel
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product
- By Distribution Channel
- Competition Analysis
- Competition Deep Dive
- Novartis AG
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Sanofi S.A.
- Cipla Limited
- KPC Pharmaceuticals Incorporated
- Fosun Pharmaceutical (Guilin Pharma) Company Limited
- Ajanta Pharma Limited
- Ipca Laboratories Limited
- Desano Incorporated
- Hovid Berhad
- Mylan N.V.
- Novartis AG
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: UK Market Value (USD Million) Forecast by Region, 2021 to 2036
- Table 2: UK Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 3: UK Market Value (USD Million) Forecast by Distribution Channel, 2021 to 2036
- Table 4: UK Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 5: UK Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 6: UK Market Value (USD Million) Forecast by Distribution Channel, 2021 to 2036
List Of Figures
- Figure 1: UK Market Pricing Analysis
- Figure 2: UK Market Value (USD Million) Forecast 2021 to 2036
- Figure 3: UK Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 4: UK Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 5: UK Market Attractiveness Analysis by Product
- Figure 6: UK Market Value Share and BPS Analysis by Distribution Channel, 2026 and 2036
- Figure 7: UK Market Y to o to Y Growth Comparison by Distribution Channel, 2026 to 2036
- Figure 8: UK Market Attractiveness Analysis by Distribution Channel
- Figure 9: UK Market Value (USD Million) Share and BPS Analysis by Region, 2026 and 2036
- Figure 10: UK Market Y to o to Y Growth Comparison by Region, 2026 to 2036
- Figure 11: UK Market Attractiveness Analysis by Region
- Figure 12: UK Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 13: UK Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 14: UK Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 15: UK Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 16: UK Market Attractiveness Analysis by Product
- Figure 17: UK Market Value Share and BPS Analysis by Distribution Channel, 2026 and 2036
- Figure 18: UK Market Y to o to Y Growth Comparison by Distribution Channel, 2026 to 2036
- Figure 19: UK Market Attractiveness Analysis by Distribution Channel
- Figure 20: UK Market - Tier Structure Analysis
- Figure 21: UK Market - Company Share Analysis
- FAQs -
How big is the demand for artemisinin combination therapy in UK in 2026?
The demand for artemisinin combination therapy in UK is estimated to be valued at USD 45.2 million in 2026.
What will be the size of artemisinin combination therapy in UK in 2036?
The market size for the artemisinin combination therapy in UK is projected to reach USD 91.8 million by 2036.
How much will be the demand for artemisinin combination therapy in UK growth between 2026 and 2036?
The demand for artemisinin combination therapy in UK is expected to grow at a 7.3?GR between 2026 and 2036.
What are the key product types in the artemisinin combination therapy in UK?
The key product types in artemisinin combination therapy in UK are artemether + lumefantrine, artesunate + amodiaquine, dihydroartemisinin + piperaquine, artesunate + mefloquine, pyronaridine + artesunate and others.
Which distribution channel segment is expected to contribute significant share in the artemisinin combination therapy in UK in 2026?
In terms of distribution channel, nhs hospitals and specialist clinics segment is expected to command 48.7% share in the artemisinin combination therapy in UK in 2026.