Omeprazole Sulfone Market
Omeprazole Sulfone Market Size and Share Forecast Outlook 2025 to 2035
Omeprazole sulfone market is projected to grow from USD 380.0 million in 2025 to USD 510.0 million by 2035, at a CAGR of 3.0%. ≥ 99% will dominate with a 70.0% market share, while api intermediate will lead the application segment with a 80.0% share.
Omeprazole Sulfone Market Forecast and Outlook 2025 to 2035
The global omeprazole sulfone market is anticipated to grow from USD 380.0 million in 2025 to approximately USD 510.0 million by 2035, recording an absolute increase of USD 130.0 million over the forecast period. This translates into a total growth of 34.2%, with the market forecast to expand at a CAGR of 3.0% between 2025 and 2035. The overall market size is expected to grow by nearly 1.3 times during the same period, supported by increasing demand for high-purity pharmaceutical intermediates, rising adoption of advanced API manufacturing processes, and growing focus on quality-driven pharmaceutical production across the global pharmaceutical and research sectors.
Quick Stats for Omeprazole Sulfone Market
- Omeprazole Sulfone Market Value (2025): USD 380.0 million
- Omeprazole Sulfone Market Forecast Value (2035): USD 510.0 million
- Omeprazole Sulfone Market Forecast CAGR: 3.0%
- Leading Purity in Omeprazole Sulfone Market: ≥ 99% (70.0%)
- Key Growth Regions in Omeprazole Sulfone Market: Asia Pacific, North America, and Europe
- Key Players in Omeprazole Sulfone Market: Teva, Dr. Reddy's, Lupin, Sandoz, Aurobindo, Sun Pharma, Cipla, Hetero, Zhejiang Huahai, Amneal
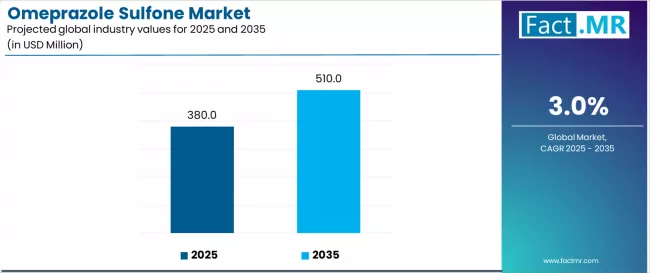
Between 2025 and 2030, the market is projected to expand from USD 380.0 million to USD 440.6 million, resulting in a value increase of USD 60.6 million, which represents 46.6% of the total forecast growth for the decade. This phase of development will be shaped by rising demand for premium pharmaceutical intermediates, increasing applications in API manufacturing optimization, and growing penetration in emerging pharmaceutical markets. Pharmaceutical manufacturers are expanding their omeprazole sulfone capabilities to address the growing demand for high-purity intermediates in various pharmaceutical segments and specialized production programs.
Omeprazole Sulfone Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value in (2025E) | USD 380.0 million |
| Forecast Value in (2035F) | USD 510.0 million |
| Forecast CAGR (2025 to 2035) | 3.0% |
From 2030 to 2035, the market is forecast to grow from USD 440.6 million to USD 510.0 million, adding another USD 69.4 million, which constitutes 53.4% of the overall ten-year expansion. This period is expected to be characterized by the expansion of advanced manufacturing platforms, the integration of cutting-edge pharmaceutical technologies, and the development of customized intermediate production systems for specific pharmaceutical applications. The growing adoption of high-purity pharmaceutical standards and manufacturing requirements will drive demand for ultra-high efficiency production systems with enhanced purity specifications and consistent quality characteristics.
Between 2020 and 2025, the market experienced steady expansion, driven by increasing recognition of pharmaceutical intermediate technologies' importance in manufacturing operations and growing acceptance of high-purity solutions in complex pharmaceutical markets. The market developed as manufacturers recognized the need for high-efficiency production systems to address pharmaceutical requirements and improve overall quality control. Research and development activities have begun to emphasize the importance of advanced intermediate technologies in achieving better efficiency and quality in pharmaceutical manufacturing processes.
Why is the Omeprazole Sulfone Market Growing?
Market expansion is being supported by the increasing demand for high-purity pharmaceutical intermediates and the corresponding need for high-efficiency production systems in omeprazole sulfone applications across global pharmaceutical and research operations. Modern pharmaceutical manufacturers are increasingly focused on advanced intermediate technologies that can improve quality control, reduce production costs, and enhance manufacturing efficiency while meeting stringent purity requirements. The proven efficacy of omeprazole sulfone in various pharmaceutical applications makes it an essential component of comprehensive API manufacturing strategies and specialized production programs.
The growing focus on pharmaceutical industry transformation and advanced quality optimization is driving demand for ultra-efficient intermediate systems that meet stringent performance specifications and manufacturing requirements for pharmaceutical applications. Pharmaceutical manufacturers' preference for reliable, high-performance production systems that can ensure consistent quality standards is creating opportunities for innovative omeprazole sulfone technologies and customized manufacturing solutions. The rising influence of regulatory requirements and pharmaceutical protocols is also contributing to increased adoption of premium-grade intermediate systems across different pharmaceutical applications and manufacturing systems requiring advanced purity technology.
Opportunity Pathways - Omeprazole Sulfone Market
The omeprazole sulfone market represents a specialized growth opportunity, expanding from USD 380.0 million in 2025 to USD 510.0 million by 2035 at a 3.0% CAGR. As manufacturers prioritize quality control, regulatory compliance, and manufacturing performance in complex pharmaceutical processes, intermediate systems have evolved from a niche pharmaceutical technology to an essential component enabling quality assurance, production optimization, and multi-stage manufacturing production across pharmaceutical operations and specialized research applications.
The convergence of pharmaceutical expansion, increasing intermediate adoption, specialized manufacturing organization growth, and purity requirements creates momentum in demand. High-purity formulations offering superior performance characteristics, cost-effective ≥ 99% systems balancing performance with economics, and specialized pharmaceutical-grade variants for premium applications will capture market premiums, while geographic expansion into high-growth Asian pharmaceutical markets and emerging market penetration will drive volume leadership. Manufacturing focus on quality assurance and regulatory reliability provides structural support.
- Pathway A - High Purity Dominance: Leading with 70.0% market share, ≥ 99% purity applications drive primary demand through complex pharmaceutical workflows requiring comprehensive intermediate systems for specialized manufacturing. Advanced formulations enabling improved quality control, reduced impurity levels, and enhanced manufacturing efficiency command premium pricing from manufacturers requiring stringent purity specifications and regulatory compliance. Expected revenue pool: USD 266.0-357.0 million.
- Pathway B - API Intermediate Leadership: Dominating with 80.0% market share through an optimal balance of performance and cost-effectiveness, API intermediate serves most pharmaceutical applications while meeting manufacturing requirements. This application addresses both performance standards and economic considerations, making it the preferred choice for pharmaceutical operations seeking reliable intermediate performance. Opportunity: USD 304.0-408.0 million.
- Pathway C - Asian Market Acceleration: India (4.0% CAGR) and China (3.4% CAGR) lead global growth through pharmaceutical infrastructure expansion, manufacturing capability development, and domestic intermediate demand. Strategic partnerships with local manufacturers, regulatory compliance expertise, and supply chain localization enable the expansion of intermediate technology in major pharmaceutical hubs. Geographic expansion upside: USD 80.0-120.0 million.
- Pathway D - Pharmaceutical Grade Premium Segment: Pharmaceutical grade serves specialized applications requiring exceptional purity specifications for critical manufacturing processes. Pharmaceutical formulations supporting consistent quality requirements, complex API applications, and regulatory-sensitive processes command significant premiums from advanced pharmaceutical organizations and specialized manufacturing facilities. Revenue potential: USD 342.0-459.0 million.
- Pathway E - Advanced Manufacturing & Technology Systems: Companies investing in sophisticated intermediate technologies, specialized production systems, and automated manufacturing processes gain competitive advantages through consistent system performance and quality reliability. Advanced capabilities enabling customized specifications and rapid deployment capture premium pharmaceutical partnerships. Technology premium: USD 50.0-80.0 million.
- Pathway F - Supply Chain Optimization & Reliability: Specialized distribution networks, strategic inventory management, and reliable supply chain systems create competitive differentiation in pharmaceutical markets requiring consistent intermediate availability. Companies offering guaranteed supply security, technical support, and regulatory documentation gain preferred supplier status with compliance-focused manufacturers. Supply chain value: USD 40.0-70.0 million.
- Pathway G - Emerging Applications & Market Development: Beyond traditional API intermediates, omeprazole sulfone in specialty research, advanced pharmaceutical formulations, and novel manufacturing systems represent growth opportunities. Companies developing new applications, supporting R&D initiatives, and expanding into adjacent pharmaceutical markets capture incremental demand while diversifying revenue streams. Emerging opportunity: USD 30.0-60.0 million.
Segmental Analysis
The market is segmented by purity, application, grade, and region. By purity, the market is divided into ≥ 99% and < 99%. Based on application, the market is categorized into API intermediate and research. By grade, the market is divided into pharma and lab. Regionally, the market is divided into Asia Pacific, North America, Europe, Latin America, Middle East & Africa.
By Purity, ≥ 99% Segment Accounts for 70.0% Market Share
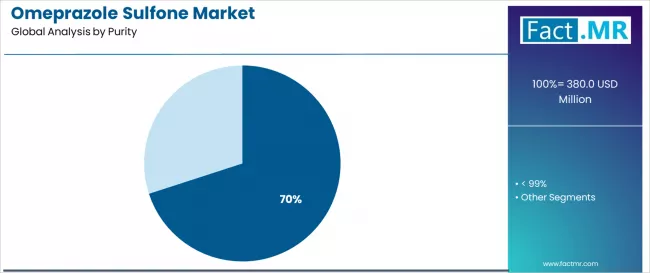
The ≥ 99% purity segment is projected to account for 70.0% of the market in 2025, reaffirming its position as the category's dominant purity level. Manufacturers increasingly recognize the optimal balance of performance and regulatory compliance offered by ≥ 99% purity for most pharmaceutical applications, particularly in API intermediate production and manufacturing processes. This purity level addresses both performance requirements and regulatory considerations while providing reliable quality across diverse pharmaceutical applications.
This purity level forms the foundation of most pharmaceutical protocols for intermediate applications, as it represents the most widely accepted and commercially viable level of omeprazole sulfone technology in the industry. Quality control standards and extensive regulatory testing continue to strengthen confidence in ≥ 99% purity formulations among pharmaceutical and manufacturing providers. With increasing recognition of the quality-performance optimization requirements in pharmaceutical manufacturing, ≥ 99% purity systems align with both operational efficiency and regulatory compliance goals, making them the central growth driver of comprehensive pharmaceutical manufacturing strategies.
By Application, API Intermediate Segment Accounts for 80.0% Market Share
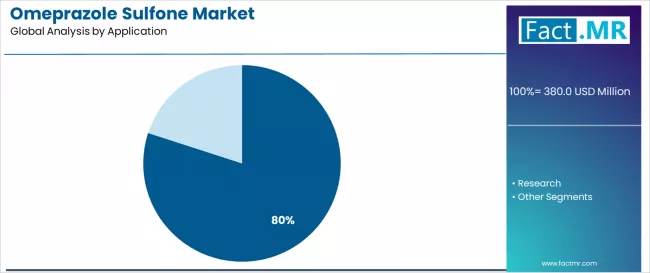
API intermediate is projected to represent 80.0% of omeprazole sulfone demand in 2025, highlighting its role as the primary application segment driving market adoption and growth. Manufacturers recognize that API intermediate requirements, including complex pharmaceutical processes, specialized manufacturing needs, and multi-stage production systems, often require advanced intermediate services that standard pharmaceutical technologies cannot adequately provide. Omeprazole sulfone offers enhanced manufacturing efficiency and regulatory compliance in API intermediate pharmaceutical applications.
The segment is supported by the growing nature of API intermediate adoption, requiring sophisticated production systems, and the increasing recognition that advanced intermediate technologies can improve pharmaceutical performance and manufacturing outcomes. Manufacturers are increasingly adopting evidence-based pharmaceutical guidelines that recommend specific intermediate services for optimal production efficiency. As understanding of pharmaceutical complexity advances and manufacturing requirements become more stringent, intermediate services will continue to play a crucial role in comprehensive pharmaceutical strategies within the API intermediate market.
What are the Drivers, Restraints, and Key Trends of the Omeprazole Sulfone Market?
The omeprazole sulfone market is advancing steadily due to increasing recognition of pharmaceutical intermediate technologies' importance and growing demand for high-purity production systems across the pharmaceutical and research sectors. The market faces challenges, including complex regulatory processes, potential for purity variations during production and storage, and concerns about supply chain consistency for specialized pharmaceutical equipment. Innovation in intermediate technologies and customized pharmaceutical protocols continues to influence product development and market expansion patterns.
Expansion of Advanced Manufacturing Facilities and Intermediate Technologies
The growing adoption of advanced manufacturing facilities is enabling the development of more sophisticated intermediate production and quality control systems that can meet stringent pharmaceutical requirements. Specialized manufacturing facilities offer comprehensive intermediate services, including advanced purification and quality processes that are particularly important for achieving high-purity requirements in pharmaceutical applications. Advanced manufacturing channels provide access to premium services that can optimize production efficiency and reduce manufacturing costs while maintaining cost-effectiveness for large-scale pharmaceutical operations.
Integration of Digital Manufacturing Systems and Quality Management Systems
Modern pharmaceutical organizations are incorporating digital technologies such as real-time quality monitoring, automated purification systems, and supply chain integration to enhance intermediate production and distribution processes. These technologies improve production performance, enable continuous quality monitoring, and provide better coordination between suppliers and manufacturers throughout the intermediate cycle. Advanced digital platforms also enable customized purity specifications and early identification of potential quality deviations or supply disruptions, supporting reliable pharmaceutical production.
Analysis of the Omeprazole Sulfone Market by Key Country
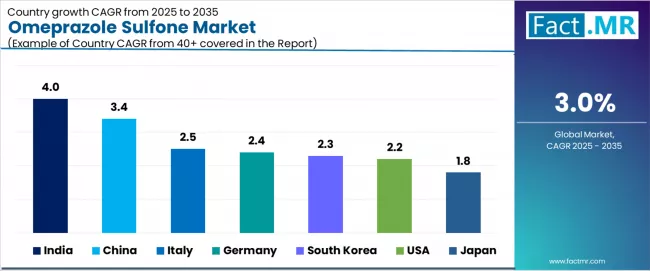
| Country | CAGR (2025-2035) |
|---|---|
| India | 4.0% |
| China | 3.4% |
| Italy | 2.5% |
| Germany | 2.4% |
| South Korea | 2.3% |
| USA | 2.2% |
| Japan | 1.8% |
The market is experiencing varied growth globally, with India leading at a 4.0% CAGR through 2035, driven by the expansion of pharmaceutical infrastructure development, increasing manufacturing capability capabilities, and growing domestic demand for high-purity intermediate systems. China follows at 3.4%, supported by pharmaceutical expansion, growing recognition of advanced intermediate technology importance, and expanding manufacturing capacity. Italy records 2.5% growth, while Germany shows 2.4% growth, both with focus on developing the pharmaceutical technology and manufacturing industries. South Korea demonstrates 2.3% growth with focus on pharmaceutical manufacturing adoption. The USA shows 2.2% growth, representing a mature market with established manufacturing patterns and regulatory frameworks. Japan demonstrates 1.8% growth, focusing pharmaceutical infrastructure expansion and systematic manufacturing approaches.
India Demonstrates Growing Market Potential with Pharmaceutical Infrastructure Development
India is projected to exhibit robust growth with a CAGR of 4.0% through 2035, driven by ongoing pharmaceutical expansion and increasing recognition of high-purity intermediate systems as essential manufacturing components for complex pharmaceutical processes. The country's expanding pharmaceutical infrastructure and growing availability of specialized manufacturing capabilities are creating significant opportunities for intermediate adoption across both domestic and export-oriented pharmaceutical facilities. Major international and domestic pharmaceutical companies are establishing comprehensive production and distribution networks to serve the growing population of manufacturers and pharmaceutical facilities requiring high-performance intermediate systems across API and research applications throughout India's major pharmaceutical hubs.
The Indian government's strategic focus on pharmaceutical manufacturing modernization and industry advancement is driving substantial investments in specialized production capabilities. This policy support, combined with the country's large domestic pharmaceutical market and expanding manufacturing requirements, creates a favorable environment for the omeprazole sulfone market development. Indian manufacturers are increasingly focusing on high-value pharmaceutical technologies to improve manufacturing capabilities, with intermediate services representing a key component in this pharmaceutical transformation.
- Government initiatives supporting pharmaceutical development and manufacturing modernization are driving demand for high-purity intermediate systems throughout major pharmaceutical and manufacturing centers, including Mumbai, Hyderabad, and Bangalore regions.
- Manufacturing capacity expansion and production system development are supporting appropriate utilization of intermediate services among manufacturers and pharmaceutical facilities nationwide, with particular growth in API manufacturing operations and research engagement services.
China Demonstrates Exceptional Market Potential with Manufacturing Growth
China is expanding at a CAGR of 3.4%, supported by increasing pharmaceutical accessibility, growing manufacturing awareness, and developing technology market presence across the country's major pharmaceutical clusters. The country's large pharmaceutical sector and increasing recognition of advanced intermediate systems are driving demand for effective high-purity manufacturing solutions in both API production and research applications. International pharmaceutical companies and domestic providers are establishing comprehensive distribution channels to serve the growing demand for quality intermediate systems while supporting the country's position as an emerging pharmaceutical technology market.
China's pharmaceutical sector continues to benefit from favorable manufacturing policies, expanding pharmaceutical capabilities, and cost-competitive production infrastructure development. The country's focus on becoming a global pharmaceutical technology hub is driving investments in specialized intermediate technology and manufacturing infrastructure. This development is particularly important for intermediate applications, as manufacturers seek reliable domestic sources for critical production technologies to reduce import dependency and improve supply chain security.
- Rising awareness about advanced manufacturing options and improving pharmaceutical capabilities are creating opportunities for specialized intermediate systems across API production and research settings in major hubs like Beijing, Shanghai, and Guangzhou.
- Growing pharmaceutical infrastructure development and technology adoption are supporting increased access to high-purity intermediate services among organizations requiring comprehensive manufacturing capabilities, particularly in API production and pharmaceutical research organizations.
USA Maintains Technology Leadership
The USA's advanced pharmaceutical technology market demonstrates sophisticated manufacturing deployment with documented intermediate effectiveness in pharmaceutical departments and research centers through integration with existing manufacturing systems and production infrastructure. The country leverages manufacturing expertise in pharmaceutical technology and production systems integration to maintain a 2.2% CAGR through 2035. Manufacturing centers, including major metropolitan areas, showcase premium installations where intermediate systems integrate with comprehensive pharmaceutical information systems and production platforms to optimize manufacturing accuracy and operational workflow effectiveness.
American manufacturers prioritize system reliability and regulatory compliance in pharmaceutical development, creating demand for premium systems with advanced features, including quality validation and integration with US pharmaceutical standards. The market benefits from established pharmaceutical industry infrastructure and a willingness to invest in advanced intermediate technologies that provide long-term operational benefits and compliance with regulatory requirements.
Germany Shows Strong Regional Leadership
Germany's market expansion benefits from diverse pharmaceutical demand, including manufacturing modernization in major cities, production development programs, and government pharmaceutical programs that increasingly incorporate intermediate solutions for manufacturing enhancement applications. The country maintains a 2.4% CAGR through 2035, driven by rising manufacturing awareness and increasing adoption of production benefits, including superior manufacturing capabilities and reduced complexity.
Market dynamics focus on cost-effective intermediate solutions that balance advanced manufacturing features with affordability considerations important to German pharmaceutical operators. Growing pharmaceutical infrastructure creates demand for modern production systems in new manufacturing facilities and pharmaceutical equipment modernization projects.
Strategic Market Considerations:
- Pharmaceutical and API manufacturing segments leading growth with focus on production enhancement and operational efficiency applications
- Regional manufacturing requirements are driving a diverse product portfolio from basic intermediate systems to advanced production platforms
- Import dependency challenges offset by potential local development partnerships with international manufacturing companies
- Government pharmaceutical initiatives beginning to influence procurement standards and manufacturing requirements
Italy Shows Steady Market Growth
Italy demonstrates steady market development with a 2.5% CAGR through 2035, distinguished by pharmaceutical operators' preference for high-quality intermediate systems that integrate seamlessly with existing manufacturing equipment and provide reliable long-term operation in specialized pharmaceutical applications. The market prioritizes advanced features, including precision production algorithms, quality validation, and integration with comprehensive pharmaceutical platforms that reflect Italian manufacturing expectations for technological advancement and operational excellence.
Italian manufacturers focus on system reliability and precision in intermediate development, creating demand for premium systems with advanced features including automated quality monitoring and comprehensive technical support. The market benefits from established pharmaceutical research infrastructure and investment in production technology that provides long-term manufacturing benefits.
Strategic Market Indicators:
- Premium focus on precision systems with advanced production algorithms and high-reliability capabilities
- Integration requirements with existing pharmaceutical information systems and manufacturing management platforms
- Focus on intermediate reliability and long-term performance in pharmaceutical applications
South Korea Emphasizes Digital Innovation
South Korea demonstrates strong market development with a 2.3% CAGR through 2035, driven by advanced digital infrastructure and manufacturing preference for technology-integrated intermediate services. The country's sophisticated pharmaceutical ecosystem and high technology penetration rates are creating significant opportunities for intermediate adoption across both domestic and technology-driven pharmaceutical facilities. Major international and domestic pharmaceutical companies are establishing comprehensive digital production and distribution networks to serve the growing population of tech-savvy manufacturers and pharmaceutical facilities requiring high-performance intermediate systems across specialized and pharmaceutical applications throughout South Korea's major pharmaceutical hubs.
The South Korean government's strategic focus on digital transformation and pharmaceutical advancement is driving substantial investments in specialized production capabilities. This policy support, combined with the country's digitally advanced manufacturing base and expanding technology requirements, creates a favorable environment for the omeprazole sulfone market development. South Korean manufacturers are increasingly focusing on high-value digital pharmaceutical technologies to improve manufacturing capabilities, with intermediate services representing a key component in this pharmaceutical transformation.
- Government initiatives supporting digital pharmaceutical development and technology modernization are driving demand for high-purity intermediate systems throughout major pharmaceutical and manufacturing centers, including Seoul, Busan, and Incheon regions.
- Technology capacity expansion and digital production system development are supporting appropriate utilization of intermediate services among manufacturers and pharmaceutical facilities nationwide, with particular growth in automated operations and digital manufacturing engagement services.
Japan Emphasizes Precision and Integration Excellence
Japan demonstrates steady market development with a 1.8% CAGR through 2035, distinguished by pharmaceutical operators' preference for high-quality intermediate systems that integrate seamlessly with existing manufacturing equipment and provide reliable long-term operation in specialized pharmaceutical applications. The market prioritizes advanced features, including precision production algorithms, quality validation, and integration with comprehensive pharmaceutical platforms that reflect Japanese manufacturing expectations for technological advancement and operational excellence.
Japanese manufacturers focus on system reliability and precision in intermediate development, creating demand for premium systems with advanced features including automated quality monitoring and comprehensive technical support. The market benefits from established pharmaceutical research infrastructure and investment in production technology that provides long-term manufacturing benefits.
Strategic Market Indicators:
- Premium focus on precision systems with advanced production algorithms and high-reliability capabilities
- Integration requirements with existing pharmaceutical information systems and manufacturing management platforms
- Focus on intermediate reliability and long-term performance in pharmaceutical applications
Europe Market Split by Country
The omeprazole sulfone market in Europe is projected to show steady growth, with individual country performance varying across the region. Germany is expected to maintain its leadership position with a market value of USD 85.0 million in 2025, supported by its advanced pharmaceutical infrastructure, precision manufacturing capabilities, and strong research presence throughout major pharmaceutical regions.
UK follows with USD 60.0 million in 2025, driven by advanced production protocols, pharmaceutical innovation integration, and expanding specialty manufacturing networks serving both domestic and international markets. France holds USD 50.0 million in 2025, supported by pharmaceutical infrastructure expansion and growing adoption of high-purity intermediate systems. Italy commands USD 40.0 million in 2025, while Spain accounts for USD 30.0 million in 2025. The Rest of Europe region, including Nordic countries, Eastern Europe, and smaller Western European markets, holds USD 115.0 million in 2025, representing diverse market opportunities with established pharmaceutical and manufacturing capabilities.
Competitive Landscape of the Omeprazole Sulfone Market
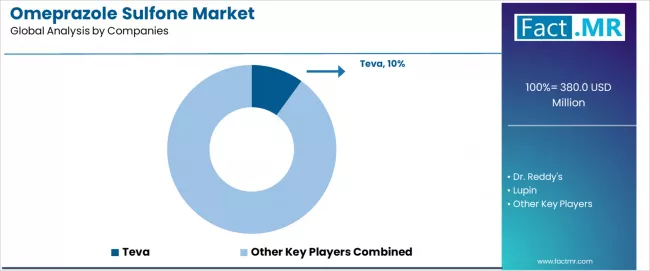
The market is characterized by competition among established pharmaceutical companies, specialty intermediate companies, and manufacturing technology suppliers focused on delivering high-purity, consistent, and reliable intermediate systems. Companies are investing in production technology advancement, quality control enhancement, strategic partnerships, and customer technical support to deliver effective, efficient, and reliable intermediate solutions that meet stringent pharmaceutical and research requirements. Quality optimization, purity validation protocols, and supply chain strategies are central to strengthening product portfolios and market presence.
Teva leads the market with comprehensive high-purity intermediate system offerings with a focus on quality consistency and production reliability for pharmaceutical applications, holding a 10.0% market share. Dr. Reddy's provides specialized manufacturing systems with focus on API intermediate applications and comprehensive technical support services. Lupin focuses on advanced intermediate technologies and customized pharmaceutical solutions for production systems serving global markets. Sandoz delivers established manufacturing systems with strong quality control systems and customer service capabilities.
Aurobindo operates with a focus on bringing innovative intermediate technologies to specialized pharmaceutical applications and emerging markets. Sun Pharma provides comprehensive production system portfolios, including advanced intermediate services, across multiple pharmaceutical applications and manufacturing processes. Cipla specializes in customized manufacturing solutions and quality management systems for pharmaceutical systems with focus on regulatory compliance. Hetero provides reliable supply chain solutions and technical expertise to enhance market accessibility and customer access to essential intermediate systems.
Key Players in the Omeprazole Sulfone Market
- Teva
- Dr. Reddy's
- Lupin
- Sandoz
- Aurobindo
- Sun Pharma
- Cipla
- Hetero
- Zhejiang Huahai
- Amneal
- Torrent
- Alkem
- Zydus
- Jubilant
- Apotex
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 380.0 million |
| Purity | ≥ 99%, < 99% |
| Application | API Intermediate, Research |
| Grade | Pharma, Lab |
| Regions Covered | Asia Pacific, North America, Europe, Latin America, Middle East & Africa |
| Countries Covered | China, USA, Germany, Japan, India, South Korea, Italy, France, UK, Spain and 50+ countries |
| Key Companies Profiled | Teva, Dr. Reddy's, Lupin, Sandoz, Aurobindo, Sun Pharma, Cipla, and Hetero |
| Additional Attributes | Dollar sales by purity and application, regional demand trends, competitive landscape, pharmaceutical provider preferences for specific intermediate systems, integration with specialty manufacturing supply chains, innovations in production technologies, quality monitoring, and manufacturing optimization |
Omeprazole Sulfone Market by Segments
-
Purity :
- ≥ 99%
- < 99%
-
Application :
- API Intermediate
- Research
-
Grade :
- Pharma
- Lab
-
Region :
-
Asia Pacific
- China
- India
- Japan
- South Korea
- Australia & New Zealand
- Thailand
- Singapore
- Malaysia
- Indonesia
- Philippines
- Vietnam
- Taiwan
- Hong Kong
- ASEAN
- Rest of Asia Pacific
-
North America
- United States
- Canada
- Mexico
-
Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Netherlands
- Belgium
- Switzerland
- Austria
- Sweden
- Norway
- Denmark
- Finland
- Poland
- Czech Republic
- Hungary
- Romania
- Portugal
- Ireland
- Greece
- Nordic
- BENELUX
- Rest of Europe
-
Latin America
- Brazil
- Argentina
- Chile
- Mexico
- Colombia
- Peru
- Venezuela
- Ecuador
- Uruguay
- Rest of Latin America
-
Middle East & Africa
- Kingdom of Saudi Arabia
- United Arab Emirates
- Other GCC Countries
- Turkey
- South Africa
- Egypt
- Nigeria
- Morocco
- Israel
- Kenya
- Other African Countries
- Rest of Middle East & Africa
-
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Purity
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Purity , 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Purity , 2025 to 2035
- ≥ 99%
- < 99%
- Y to o to Y Growth Trend Analysis By Purity , 2020 to 2024
- Absolute $ Opportunity Analysis By Purity , 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- API Intermediate
- Research
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Purity
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Purity
- By Application
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Purity
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Purity
- By Application
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Purity
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Purity
- By Application
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Purity
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Purity
- By Application
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Purity
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Purity
- By Application
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Purity
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Purity
- By Application
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Purity
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Purity
- By Application
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Purity
- By Application
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Purity
- By Application
- Competition Analysis
- Competition Deep Dive
- Teva
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Dr. Reddy's
- Lupin
- Sandoz
- Aurobindo
- Sun Pharma
- Cipla
- Hetero
- Zhejiang Huahai
- Amneal
- Torrent
- Alkem
- Zydus
- Jubilant
- Apotex
- Teva
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Purity , 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Purity , 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Purity , 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Purity , 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Purity , 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Purity , 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Purity , 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Purity , 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by Application, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Purity , 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Purity , 2025-2035
- Figure 5: Global Market Attractiveness Analysis by Purity
- Figure 6: Global Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 8: Global Market Attractiveness Analysis by Application
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025-2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025-2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025-2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025-2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025-2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Purity , 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Purity , 2025-2035
- Figure 22: North America Market Attractiveness Analysis by Purity
- Figure 23: North America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 25: North America Market Attractiveness Analysis by Application
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Purity , 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Purity , 2025-2035
- Figure 29: Latin America Market Attractiveness Analysis by Purity
- Figure 30: Latin America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 32: Latin America Market Attractiveness Analysis by Application
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Purity , 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Purity , 2025-2035
- Figure 36: Western Europe Market Attractiveness Analysis by Purity
- Figure 37: Western Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 39: Western Europe Market Attractiveness Analysis by Application
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Purity , 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Purity , 2025-2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Purity
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by Application
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Purity , 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Purity , 2025-2035
- Figure 50: East Asia Market Attractiveness Analysis by Purity
- Figure 51: East Asia Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 53: East Asia Market Attractiveness Analysis by Application
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Purity , 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Purity , 2025-2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Purity
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Purity , 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Purity , 2025-2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Purity
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the omeprazole sulfone market in 2025?
The global omeprazole sulfone market is estimated to be valued at USD 380.0 million in 2025.
What will be the size of omeprazole sulfone market in 2035?
The market size for the omeprazole sulfone market is projected to reach USD 510.0 million by 2035.
How much will be the omeprazole sulfone market growth between 2025 and 2035?
The omeprazole sulfone market is expected to grow at a 3.0% CAGR between 2025 and 2035.
What are the key product types in the omeprazole sulfone market?
The key product types in omeprazole sulfone market are ≥ 99% and < 99%.
Which application segment to contribute significant share in the omeprazole sulfone market in 2025?
In terms of application, api intermediate segment to command 80.0% share in the omeprazole sulfone market in 2025.