Demand for Cancer and Tumor Biomarker-based Assay in UK
Demand for Cancer and Tumor Biomarker-based Assay in UK Size and Share Forecast Outlook 2025 to 2035
Demand for cancer and tumor biomarker-based assay in UK is projected to grow from USD 1.2 billion in 2025 to USD 2.9 billion by 2035, at a CAGR of 9.2%. Genetic/Genomic Biomarkers will dominate with a 68.1% market share, while breast cancer will lead the cancer type segment with a 24.7% share.
Demand for Cancer and Tumor Biomarker-based Assay in UK 2025 to 2035
The demand for cancer and tumor biomarker-based assay in the UK is projected to grow from USD 1.2 billion in 2025 to approximately USD 2.9 billion by 2035, with demand forecast to expand at a compound annual growth rate (CAGR) of 9.2% between 2025 and 2035.
Quick Stats for UK Cancer and Tumor Biomarker-based Assay Industry
- UK Cancer and Tumor Biomarker-based Assay Sales Value (2025): USD 1.2 billion
- UK Cancer and Tumor Biomarker-based Assay Forecast Value (2035): USD 2.9 billion
- UK Cancer and Tumor Biomarker-based Assay Forecast CAGR: 9.2%
- Leading Application in UK Cancer and Tumor Biomarker-based Assay Industry: Genetic/Genomic Biomarkers (68.1%)
- Key Growth Regions in UK Cancer and Tumor Biomarker-based Assay Industry: England, Scotland, Wales, and Northern Ireland
- Regional Leadership: England holds the leading position in demand
- Key Players in UK Cancer and Tumor Biomarker-based Assay Industry: F. Hoffmann-La Roche Limited, Thermo Fisher Scientific Incorporated, Illumina Incorporated, QIAGEN N.V., Guardant Health Incorporated, Exact Sciences Corporation, Bio-Rad Laboratories Incorporated, Agilent Technologies Incorporated, Sysmex Corporation, Abbott Laboratories, bioMérieux S.A.
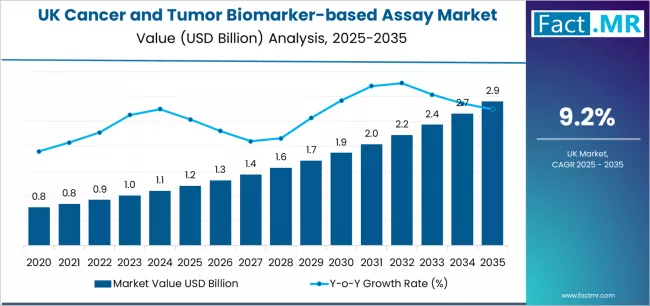
Growth is driven by expanding precision oncology requirements and increasing adoption of companion diagnostic solutions across UK healthcare sectors, particularly in England where major cancer centers and diagnostic laboratories are accelerating biomarker testing deployment. Increasing integration of liquid biopsy technologies in clinical applications and growing adoption of comprehensive genomic profiling approaches continue to drive demand. Healthcare providers and diagnostic laboratories are expanding their biomarker testing investments to address the growing complexity of modern cancer treatment requirements and personalized medicine specifications, with UK facilities leading investments in advanced molecular diagnostic systems.
The genetic/genomic biomarkers segment is projected to register 68.1% of cancer and tumor biomarker-based assay demand in 2025. Cancer and tumor biomarker-based assays are widely used in genetic/genomic configurations because they enable precise molecular characterization, reliable testing performance, and proven clinical utility that enhance treatment selection quality. They also support diverse therapeutic applications and oncologist preferences across hospital environments, improving diagnostic accuracy and effectiveness. Breast cancer applications are expected to account for 24.7% of cancer and tumor biomarker-based assay demand in 2025. Cancer and tumor biomarker-based assays are widely used for breast cancer because they provide proven treatment optimization and comprehensive diagnostic support for demanding clinical requirements. Their integration enhances therapeutic decision-making, biomarker interpretation capabilities, and patient outcomes by enabling improved treatment personalization and authentic clinical guidance during cancer care.
From 2030 to 2035, demand is forecast to grow from USD 1.9 billion to USD 2.9 billion, adding another USD 1.0 billion, which constitutes 58.8% of the overall ten-year expansion. This period is expected to be characterized by expansion of artificial intelligence integration, development of advanced liquid biopsy platforms and specialized companion diagnostics, and implementation of quality assurance systems across different oncology applications. The growing adoption of precision medicine principles and enhanced genomic testing requirements, particularly in England and Scotland regions, will drive demand for more sophisticated biomarker-based assay systems and integrated diagnostic platforms.
The cancer and tumor biomarker-based assay industry is experiencing robust growth in the UK primarily due to the increasing adoption of advanced molecular diagnostic alternatives and the expansion of personalized medicine initiatives. The country's emphasis on cancer care advancement and healthcare innovation necessitates investment in effective biomarker testing systems (diagnostic support, treatment guidance) and advanced molecular products (accuracy enhancement, clinical validation) for oncology operations and therapeutic functions.
UK Cancer and Tumor Biomarker-based Assay Industry Key Takeaways
| Metric | Value |
|---|---|
| UK Cancer and Tumor Biomarker-based Assay Sales Value (2025) | USD 1.2 billion |
| UK Cancer and Tumor Biomarker-based Assay Forecast Value (2035) | USD 2.9 billion |
| UK Cancer and Tumor Biomarker-based Assay Forecast CAGR (2025-2035) | 9.2% |
Why is the UK Cancer and Tumor Biomarker-based Assay Industry Growing?
Modern oncologists and pathologists rely on professional biomarker-based assay systems to ensure diagnostic competitiveness, treatment optimization, and optimal pathway achievement toward therapeutic goals. Advanced clinical requirements necessitate comprehensive biomarker testing solutions including specialized genomic profiling capabilities, molecular diagnostic support systems, and clinical validation infrastructure to address diverse cancer applications and treatment specifications.
Oncologists and pathologists are emphasizing precision medicine and integrated diagnostic solutions to enhance patient outcomes, access treatment optimization trends, and demonstrate clinical leadership in competitive healthcare environments. Advanced quality policies and biomarker testing performance requirements are establishing standardized diagnostic pathways that require professional systems and clinical assurance, with UK facilities often pioneering large-scale implementation of advanced molecular diagnostic technologies.
Opportunity Pathways - Demand for Cancer and Tumor Biomarker-based Assay in the UK
The cancer and tumor biomarker-based assay demand in the UK is positioned for robust expansion, growing from USD 1.2 billion in 2025 to USD 2.9 billion by 2035, reflecting an 9.2% CAGR. Rising adoption of professional biomarker testing systems in hospital facilities, diagnostic laboratories, and oncology applications is driving growth as practitioners seek molecular diagnostic solutions that maximize treatment effectiveness and comply with stringent clinical standards. Additionally, demand from precision medicine applications and advanced genomic testing implementations strengthens opportunities for both sophisticated diagnostic platforms and integrated molecular solutions.
Suppliers focusing on oncology implementations, diagnostic integration, and advanced biomarker capabilities stand to gain from evolving healthcare standards and practitioner expectations for effective molecular diagnostics, clinical validation, and therapeutic guidance.
- Pathway A - Hospital Implementations and Oncology Applications. Healthcare providers face increasing demands for reliable biomarker testing solutions in modern hospital facilities. Molecular diagnostic systems enable enhanced treatment effectiveness and comprehensive clinical capabilities without compromising diagnostic functionality. Solutions targeting hospital operations, cancer centers, and specialty facilities can achieve strong adoption rates through treatment optimization and clinical outcome improvements. Estimated revenue opportunity: USD 8.7-12.1 million.
- Pathway B - Genetic/Genomic Biomarker Applications and Molecular Testing. Diagnostic companies are increasingly adopting genetic/genomic biomarker systems for consistent molecular diagnostic utilization. Collaborations with genomic platforms for integrated testing solutions can unlock large-volume supply contracts and long-term partnerships in clinical applications. Estimated revenue opportunity: USD 7.3-10.2 million.
- Pathway C - Liquid Biopsy and Companion Diagnostic Applications. The growth in precision medicine, personalized therapy, and diagnostic establishments creates robust demand for biomarker testing systems ensuring effectiveness in oncology processes. Suppliers offering liquid biopsy solutions for companion diagnostic applications can build relationships with oncologists and diagnostic integrators. Estimated revenue opportunity: USD 5.8-8.1 million.
- Pathway D - Comprehensive Genomic Profiling and Testing Applications. Clinical requirements and modern diagnostic demands are driving preference for comprehensive genomic profiling platforms with superior molecular capabilities. Suppliers offering integrated genomic solutions with exceptional testing characteristics can differentiate offerings and attract quality-focused organizations. Estimated revenue opportunity: USD 4.2-6.0 million.
- Pathway E - Specialized Cancer Testing and Clinical Solutions. Critical oncology applications require specialized biomarker configurations with advanced molecular features and enhanced diagnostic properties. Suppliers investing in clinical development can secure advantages in serving demanding oncology applications. Estimated revenue opportunity: USD 3.4-4.8 million.
- Pathway F - Technical Services and Laboratory Networks. Comprehensive laboratory networks offering technical support, training, and ongoing service support create recurring revenue opportunities. Companies building strong expertise capabilities can capture ongoing relationships and enhance customer satisfaction across diagnostic facilities. Estimated revenue opportunity: USD 2.9-4.1 million.
Segmental Analysis
The industry is segmented by cancer type, biomarker type, end use, and region. By cancer type, the industry is divided into breast cancer, lung cancer, colorectal cancer, prostate cancer, and ovarian cancer & others categories. In terms of biomarker type, industry is segmented into genetic/genomic biomarkers, protein biomarkers, epigenetic biomarkers, and others. By end use, industry is divided into hospitals & cancer centers, diagnostic laboratories, and others. Regionally, the industry is divided into England, Scotland, Wales, and Northern Ireland.
Why do Genetic/Genomic Biomarkers Account for High Share of 68.1%?
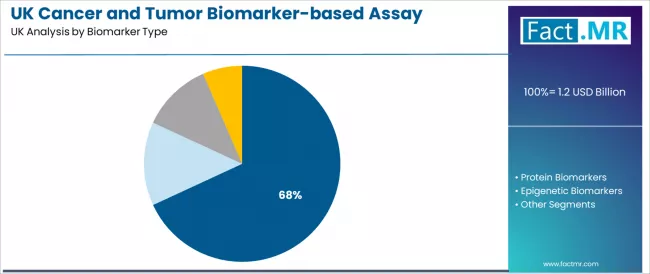
The genetic/genomic biomarkers segment is projected to account for 68.1% of cancer and tumor biomarker-based assay demand in 2025, making it the leading biomarker category across the sector. This dominance reflects the molecular diagnostic performance requirements and proven capabilities of genetic/genomic systems for existing oncology operations and clinical applications where treatment precision is optimized through established testing capabilities and integrated quality architecture. In the UK, where substantial cancer populations require biomarker testing integration without complete diagnostic modification, genetic/genomic platforms provide practical pathways for molecular enhancement while maintaining clinical integrity. Continuous innovations are improving testing consistency, genomic characteristics, and diagnostic integration parameters, enabling healthcare providers to achieve high performance standards while maximizing reliability. The segment's strong position is reinforced by the extensive existing clinical infrastructure requiring biomarker adoption and growing availability of genomic suppliers with proven commercial experience.
- Molecular compatibility and existing clinical integration make genetic/genomic platforms the preferred biomarker type for enhancing oncology operations and diagnostic installations.
- Performance reliability and commercial demonstration track records are enhancing clinician confidence and system viability across large-scale adoption initiatives.
Why do Breast Cancer Account for High Share of 24.7%?
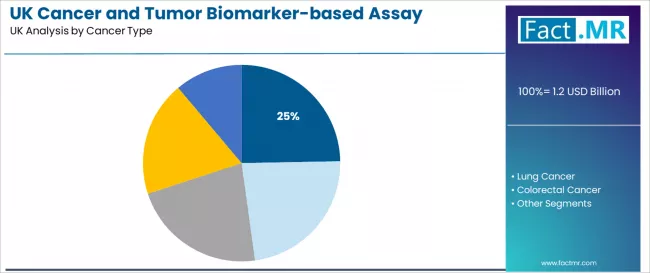
Breast cancer applications are expected to represent 24.7% of cancer and tumor biomarker-based assay demand in 2025, demonstrating the significant role of breast cancer treatment and diagnostics requiring comprehensive molecular diagnostic support solutions. Breast cancer facilities including major oncology centers, cancer hospitals, diagnostic providers, and treatment programs generate consistent demand for biomarker testing systems that are clinically and operationally favorable for treatment applications. The segment benefits from diagnostic characteristics that provide treatment optimization and therapeutic capabilities for breast cancer care. In the UK, where breast cancer treatment represents substantial portions of oncology services, clinical excellence requires biomarker testing integration across diverse healthcare facilities. In England regions, where breast cancer concentrations are significant, biomarker demand is elevated by emphasis on maintaining treatment excellence while achieving therapeutic targets.
- Treatment requirements and diagnostic optimization drive consistent demand across major breast cancer facilities, oncology centers, diagnostic providers, and treatment programs.
- The integration of biomarker-based solutions enhances the accuracy, personalization, and effectiveness of breast cancer treatment protocols, further supporting the sector's need for high-performance molecular diagnostics in both clinical and research applications.
What are the Drivers, Restraints, and Key Trends in the UK Cancer and Tumor Biomarker-based Assay Industry?
The country's cancer and tumor biomarker-based assay demand is advancing rapidly due to increasing precision medicine requirements and growing recognition of molecular diagnostic necessity for healthcare development, with England region serving as a key driver of innovation and application development. The sector faces challenges including competition from alternative diagnostic systems, need for specialized clinical validation development, and ongoing concerns regarding testing costs and reimbursement considerations. National healthcare guidelines and regional-level clinical initiatives, particularly oncology programs in England and Scotland regions, continue to influence biomarker testing selection and adoption timelines.
Expansion of Precision Medicine Requirements and Treatment Standards
The enhancement of clinical protocols, gaining particular significance through therapeutic standards and advanced healthcare programs, is enabling biomarker testing suppliers to achieve differentiation without prohibitive development costs, providing predictable demand patterns through molecular requirements and treatment preferences. Enhanced quality standards offering substantial opportunities for professional biomarker systems and integrated applications provide foundational dynamics while allowing suppliers to secure clinical agreements and therapeutic partnerships. These trends are particularly valuable for first-mover suppliers and advanced system development that require substantial technical investments without immediate cost advantages.
Incorporation of Liquid Biopsy Technologies and Clinical Validation Systems
Modern biomarker suppliers and diagnostic companies are establishing advanced liquid biopsy networks and centralized quality management facilities that improve molecular effectiveness through technical standardization and performance validation. Integration of genomic optimization systems, high-precision testing technologies, and coordinated quality management enables more efficient biomarker testing across multiple clinical locations. Advanced diagnostic concepts also support next-generation oncology applications including specialized molecular integration, treatment optimization, and regional biomarker supply networks that optimize system-level economics while enabling comprehensive performance monitoring across healthcare regions, with UK developments increasingly adopting collaborative research models to reduce individual supplier costs and accelerate validation.
Analysis of UK Cancer and Tumor Biomarker-based Assay Industry by Key Region
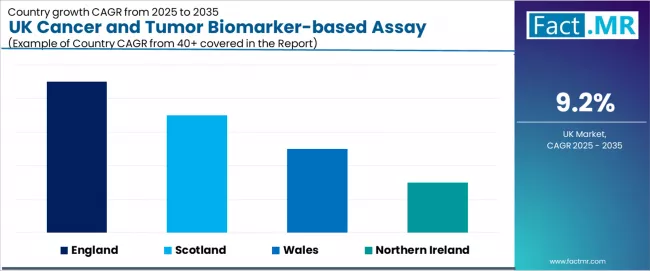
| Region | CAGR (2025-2035) |
|---|---|
| England | 9.1% |
| Scotland | 8.9% |
| Wales | 8.7% |
| Northern Ireland | 8.5% |
The UK cancer and tumor biomarker-based assay industry is witnessing robust growth, supported by rising precision medicine requirements, expanding clinical testing initiatives, and the deployment of advanced molecular diagnostic technologies across regions. England leads the nation with a 9.1% CAGR, reflecting progressive healthcare trends, substantial clinical innovation, and early adoption of professional biomarker testing systems. Scotland follows with an 8.9% CAGR, driven by extensive healthcare infrastructure, favorable clinical demographics, and concentration of specialized operations that enhance application development. Wales grows at 8.7%, as healthcare modernization and clinical opportunities increasingly drive biomarker testing adoption. Northern Ireland demonstrates growth at 8.5%, supported by expanding healthcare facilities and regional clinical initiatives.
Why Does England Lead Cancer and Tumor Biomarker-based Assay Demand?
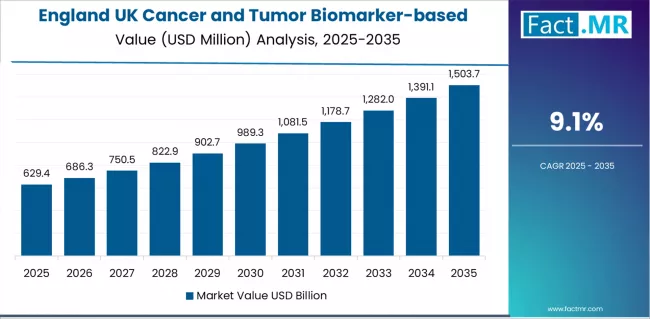
Demand for cancer and tumor biomarker-based assay in England is projected to exhibit strong growth with a CAGR of 9.1% through 2035, driven by progressive healthcare preferences, substantial clinical development creating advanced biomarker opportunities, and concentration of medical advancement across London and surrounding metropolitan areas.
As the dominant region with extensive oncology infrastructure and quality-focused healthcare policies, England's emphasis on comprehensive diagnostic excellence and clinical leadership is creating significant demand for professional biomarker testing systems with proven effectiveness and reliable application potential. Major molecular diagnostic suppliers and healthcare companies are establishing comprehensive clinical development programs to support innovation advancement and advanced system deployment across diverse applications.
- Healthcare development trends and clinical preferences are requiring comprehensive molecular diagnostic strategies and biomarker solutions, driving demand for systems with demonstrated performance enhancement capabilities and permanent quality assurance throughout diverse clinical operations.
- Innovation ecosystem strength and healthcare capital availability are supporting deployment of next-generation biomarker testing technologies and novel application pathways that enhance commercial viability, reduce testing costs, and create new molecular opportunities across oncology and quality-focused applications, positioning England as a national healthcare leadership region.
Why Does Scotland Show Strong Growth?
Demand for cancer and tumor biomarker-based assay in Scotland is expanding at a CAGR of 8.9%, supported by extensive healthcare facilities including large-scale clinical programs, diagnostic operations, and medical companies generating concentrated demand favorable for biomarker testing systems. The region's healthcare characteristics, featuring substantial medical infrastructure and clinical requirements ideal for biomarker integration, provide natural advantages.
Clinical expertise concentrated in Edinburgh, Glasgow, and regional healthcare corridors facilitates application development and molecular diagnostic management. Biomarker suppliers and diagnostic companies are implementing comprehensive healthcare strategies to serve expanding quality-focused requirements throughout Scotland.
- Clinical concentration and favorable application economics are creating opportunities for specialized biomarker suppliers that can integrate molecular systems with existing healthcare operations.
- Biomarker positioning and healthcare awareness are building regional competitive advantages in clinical applications, enabling comprehensive medical development and diagnostic cluster enhancement that meets molecular targets while accessing advanced pricing opportunities.
Why Does Wales Show Consistent Expansion?
Demand for cancer and tumor biomarker-based assay in Wales is growing at a CAGR of 8.7%, driven by substantial healthcare facilities from clinical operations, diagnostic distributors, and regional practitioners requiring biomarker pathways.
The region's medical base, supporting critical healthcare operations, is increasingly adopting biomarker testing technologies to maintain competitiveness while meeting diagnostic expectations. Diagnostic companies and biomarker suppliers are investing in molecular integration systems and regional supply infrastructure to address growing healthcare management requirements.
- Healthcare modernization imperatives and clinical competitiveness concerns are facilitating adoption of biomarker technologies that enable continued medical operations while achieving molecular enhancement across clinical operations, diagnostic distributors, and practitioner facilities.
- Diagnostic optimization opportunities including regional healthcare development and biomarker utilization for enhanced clinical operations are creating unique regional advantages and diversified application types throughout Wales healthcare operations.
What Factors Underpin Cancer and Tumor Biomarker-based Assay Demand in Northern Ireland?
Demand for cancer and tumor biomarker-based assay in Northern Ireland is advancing at a CAGR of 8.5%, supported by expanding healthcare facilities, regional clinical development including diagnostic and treatment operations, and growing emphasis on molecular solutions across the region.
Healthcare modernization and clinical facility expansion are driving consideration of biomarker testing systems as molecular enhancement pathways. Diagnostic companies and biomarker suppliers are developing regional capabilities to support emerging molecular deployment requirements.
- Healthcare expansion and clinical diversification are creating economic drivers for biomarker technologies and system adoption across medical and commercial facilities seeking competitive differentiation pathways.
- Regional healthcare cooperation and coordinated clinical development are establishing consistent biomarker environments and shared molecular infrastructure that support multi-regional treatment projects throughout Northern Ireland healthcare operations.
Competitive Landscape of UK Cancer and Tumor Biomarker-based Assay Industry
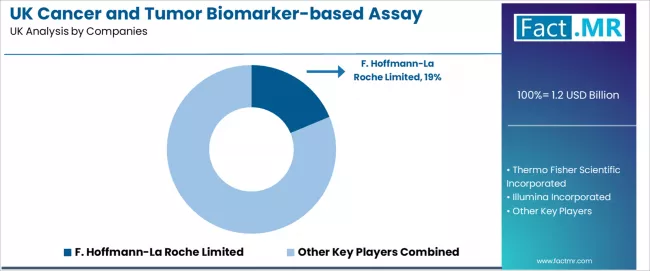
UK cancer and tumor biomarker-based assay industry is defined by competition among specialized diagnostic manufacturers, molecular testing companies, and integrated solution providers, with major healthcare corporations maintaining significant influence through research resources and application development capabilities. Companies are investing in testing advancement, clinical validation infrastructure optimization, distribution network structures, and comprehensive technical support services to deliver effective, reliable, and accessible molecular diagnostic solutions across UK oncology and clinical applications. Strategic partnerships, research infrastructure development, and first-mover application execution are central to strengthening competitive positioning and presence across oncology, quality-focused, and clinical molecular applications.
F. Hoffmann-La Roche Limited leads with an 18.7% share, offering comprehensive tissue and liquid biopsy solutions including testing development, clinical validation, and distribution services with focus on oncology applications, healthcare, and accessibility across UK operations. Thermo Fisher Scientific Incorporated delivers full-service molecular supply including diagnostic distribution, custom integration, and technical support serving UK and international clinical projects.
Illumina Incorporated emphasizes comprehensive advanced solutions with integrated sequencing capabilities, quality management, and genomic features leveraging molecular sector expertise. QIAGEN N.V. offers biomarker application development and molecular optimization operations for oncology and healthcare applications across UK operations.
Key Players in UK Cancer and Tumor Biomarker-based Assay Industry
- F. Hoffmann-La Roche Limited
- Thermo Fisher Scientific Incorporated
- Illumina Incorporated
- QIAGEN N.V.
- Guardant Health Incorporated
- Exact Sciences Corporation
- Bio-Rad Laboratories Incorporated
- Agilent Technologies Incorporated
- Sysmex Corporation
- Abbott Laboratories
- bioMérieux S.A.
Scope of the Report
| Item | Value |
|---|---|
| Quantitative Units | USD 2.9 billion |
| Cancer Type | Breast Cancer, Lung Cancer, Colorectal Cancer, Prostate Cancer, Ovarian Cancer & Others |
| Biomarker Type | Genetic/Genomic Biomarkers, Protein Biomarkers, Epigenetic Biomarkers, Others |
| End Use | Hospitals & Cancer Centers, Diagnostic Laboratories, Others |
| Regions Covered | England, Scotland, Wales, Northern Ireland |
| Key Companies | F. Hoffmann-La Roche Limited, Thermo Fisher Scientific Incorporated, Illumina Incorporated, QIAGEN N.V., Guardant Health Incorporated, Exact Sciences Corporation, Bio-Rad Laboratories Incorporated, Agilent Technologies Incorporated, Sysmex Corporation, Abbott Laboratories, bioMérieux S.A., Regional biomarker specialists |
| Additional Attributes | Sales by cancer type and biomarker type segment; regional demand trends across England, Scotland, Wales, and Northern Ireland; competitive landscape with established biomarker suppliers and specialized molecular developers; quality preferences for genetic/genomic biomarkers versus protein biomarker technologies; integration with healthcare programs and advanced clinical policies, particularly advanced in the England region |
UK Cancer and Tumor Biomarker-based Assay Industry by Segments
-
Cancer Type :
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Ovarian Cancer & Others
-
Biomarker Type :
- Genetic/Genomic Biomarkers
- Protein Biomarkers
- Epigenetic Biomarkers
- Others
-
End Use :
- Hospitals & Cancer Centers
- Diagnostic Laboratories
- Others
-
Region :
- England
- Scotland
- Wales
- Northern Ireland
Table of Content
- Executive Summary
- UK Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- UK Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- UK Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- UK Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Biomarker Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Biomarker Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Biomarker Type, 2025 to 2035
- Genetic/Genomic Biomarkers
- Protein Biomarkers
- Epigenetic Biomarkers
- Others
- Y to o to Y Growth Trend Analysis By Biomarker Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Biomarker Type, 2025 to 2035
- UK Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Cancer Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Cancer Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Cancer Type, 2025 to 2035
- Breast Cancer
- Lung Cancer
- Colorectal Cancer
- Prostate Cancer
- Ovarian Cancer & Others
- Y to o to Y Growth Trend Analysis By Cancer Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Cancer Type, 2025 to 2035
- UK Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- UK
- Market Attractiveness Analysis By Region
- UK Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- By Biomarker Type
- By Cancer Type
- Market Attractiveness Analysis
- By Country
- By Biomarker Type
- By Cancer Type
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Biomarker Type
- By Cancer Type
- Competition Analysis
- Competition Deep Dive
- F. Hoffmann-La Roche Limited
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Thermo Fisher Scientific Incorporated
- Illumina Incorporated
- QIAGEN N.V.
- Guardant Health Incorporated
- Exact Sciences Corporation
- Bio-Rad Laboratories Incorporated
- Agilent Technologies Incorporated
- Sysmex Corporation
- Abbott Laboratories
- bioMérieux S.A.
- F. Hoffmann-La Roche Limited
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: UK Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: UK Market Value (USD Million) Forecast by Biomarker Type, 2020 to 2035
- Table 3: UK Market Value (USD Million) Forecast by Cancer Type, 2020 to 2035
- Table 4: UK Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: UK Market Value (USD Million) Forecast by Biomarker Type, 2020 to 2035
- Table 6: UK Market Value (USD Million) Forecast by Cancer Type, 2020 to 2035
List Of Figures
- Figure 1: UK Market Pricing Analysis
- Figure 2: UK Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: UK Market Value Share and BPS Analysis by Biomarker Type, 2025 and 2035
- Figure 4: UK Market Y to o to Y Growth Comparison by Biomarker Type, 2025 to 2035
- Figure 5: UK Market Attractiveness Analysis by Biomarker Type
- Figure 6: UK Market Value Share and BPS Analysis by Cancer Type, 2025 and 2035
- Figure 7: UK Market Y to o to Y Growth Comparison by Cancer Type, 2025 to 2035
- Figure 8: UK Market Attractiveness Analysis by Cancer Type
- Figure 9: UK Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: UK Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: UK Market Attractiveness Analysis by Region
- Figure 12: UK Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: UK Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 14: UK Market Value Share and BPS Analysis by Biomarker Type, 2025 and 2035
- Figure 15: UK Market Y to o to Y Growth Comparison by Biomarker Type, 2025 to 2035
- Figure 16: UK Market Attractiveness Analysis by Biomarker Type
- Figure 17: UK Market Value Share and BPS Analysis by Cancer Type, 2025 and 2035
- Figure 18: UK Market Y to o to Y Growth Comparison by Cancer Type, 2025 to 2035
- Figure 19: UK Market Attractiveness Analysis by Cancer Type
- Figure 20: UK Market - Tier Structure Analysis
- Figure 21: UK Market - Company Share Analysis
- FAQs -
How big is the demand for cancer and tumor biomarker-based assay in UK in 2025?
The demand for cancer and tumor biomarker-based assay in UK is estimated to be valued at USD 1.2 billion in 2025.
What will be the size of cancer and tumor biomarker-based assay in UK in 2035?
The market size for the cancer and tumor biomarker-based assay in UK is projected to reach USD 2.9 billion by 2035.
How much will be the demand for cancer and tumor biomarker-based assay in UK growth between 2025 and 2035?
The demand for cancer and tumor biomarker-based assay in UK is expected to grow at a 9.2% CAGR between 2025 and 2035.
What are the key product types in the cancer and tumor biomarker-based assay in UK?
The key product types in cancer and tumor biomarker-based assay in UK are genetic/genomic biomarkers, protein biomarkers, epigenetic biomarkers and others.
Which cancer type segment is expected to contribute significant share in the cancer and tumor biomarker-based assay in UK in 2025?
In terms of cancer type, breast cancer segment is expected to command 24.7% share in the cancer and tumor biomarker-based assay in UK in 2025.