Demand for Thyroid Cancer Diagnostics in UK
Demand for Thyroid Cancer Diagnostics in UK Size and Share Forecast Outlook 2026 to 2036
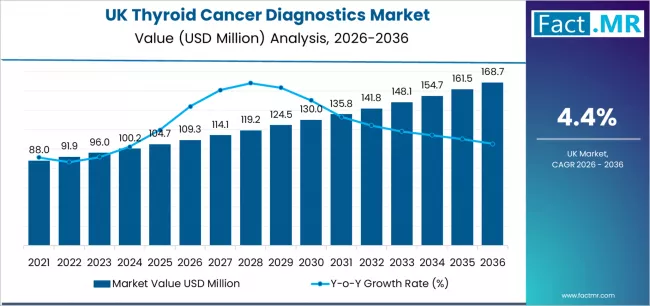
Demand for thyroid cancer diagnostics in UK is projected to grow from USD 109.3 million in 2026 to USD 168.7 million by 2036, at a CAGR of 4.4%. Papillary Carcinoma will dominate with a 59.2% market share, while imaging will lead the diagnostic technique segment with a 43.5% share.
Demand for Thyroid Cancer Diagnostics in UK 2026 to 2036
The demand for thyroid cancer diagnostics in the UK is projected to rise from USD 109.3 million in 2026 to about USD 168.7 million in 2036, reflecting a projected CAGR of 4.4% during the period. The increase represents growth of roughly 54.3% from the 2026 baseline. Papillary carcinoma is expected to account for 59.2% of national diagnostic demand in 2026, and imaging modalities are projected to hold 43.5% of total requirements. NHS screening expansion continues to strengthen early detection pathways, and higher nodule identification rates are emerging as ultrasound access widens across major clinical regions.
Key Takeaways from UK Thyroid Cancer Diagnostics Industry Analysis
- UK Thyroid Cancer Diagnostics Sales Value (2026): USD 109.3 million
- UK Thyroid Cancer Diagnostics Forecast Value (2036): USD 168.7 million
- UK Thyroid Cancer Diagnostics Forecast CAGR: 4.4%
- Leading Cancer Type in UK Thyroid Cancer Diagnostics Industry: Papillary Carcinoma (59.2%)
- Key Growth Regions in UK Thyroid Cancer Diagnostics Industry: England, Scotland, Wales, and Northern Ireland
- Regional Leadership: England holds the leading position in demand
- Key Players in UK Thyroid Cancer Diagnostics Industry: Abbott Laboratories, F. Hoffmann-La Roche Limited, Thermo Fisher Scientific Incorporated, Siemens Healthineers GmbH, Bio-Rad Laboratories Incorporated, GE HealthCare Technologies Incorporated, Hologic Incorporated, Koninklijke Philips N.V., Toshiba Corporation, Agilent Technologies Incorporated, JSW Steel Limited
England’s specialist cancer centres and university hospitals are adopting advanced diagnostic protocols that support precision-led endocrinology workflows. Molecular tools and AI-assisted imaging platforms are gaining traction as clinical teams seek greater accuracy in risk classification and nodule assessment. Papillary carcinoma applications remain central to diagnostic activity, as cytological clarity and early-stage identification shape most clinical decisions. Imaging retains a substantial share since clinicians depend on structured visual evaluation for reliable triage, staging, and procedural alignment.
A new phase of development is forming as liquid biopsy platforms, refined molecular formulations, and AI-driven evaluation pathways become more accessible across UK facilities. These technologies encourage regional providers, particularly in England and Scotland, to reassess diagnostic configurations that support tailored intervention planning. The UK thyroid cancer diagnostics industry continues to advance as clinical operators prioritise early detection quality, consistent performance across imaging and molecular systems, and dependable evaluation standards within NHS environments.
UK Thyroid Cancer Diagnostics Industry
| Metric | Value |
|---|---|
| UK Thyroid Cancer Diagnostics Sales Value (2026) | USD 109.3 million |
| UK Thyroid Cancer Diagnostics Forecast Value (2036) | USD 168.7 million |
| UK Thyroid Cancer Diagnostics Forecast CAGR (2026-2036) | 4.4% |
Category
| Category | Segments |
|---|---|
| Cancer Type | Papillary Carcinoma, Follicular Carcinoma, Medullary Carcinoma, Anaplastic Variants |
| Diagnostic Technique | Imaging, Biopsy, Blood Tests, Others |
| Country | England, Scotland, Wales, Northern Ireland |
Segmental Analysis
Which Thyroid Cancer Type Accounts for Maximum Diagnostics in the UK?
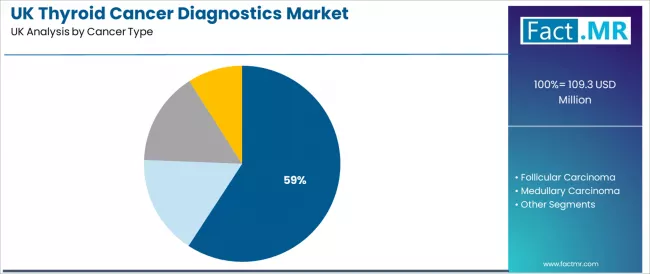
Papillary carcinoma is projected to represent 59.2% of thyroid cancer diagnostic demand in 2026 due to its high epidemiological prevalence and alignment with established UK clinical workflows. NHS facilities rely on diagnostic methods that support cytological accuracy, operational consistency, and streamlined integration across existing systems. Advancements in pathological evaluation continue to strengthen reliability and improve diagnostic precision in routine settings.
- Cytological compatibility and established clinical pathways support widespread diagnostic use.
- Proven performance reinforces clinician confidence across large-scale diagnostic programs.
Which Diagnostic Technique is Preferred for Detecting Thyroid Cancer in the UK?
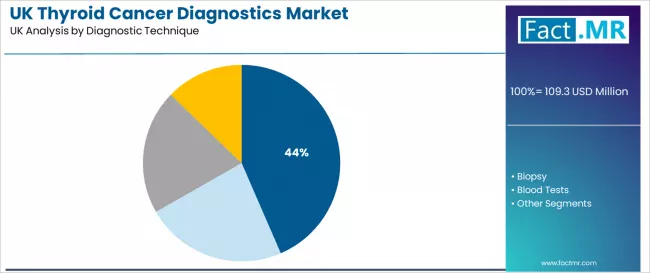
Imaging is expected to account for 43.5% of thyroid cancer diagnostic demand in 2026, reflecting its central role in clinical evaluation and early detection. UK healthcare facilities depend on imaging modalities that deliver consistent diagnostic clarity and integrate effectively with screening workflows. Concentrated imaging capacity in England strengthens adoption, supported by operational requirements for accuracy and reliability.
- Clinical evaluation requirements sustain strong demand across imaging facilities and screening programs.
- Integration enhances diagnostic accuracy and supports performance consistency across healthcare environments.
What are the Drivers, Restraints, and Key Trends in the UK Thyroid Cancer Diagnostics Industry?
Demand for thyroid cancer diagnostics in the UK is rising as clinicians emphasise early detection and require tools that deliver higher accuracy across screening pathways. England continues to lead adoption through strong clinical innovation and expanding diagnostic programmes.
Restraints include NHS budget pressures, competition from alternative testing methods, and the need for more advanced accuracy-validation capabilities. Evolving regulatory expectations also influence technology selection and workflow design.
Key trends highlight growing integration of AI-enabled imaging systems, wider use of validation frameworks, and deeper collaboration with clinical programmes in England and Scotland. These developments strengthen diagnostic consistency, support compliance requirements, and help suppliers build durable relationships within the UK healthcare ecosystem.
Analysis of UK Thyroid Cancer Diagnostics Industry by Key Countries
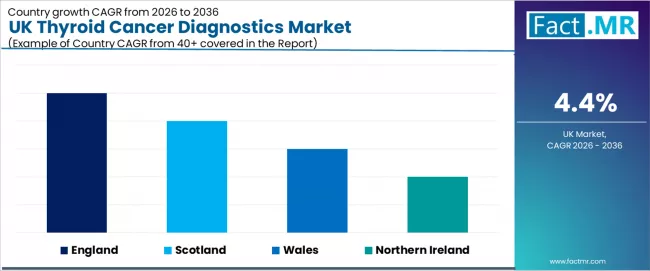
| Region | CAGR (2026 to 2036) |
|---|---|
| England | 4.9% |
| Scotland | 4.8% |
| Wales | 4.7% |
| Northern Ireland | 4.6% |
Why does England lead Thyroid Cancer Diagnostics Demand Across Its Advanced Clinical Ecosystem?
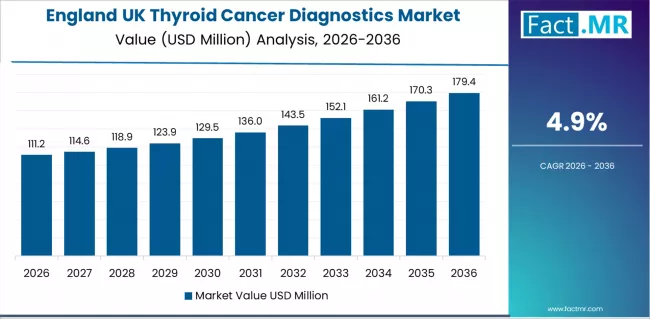
Thyroid cancer diagnostics demand in England is rising at a 4.9% CAGR through 2036, supported by extensive clinical networks and evolving diagnostic requirements across major metropolitan regions. The region’s structured healthcare infrastructure and efficiency-oriented policies create favourable conditions for consistent adoption of advanced diagnostic systems. Suppliers and healthcare organisations are strengthening development programs that improve analytical reliability, operational continuity, and regulatory alignment across varied medical applications.
- Growing clinical activity and shifting diagnostic expectations are shaping demand for systems that deliver consistent performance and stable interpretation accuracy.
- Innovation capacity and healthcare capital availability are supporting technologies that enhance feasibility and reduce diagnostic costs.
What is Driving Scotland’s Expanding Demand for Thyroid Cancer Diagnostics Within Key Healthcare Corridors?
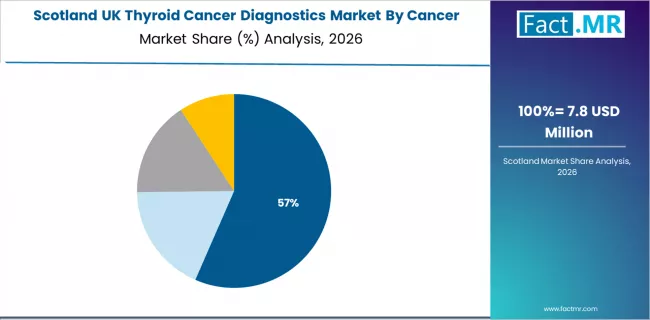
Thyroid cancer diagnostics demand in Scotland is increasing at a 4.8% CAGR, shaped by concentrated healthcare capabilities across Edinburgh, Glasgow, and surrounding clinical regions. The region’s established medical base supports integration of diagnostic systems across hospitals, outpatient centres, and specialised care programs. Suppliers and healthcare providers are refining diagnostic pathways that strengthen result accuracy and align with evolving clinical priorities.
- Dense healthcare networks and favourable operational structures support specialised suppliers aligned with diagnostic management needs.
- Heightened awareness of diagnostic precision is reinforcing demand for systems offering predictable performance and consistent clinical output.
How is Wales Expanding Thyroid Cancer Diagnostics Adoption Through Healthcare Modernization?
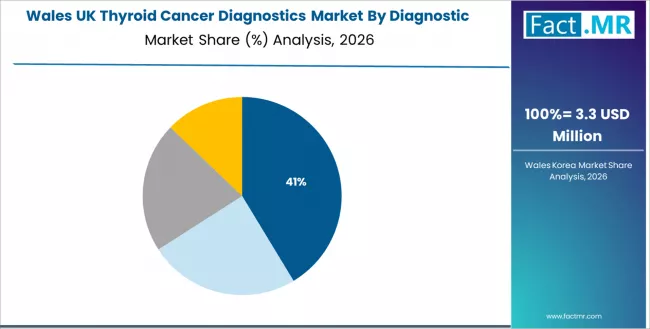
Thyroid cancer diagnostics demand in Wales is advancing at a 4.7% CAGR, driven by hospital upgrades and increasing utilisation of analytical systems across regional healthcare operations. Healthcare providers are developing diagnostic integration models that improve accuracy while accommodating changing medical requirements. Suppliers are expanding regional capabilities that align system deployment with emerging facility needs.
- Modernization priorities and competitive healthcare requirements are encouraging utilisation of systems that support clinical continuity and enhanced diagnostic confidence.
- Regional optimisation and development efforts are creating stable opportunities for suppliers expanding across Wales’ healthcare landscape.
What Strengthens Thyroid Cancer Diagnostics Demand across Northern Ireland’s Developing Healthcare Networks?
Thyroid cancer diagnostics demand in Northern Ireland is rising at a 4.6% CAGR, supported by expanding clinical capacity and growing adoption of precision-oriented diagnostic systems across medical facilities. Providers are integrating advanced diagnostics to reinforce accuracy, improve care pathways, and support regulatory expectations. Suppliers are building deployment capabilities that address diversified diagnostic needs across the region.
- Healthcare expansion and treatment diversification are shaping demand for systems that strengthen competitive positioning and clinical reliability.
- Coordinated regional development and shared clinical infrastructure are enabling consistent utilisation of diagnostic technologies across Northern Ireland’s healthcare corridors.
Competitive Landscape of UK Thyroid Cancer Diagnostics Industry
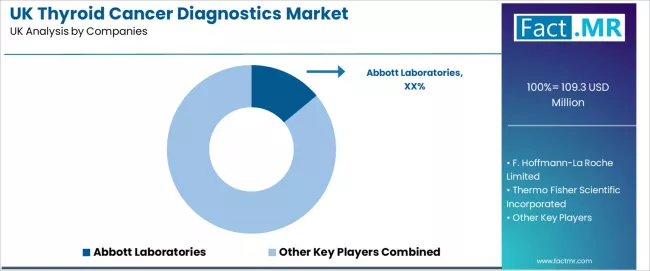
The UK thyroid cancer diagnostics industry is shaped by competition among diagnostic equipment manufacturers, molecular testing companies, and integrated solution providers. Major healthcare corporations retain influence through research capability, application development, and established distribution networks. Companies continue investing in imaging improvement, accuracy assurance systems, distribution efficiency, and technical validation to support clinical and healthcare applications across the country. Strategic partnerships, expanded research capacity, and early deployment remain central to strengthening competitive positioning within UK diagnostic markets.
Abbott Laboratories maintains a broad presence supported by imaging development, accuracy management infrastructure, and distribution coverage across clinical settings. F. Hoffmann-La Roche Limited provides full-service molecular testing supply, including diagnostic distribution, customized integration, and technical support for domestic and international clinical projects.
Thermo Fisher Scientific Incorporated leverages advanced processing capabilities, accuracy assurance platforms, and molecular expertise to deliver comprehensive diagnostic solutions. Siemens Healthineers GmbH focuses on application development and clinical workflow optimization for UK hospital and healthcare users.
Industry competitiveness depends on resilient supply networks, research-driven diagnostic innovation, and consistent performance across diverse clinical environments. Advances in molecular sensitivity, imaging efficiency, and workflow integration are expected to influence long-term development within the UK thyroid cancer diagnostics landscape.
Key Players in UK Thyroid Cancer Diagnostics Industry
- Abbott Laboratories
- F. Hoffmann-La Roche Limited
- Thermo Fisher Scientific Incorporated
- Siemens Healthineers GmbH
- Bio-Rad Laboratories Incorporated
- GE HealthCare Technologies Incorporated
- Hologic Incorporated
- Koninklijke Philips N.V.
- Toshiba Corporation
- Agilent Technologies Incorporated
Scope of the Report
| Item | Value |
|---|---|
| Quantitative Units | USD 168.7 million |
| Cancer Type | Papillary Carcinoma, Follicular Carcinoma, Medullary Carcinoma, Anaplastic Variants |
| Diagnostic Technique | Imaging, Biopsy, Blood Tests, Others |
| Regions Covered | England, Scotland, Wales, Northern Ireland |
| Key Companies | Abbott Laboratories, F. Hoffmann-La Roche Limited, Thermo Fisher Scientific Incorporated, Siemens Healthineers GmbH, Bio-Rad Laboratories Incorporated, GE HealthCare Technologies Incorporated, Hologic Incorporated, Koninklijke Philips N.V., Toshiba Corporation, Agilent Technologies Incorporated, JSW Steel Limited, Regional thyroid cancer diagnostic specialists |
| Additional Attributes | Sales by cancer type and diagnostic technique segment; regional demand trends across England, Scotland, Wales, and Northern Ireland; competitive landscape with established thyroid cancer diagnostic suppliers and specialized diagnostic developers; accuracy preferences for imaging versus molecular technologies; integration with NHS programs and advanced clinical policies, particularly advanced in the England region |
UK Thyroid Cancer Diagnostics Industry by Segments
-
Cancer Type :
- Papillary Carcinoma
- Follicular Carcinoma
- Medullary Carcinoma
- Anaplastic Variants
-
Diagnostic Technique :
- Imaging
- Biopsy
- Blood Tests
- Others
-
Country :
- England
- Scotland
- Wales
- Northern Ireland
Table of Content
- Executive Summary
- UK Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- UK Market Analysis 2021 to 2025 and Forecast, 2026 to 2036
- Historical Market Size Value (USD Million) Analysis, 2021 to 2025
- Current and Future Market Size Value (USD Million) Projections, 2026 to 2036
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- UK Market Pricing Analysis 2021 to 2025 and Forecast 2026 to 2036
- UK Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Cancer Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Cancer Type, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Cancer Type, 2026 to 2036
- Papillary Carcinoma
- Follicular Carcinoma
- Medullary Carcinoma
- Anaplastic Variants
- Y to o to Y Growth Trend Analysis By Cancer Type, 2021 to 2025
- Absolute $ Opportunity Analysis By Cancer Type, 2026 to 2036
- UK Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Diagnostic Technique
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Diagnostic Technique, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Diagnostic Technique, 2026 to 2036
- Imaging
- Biopsy
- Blood Tests
- Others
- Y to o to Y Growth Trend Analysis By Diagnostic Technique, 2021 to 2025
- Absolute $ Opportunity Analysis By Diagnostic Technique, 2026 to 2036
- UK Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2021 to 2025
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2026 to 2036
- UK
- Market Attractiveness Analysis By Region
- UK Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- By Cancer Type
- By Diagnostic Technique
- Market Attractiveness Analysis
- By Country
- By Cancer Type
- By Diagnostic Technique
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Cancer Type
- By Diagnostic Technique
- Competition Analysis
- Competition Deep Dive
- Abbott Laboratories
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- F. Hoffmann-La Roche Limited
- Thermo Fisher Scientific Incorporated
- Siemens Healthineers GmbH
- Bio-Rad Laboratories Incorporated
- GE HealthCare Technologies Incorporated
- Hologic Incorporated
- Koninklijke Philips N.V.
- Toshiba Corporation
- Agilent Technologies Incorporated
- Abbott Laboratories
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: UK Market Value (USD Million) Forecast by Region, 2021 to 2036
- Table 2: UK Market Value (USD Million) Forecast by Cancer Type, 2021 to 2036
- Table 3: UK Market Value (USD Million) Forecast by Diagnostic Technique, 2021 to 2036
- Table 4: UK Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 5: UK Market Value (USD Million) Forecast by Cancer Type, 2021 to 2036
- Table 6: UK Market Value (USD Million) Forecast by Diagnostic Technique, 2021 to 2036
List Of Figures
- Figure 1: UK Market Pricing Analysis
- Figure 2: UK Market Value (USD Million) Forecast 2021 to 2036
- Figure 3: UK Market Value Share and BPS Analysis by Cancer Type, 2026 and 2036
- Figure 4: UK Market Y to o to Y Growth Comparison by Cancer Type, 2026 to 2036
- Figure 5: UK Market Attractiveness Analysis by Cancer Type
- Figure 6: UK Market Value Share and BPS Analysis by Diagnostic Technique, 2026 and 2036
- Figure 7: UK Market Y to o to Y Growth Comparison by Diagnostic Technique, 2026 to 2036
- Figure 8: UK Market Attractiveness Analysis by Diagnostic Technique
- Figure 9: UK Market Value (USD Million) Share and BPS Analysis by Region, 2026 and 2036
- Figure 10: UK Market Y to o to Y Growth Comparison by Region, 2026 to 2036
- Figure 11: UK Market Attractiveness Analysis by Region
- Figure 12: UK Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 13: UK Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 14: UK Market Value Share and BPS Analysis by Cancer Type, 2026 and 2036
- Figure 15: UK Market Y to o to Y Growth Comparison by Cancer Type, 2026 to 2036
- Figure 16: UK Market Attractiveness Analysis by Cancer Type
- Figure 17: UK Market Value Share and BPS Analysis by Diagnostic Technique, 2026 and 2036
- Figure 18: UK Market Y to o to Y Growth Comparison by Diagnostic Technique, 2026 to 2036
- Figure 19: UK Market Attractiveness Analysis by Diagnostic Technique
- Figure 20: UK Market - Tier Structure Analysis
- Figure 21: UK Market - Company Share Analysis
- FAQs -
How big is the demand for thyroid cancer diagnostics in UK in 2026?
The demand for thyroid cancer diagnostics in UK is estimated to be valued at USD 109.3 million in 2026.
What will be the size of thyroid cancer diagnostics in UK in 2036?
The market size for the thyroid cancer diagnostics in UK is projected to reach USD 168.7 million by 2036.
How much will be the demand for thyroid cancer diagnostics in UK growth between 2026 and 2036?
The demand for thyroid cancer diagnostics in UK is expected to grow at a 4.4?GR between 2026 and 2036.
What are the key product types in the thyroid cancer diagnostics in UK?
The key product types in thyroid cancer diagnostics in UK are papillary carcinoma, follicular carcinoma, medullary carcinoma and anaplastic variants.
Which diagnostic technique segment is expected to contribute significant share in the thyroid cancer diagnostics in UK in 2026?
In terms of diagnostic technique, imaging segment is expected to command 43.5% share in the thyroid cancer diagnostics in UK in 2026.