Demand for Ventricular Assist Device in USA
Demand for Ventricular Assist Device in USA Size and Share Forecast Outlook 2025 to 2035
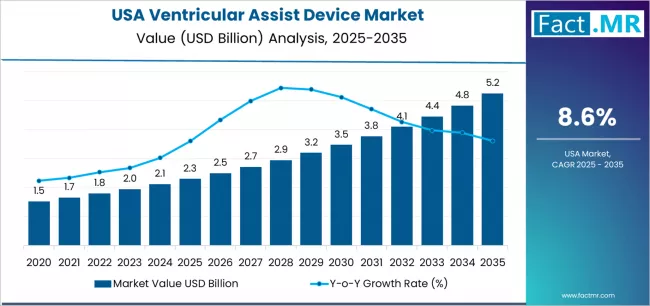
Demand for ventricular assist device in USA is projected to grow from USD 2.3 billion in 2025 to USD 5.2 billion by 2035, at a CAGR of 8.6%. Left Ventricular Assist Device will dominate with a 75.2% market share, while destination therapy will lead the application segment with a 41.6% share.
Demand for Ventricular Assist Device in USA 2025 to 2035
Demand for ventricular assist devices in the USA is projected to grow from USD 2.30 billion in 2025 to approximately USD 5.24 billion by 2035, recording an absolute increase of USD 2.94 billion over the forecast period. This translates into a total growth of 127.8%, with demand forecast to expand at a compound annual growth rate (CAGR) of 8.6% between 2025 and 2035.
The overall demand is expected to grow by 2.3 times during the same period, supported by increasing heart failure prevalence, rising adoption of destination therapy protocols, advancing magnetic levitation technology, and expanding cardiac surgery infrastructure throughout the USA.
Quick Stats for USA Ventricular Assist Device Industry
- USA Ventricular Assist Device Sales Value (2025): USD 2.30 billion
- USA Ventricular Assist Device Forecast Value (2035): USD 5.24 billion
- USA Ventricular Assist Device Forecast CAGR: 8.6%
- Leading Product in USA Ventricular Assist Device Industry: Left Ventricular Assist Device (75.2%)
- Key Growth Regions in USA Ventricular Assist Device Industry: West, Northeast, South, Midwest
- Regional Leadership: West holds the leading position in demand
- Key Players in USA Ventricular Assist Device Industry: Abbott Laboratories, Abiomed Incorporated, Medtronic Public Limited Company, Berlin Heart GmbH, Jarvik Heart Incorporated, Cardiac Assist Incorporated, ReliantHeart Incorporated, Sun Medical Technology Research Corporation, FineHeart SARL, CorWave SA
The left ventricular assist device segment is projected to account for 75.2% of ventricular assist device demand in 2025. Left ventricular assist devices are widely used in the USA for advanced heart failure management, destination therapy applications, and bridge-to-transplant protocols where hemodynamic support, proven clinical efficacy, and long-term durability remain essential for cardiac care applications and patient survival optimization.
The destination therapy segment is expected to represent 41.6% of ventricular assist device demand in 2025. Destination therapy applications are fundamental to the industry because they provide the essential permanent support capabilities, transplant alternative solutions, and long-term cardiac management required for end-stage heart failure patients, transplant-ineligible candidates, and advanced cardiac support systems.
Between 2020 and 2025, ventricular assist device demand in the USA experienced rapid expansion, driven by increasing cardiac surgery infrastructure development and growing recognition of mechanical circulatory support requirements for advanced heart failure management and patient survival optimization. The sector developed as cardiac surgeons and heart failure specialists, especially in major cardiac centers, recognized the need for specialized mechanical support solutions and effective hemodynamic assistance to achieve survival objectives while meeting FDA regulatory standards and clinical performance requirements. Cardiac centers and device manufacturers began emphasizing technology innovation and clinical outcomes to maintain competitive advantages and regulatory compliance.
Between 2025 and 2030, demand for ventricular assist devices in the USA is projected to expand from USD 2.30 billion to USD 3.54 billion, resulting in a value increase of USD 1.24 billion, which represents 42.2% of the total forecast growth for the decade. This phase of growth will be shaped by accelerating destination therapy adoption, rising magnetic levitation technology advancement, and growing clinical evidence requirements for advanced cardiac support systems across USA regions, particularly in areas where cardiac surgery infrastructure and heart failure management initiatives are accelerating device adoption. Increasing integration of continuous flow pump systems and growing adoption of implantable platforms continue to drive demand.
Cardiac surgeons and device manufacturers are expanding their clinical capabilities to address the growing complexity of modern heart failure requirements and hemodynamic support standards, with USA operations leading investments in conventional surgical technique enhancement methods and efficient patient management optimization systems.
From 2030 to 2035, demand is forecast to grow from USD 3.54 billion to USD 5.24 billion, adding another USD 1.70 billion, which constitutes 57.8% of the overall ten-year expansion. This period is expected to be characterized by expansion of specialized cardiac applications, development of enhanced hemodynamic capabilities, and implementation of comprehensive ventricular assist device technology programs across different healthcare and surgical sectors. The growing adoption of advanced pump systems and enhanced clinical outcome platforms, particularly in major cardiac centers and surgical operations, will drive demand for more sophisticated device solutions and validated hemodynamic systems.
USA Ventricular Assist Device Industry Key Takeaways
| Metric | Value |
|---|---|
| USA Ventricular Assist Device Sales Value (2025) | USD 2.30 billion |
| USA Ventricular Assist Device Forecast Value (2035) | USD 5.24 billion |
| USA Ventricular Assist Device Forecast CAGR (2025-2035) | 8.6% |
Why is the USA Ventricular Assist Device Industry Growing?
The USA ventricular assist device industry is experiencing robust growth, primarily fueled by a parallel expansion in the broader cardiac surgery and heart failure management sector. A significant surge in demand for both advanced cardiac support technology and proven hemodynamic assistance has created a larger base of cardiac surgeons, heart failure specialists, and medical companies requiring reliable device products.
Changing healthcare regulations and FDA safety standards mandate the use of efficient mechanical circulatory support systems for optimal patient outcomes and survival optimization. This cardiac-driven demand establishes a consistent, safety-based foundation.
As new operators enter the heart failure management segment and existing cardiac practices are modernized, the need for standard-issue and specialized device products forms a stable foundation for the industry's growth, ensuring a continuous stream of customers driven by clinical necessity and regulatory compliance.
Technological innovation serves as a powerful secondary engine for this growth. Modern ventricular assist device systems are no longer just basic mechanical pumps; they are advanced hemodynamic support products. The rapid adoption of specialized pump technologies has become a major selling point, significantly reducing surgical complexity and enhancing patient survival.
Beyond traditional options, manufacturers are integrating advanced formulations for seamless incorporation with digitally conscious cardiac approaches, and compatibility with various clinical monitoring requirements. These features, coupled with improvements in hemodynamic performance, system convenience, and device quality, are compelling both cardiac and surgical operators to upgrade from basic pump systems, driving a cycle of replacement and premiumization within the industry.
The industry is benefiting from evolving cardiac dynamics and a heightened focus on heart failure patient experience. An increasing emphasis on patient survival, particularly exploration of advanced hemodynamic technologies, is pushing demand for higher-quality, more diverse device varieties.
The segment has also expanded beyond traditional cardiac channels, with growing interest from the transplant sector, cardiac care applications, and even medical device units. This diversification, combined with the rise of specialized platforms that improve accessibility for all surgeons, ensures that suppliers can reach a wider audience than ever before. This confluence of efficiency, innovation, and accessibility creates a fertile ground for continued industry expansion.
Opportunity Pathways - Demand for Ventricular Assist Devices in the USA
The ventricular assist device demand in the USA is positioned for strong and steady expansion, growing from USD 2.30 billion in 2025 to USD 5.24 billion by 2035, reflecting an 8.8% CAGR. This growth is driven by the rising adoption of advanced, hemodynamic pump systems in cardiac surgery applications, destination therapy protocols, and bridge-to-transplant implementations. Operators are seeking specialized device solutions that maximize patient survival, regulatory compliance, and surgical efficiency amidst stringent FDA standards.
Demand from continuous flow device trends, cardiac support innovations, and heart failure management implementations strengthens opportunities for both high-performance pump formulations and integrated cardiac technology solutions. Suppliers focusing on implant-grade hemodynamic performance, clinical excellence processing, and validation support stand to gain from evolving regulatory landscapes and surgeon expectations for device traceability, clinical assurance, and technical documentation completeness.
- Pathway A - Destination Therapy and Long-Term Support: Surgeons face escalating demand for reliable, effective devices for the high-volume treatment of transplant-ineligible patients. Solutions targeting permanent support systems, long-term cardiac assistance, and durable hemodynamic devices can achieve strong adoption through optimized clinical performance, durability, and magnetic levitation resistance. Estimated revenue opportunity: USD 1.8-2.4 billion.
- Pathway B - Advanced Bridge-to-Transplant Applications: The transplant industry requires increasingly sophisticated hemodynamic support and patient stabilization solutions. Collaborations with transplant centers for integrated cardiac support systems can unlock long-term supply contracts and partnerships. Estimated revenue opportunity: USD 1.6-2.2 billion.
- Pathway C - Continuous Flow and Magnetic Levitation Devices: Growth in cardiac surgery, heart failure management, and hemodynamic applications creates robust demand for advanced pump systems and clinical performance-compliant devices. Suppliers offering proven continuous flow pumps with exceptional hemodynamic performance and mechanical durability can build strategic relationships with top-tier cardiac centers. Estimated revenue opportunity: USD 1.4-1.9 billion.
- Pathway D - Surgical Training and Clinical Support: Manufacturing requirements for surgical precision, clinical resistance, and outcome durability are driving preference for specialized devices in cardiac procedures, hemodynamic management, and clinical applications. Suppliers offering systems with exceptional performance stability and clinical purity can differentiate offerings and attract quality-focused organizations. Estimated revenue opportunity: USD 1.2-1.6 billion.
- Pathway E - Implantable System Components: Critical applications in cardiac surgery, hemodynamic support, and patient management require specialized device configurations with advanced clinical resistance, durability properties, and patient-safety features. Suppliers investing in technology development for cardiac products can secure advantages in this demanding segment. Estimated revenue opportunity: USD 1.0-1.4 billion.
- Pathway F - Technical Service, Validation and Regulatory Support: Comprehensive support networks offering device selection guidance, full regulatory documentation, and surgical process validation create recurring partnership opportunities. Companies building strong technical service capabilities can capture ongoing relationships and enhance surgeon speed-to-deployment. Estimated revenue opportunity: USD 0.8-1.1 billion.
Segmental Analysis
The industry is segmented by product, application, and region. By product, the industry is divided into left ventricular assist device, right ventricular assist device, bi-ventricular assist device, and total artificial heart. In terms of application, the industry is segmented into destination therapy, bridge to transplant, bridge to recovery, with destination therapy representing a key growth and innovation hub for cardiac technologies. Regionally, the industry is divided into West, Northeast, South, and Midwest.
Why Does Left Ventricular Assist Device Account for High Share of 75.20%?
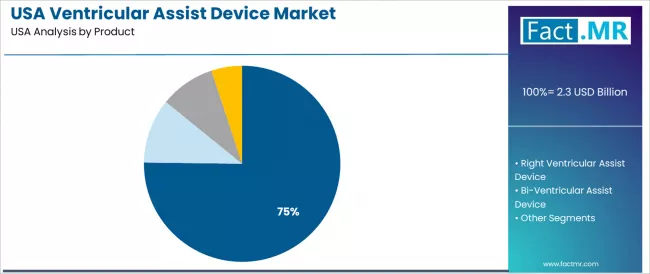
The left ventricular assist device segment is projected to account for 75.20% of ventricular assist device demand in 2025, making it the leading product type across the sector. This dominance reflects the hemodynamic support requirements and clinical acceptance needs of device systems for existing cardiac facilities and heart failure applications where cardiac performance is optimized through established clinical characteristics and integrated hemodynamic architecture.
In the USA, where substantial cardiac infrastructure requires device integration without complete system redesign, left ventricular assist device solutions provide practical pathways for cardiac enhancement while maintaining clinical preferences. Continuous innovations are improving hemodynamic performance, durability, and safety parameters, enabling cardiac operators to achieve high clinical standards while maximizing cost effectiveness.
- Clinical compatibility and existing system integration make left ventricular assist devices the preferred product type for enhancing cardiac facilities and surgical operations.
- Hemodynamic reliability and performance demonstration track records are enhancing surgeon confidence and clinical viability across large-scale adoption initiatives.
Why Does Destination Therapy Account for High Share of 41.60%?
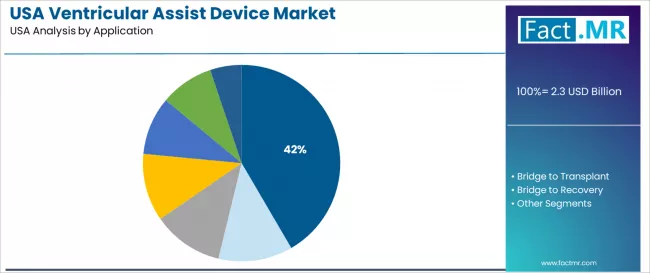
Destination therapy applications are expected to represent 41.60% of ventricular assist device demand in 2025, reflecting the critical role of permanent cardiac support requiring comprehensive survival solutions. Destination therapy operations including transplant-ineligible projects, long-term support facilities, and permanent cardiac systems generate consistent demand for devices that support efficient cardiac utilization and performance optimization.
Device systems are widely adopted for destination therapy facilities due to significant survival benefits and enhanced hemodynamic capabilities. Their reliable, high-performance operation provides effective, cost-efficient cardiac protection solutions, enhancing survival independence for heart failure patients.
- Permanent support requirements and cardiac management operations drive substantial demand for specialized devices designed for cardiac applications.
- Survival optimization and clinical efficiency demands create consistent cardiac protection requirements across major cardiac regions and surgical facilities.
What are the Drivers, Restraints, and Key Trends in the USA Ventricular Assist Device Industry?
The demand for ventricular assist devices in the USA is advancing steadily due to increasing cardiac requirements and growing recognition of advanced hemodynamic necessity for regulatory compliance, with the West region serving as a key driver of innovation and cardiac advancement.
The sector faces challenges including hemodynamic performance consistency optimization, device enhancement complexity, and ongoing concerns regarding regulatory cost considerations and approval variations.
Growth in Cardiac Infrastructure Development and Heart Failure Management Expansion Programs
The enhancement of surgical standards, gaining particular significance through heart failure trends and clinical education campaigns, is enabling device providers to achieve differentiation without prohibitive development costs, providing predictable demand patterns through cardiac requirements and surgeon preferences.
Enhanced surgical standards offering substantial opportunities for device systems and integrated applications provide foundational dynamics while allowing providers to secure cardiac facility agreements and distribution partnerships.
Deployment of Advanced Hemodynamic Capabilities and High-Performance Cardiac Systems
Modern device providers and cardiac operators are establishing advanced surgical networks and centralized clinical facilities that improve surgical efficiency through procedure standardization and hemodynamic analytics.
Integration of hemodynamic enhancement systems, high-performance device technology, and coordinated quality management enables more efficient clinical operations across multiple surgical regions.
Development of Automated Cardiac Systems and Enhanced Patient Targeting Methods
The expansion of automated cardiac systems and patient segmentation is driving development of specialized device systems with enhanced hemodynamic profiles, improved clinical characteristics, and optimized survival attributes that address current limitations and expand cardiac applications beyond traditional devices.
These specialized systems require sophisticated hemodynamic capabilities and clinical expertise that exceed traditional surgical requirements, creating specialized demand segments with differentiated survival propositions. Producers are investing in patient targeting and hemodynamic optimization to serve emerging cardiac applications while supporting innovation in clinical development and survival engagement.
Analysis of USA Ventricular Assist Device Demand by Key Region
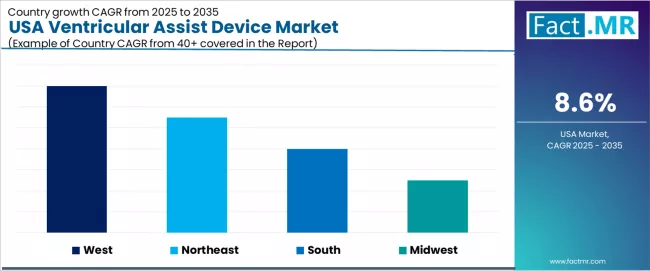
| Region | CAGR (2025 to 2035) |
|---|---|
| West | 9.20% |
| Northeast | 8.90% |
| South | 8.60% |
| Midwest | 8.30% |
The USA ventricular assist device demand is witnessing steady growth, supported by rising cardiac requirements, expanding heart failure management facility initiatives, and the deployment of advanced hemodynamic technologies across regions. West leads the nation with a 9.20% CAGR, reflecting a strong innovation-conscious base, substantial cardiac surgery development, and established cardiac advancement facilities.
Why Does the West Region Lead Ventricular Assist Device Demand?
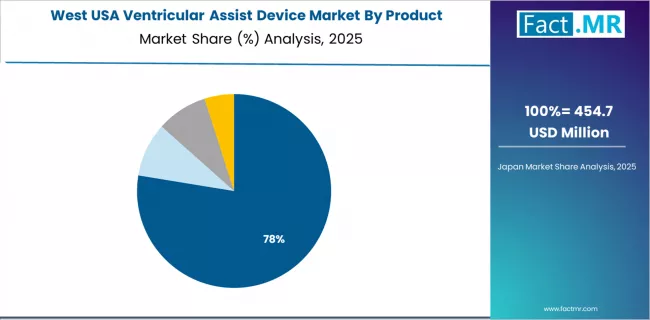
Demand for ventricular assist devices in West is projected to exhibit strong growth with a CAGR of 9.20% through 2035, driven by a strong innovation-conscious cardiac base, substantial heart failure management creating premium hemodynamic opportunities, and a concentration of surgical advancement across California, Oregon, Washington, Nevada, and surrounding states.
Advanced cardiac technology programs and hemodynamic control initiatives are expanding device adoption among surgeons, cardiac facilities, and heart failure specialists pursuing clinical optimization, survival development, and specialized cardiac projects throughout major urban hubs and surgical corridors.
- Innovation consciousness base and cardiac infrastructure capabilities are requiring comprehensive hemodynamic strategies and survival solutions, driving demand for device systems with demonstrated clinical enhancement capabilities and permanent survival assurance throughout diverse cardiac operations.
- Heart failure management and cardiac concentration are generating substantial device demand across cardiac companies, surgeons, and survival suppliers serving cardiac applications and surgical requirements.
Why Does the Northeast Region Demonstrate Strong Growth?
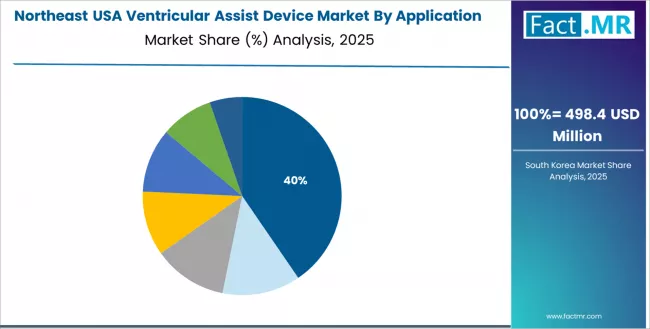
Demand for ventricular assist devices in Northeast is projected to grow with a CAGR of 8.90% through 2035, supported by established cardiac presence, comprehensive heart failure management, and strong surgical facilities across New York, Pennsylvania, Massachusetts, New Jersey, and surrounding states.
Established cardiac presence and surgical leadership are supporting device adoption throughout surgeon facilities, heart failure operations, and survival distribution centers serving clinical enhancement and hemodynamic applications.
- Strong cardiac ecosystem and surgical networks are enabling device integration across survival producers, surgeons, and hemodynamic suppliers pursuing advanced clinical development and cardiac programs.
- Premium heart failure capabilities and survival excellence are driving device demand among leading surgeon corporations, cardiac centers, and specialized survival firms focused on clinical enhancement, hemodynamic optimization, and surgical development targeting cardiac applications and advanced cardiac operations.
What Factors Underpin Ventricular Assist Device Demand in South Region?
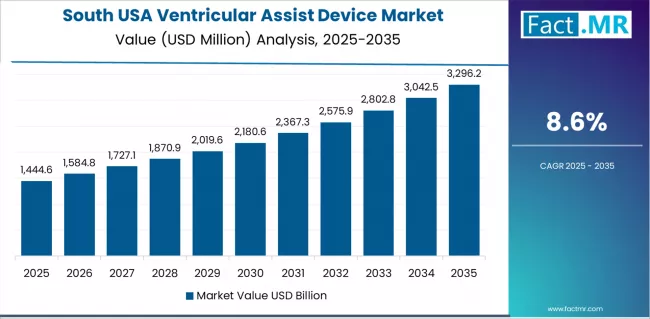
Demand for ventricular assist devices in South is forecast to advance with a CAGR of 8.60% through 2035, driven by expanding cardiac capabilities, growing hemodynamic investment, and increasing survival consciousness across Texas, Florida, Georgia, North Carolina, and surrounding states.
Rising heart failure sector development and surgical partnerships are supporting device integration across hemodynamic producers, surgeon facilities, and survival distributors pursuing clinical enhancement, cardiac expansion, and surgical initiatives throughout expanding cardiac regions and urban centers.
- Growing cardiac infrastructure and heart failure investment are creating opportunities for device adoption across emerging hemodynamic hubs, surgeon facilities, and survival distribution centers in major metropolitan areas and cardiac corridors.
- Clinical expansion and survival growth are driving device demand among cardiac operators seeking enhanced clinical capabilities and participation in advanced hemodynamic programs.
What Boosts Consistent Demand in the Midwest Region?
Demand for ventricular assist devices in Midwest is expected to expand with a CAGR of 8.30% through 2035, supported by hemodynamic surgical capabilities, cardiac infrastructure development, and growing surgeon efficiency presence across Illinois, Ohio, Wisconsin, Michigan, and surrounding states.
Survival expertise and clinical capabilities are driving device demand among hemodynamic producers, cardiac suppliers, and heart failure surgeons serving device production and clinical applications.
- Growing hemodynamic development and surgical investment are supporting device adoption across emerging clinical hubs, cardiac facilities, and surgeon centers pursuing clinical enhancement and hemodynamic programs.
- Expanding cardiac infrastructure and hemodynamic integration are creating opportunities for device utilization across survival suppliers, cardiac production facilities, and surgeon operations seeking clinical device production, survival support, and surgical capabilities throughout major cardiac regions and emerging surgeon survival centers.
Competitive Landscape of USA Ventricular Assist Device Industry
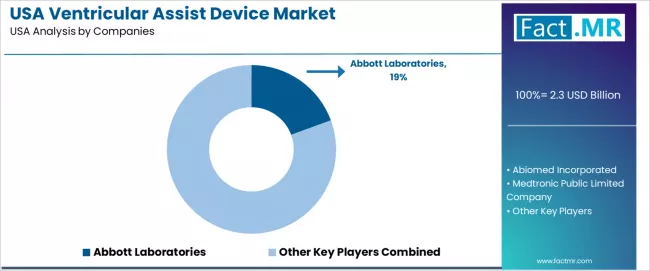
The USA ventricular assist device industry is defined by competition among established cardiac device corporations, specialized hemodynamic companies, and integrated cardiac producers, with major surgeon operators maintaining significant influence through clinical resources and surgical capabilities. Companies are investing in device advancement, hemodynamic optimization, survival acceptance technologies, and comprehensive clinical services to deliver effective, reliable, and efficient cardiac solutions across USA cardiac and surgeon applications.
Abbott Laboratories dominates with a 19.40% share, offering comprehensive hemodynamic solutions including advanced equipment, clinical enhancement technologies, and distribution services with a focus on cardiac applications, survival consistency, and hemodynamic optimization across USA operations. The company continues investing in hemodynamic programs, distribution strategies, and device innovation while expanding operational presence and advanced surgeon applications.
Abiomed Incorporated provides specialized hemodynamic solutions with emphasis on clinical development and surgical excellence. Medtronic Public Limited Company focuses on premium device development and cardiac applications. Berlin Heart GmbH emphasizes clinical development and specialized surgeon equipment production. Jarvik Heart Incorporated offers hemodynamic technology solutions and professional clinical support. Cardiac Assist Incorporated specializes in cardiac device development and distribution programs.
Key Players in USA Ventricular Assist Device Industry
- Abbott Laboratories
- Abiomed Incorporated
- Medtronic Public Limited Company
- Berlin Heart GmbH
- Jarvik Heart Incorporated
- Cardiac Assist Incorporated
- ReliantHeart Incorporated
- Sun Medical Technology Research Corporation
- FineHeart SARL
- CorWave SA
- Rogers Corporation
- Covestro AG
- Armacell International S.A.
- Zeus Company Incorporated
- Saint-Gobain Performance Plastics Corporation
Scope of the Report
| Item | Value |
|---|---|
| Quantitative Units | USD 5.24 billion |
| Product | Left Ventricular Assist Device, Right Ventricular Assist Device, Bi-Ventricular Assist Device, Total Artificial Heart |
| Application | Destination Therapy, Bridge to Transplant, Bridge to Recovery, Drug Delivery Systems, Implants, Medical Bags, Others |
| Regions Covered | West, Northeast, South, Midwest |
| Key Companies Profiled | Abbott Laboratories, Abiomed Incorporated, Medtronic Public Limited Company, Berlin Heart GmbH, Jarvik Heart Incorporated, Cardiac Assist Incorporated, ReliantHeart Incorporated, Sun Medical Technology Research Corporation, FineHeart SARL, CorWave SA, Rogers Corporation, Covestro AG, Armacell International S.A., Zeus Company Incorporated, Saint-Gobain Performance Plastics Corporation |
| Additional Attributes | Sales by product and application segment, regional demand trends across West, Northeast, South, and Midwest, competitive landscape with established cardiac device corporations and hemodynamic suppliers, surgeon facility preferences for left ventricular assist device versus total artificial heart equipment, integration with cardiac facilities and advanced hemodynamic optimization policies particularly advanced in West region |
USA Ventricular Assist Device Industry by Segments
-
Product :
- Left Ventricular Assist Device
- Right Ventricular Assist Device
- Bi-Ventricular Assist Device
- Total Artificial Heart
-
Application :
- Destination Therapy
- Bridge to Transplant
- Bridge to Recovery
- Drug Delivery Systems
- Implants
- Medical Bags
- Others
-
Region :
- West
- Northeast
- South
- Midwest
Table of Content
- Executive Summary
- USA Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- USA Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- USA Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2025 to 2035
- Left Ventricular Assist Device
- Right Ventricular Assist Device
- Bi-Ventricular Assist Device
- Total Artificial Heart
- Y to o to Y Growth Trend Analysis By Product, 2020 to 2024
- Absolute $ Opportunity Analysis By Product, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- Destination Therapy
- Bridge to Transplant
- Bridge to Recovery
- Drug Delivery Systems
- Implants
- Medical Bags
- Others
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- USA
- Market Attractiveness Analysis By Region
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- By Product
- By Application
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product
- By Application
- Competition Analysis
- Competition Deep Dive
- Abbott Laboratories
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Abiomed Incorporated
- Medtronic Public Limited Company
- Berlin Heart GmbH
- Jarvik Heart Incorporated
- Cardiac Assist Incorporated
- ReliantHeart Incorporated
- Sun Medical Technology Research Corporation
- FineHeart SARL
- CorWave SA
- Rogers Corporation
- Covestro AG
- Armacell International S.A.
- Zeus Company Incorporated
- Saint-Gobain Performance Plastics Corporation
- Abbott Laboratories
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: USA Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 3: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: USA Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: USA Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 6: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
List Of Figures
- Figure 1: USA Market Pricing Analysis
- Figure 2: USA Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: USA Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 4: USA Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 5: USA Market Attractiveness Analysis by Product
- Figure 6: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: USA Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 8: USA Market Attractiveness Analysis by Application
- Figure 9: USA Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: USA Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: USA Market Attractiveness Analysis by Region
- Figure 12: USA Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: USA Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 14: USA Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 15: USA Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 16: USA Market Attractiveness Analysis by Product
- Figure 17: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 18: USA Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 19: USA Market Attractiveness Analysis by Application
- Figure 20: USA Market - Tier Structure Analysis
- Figure 21: USA Market - Company Share Analysis
- FAQs -
How big is the demand for ventricular assist device in USA in 2025?
The demand for ventricular assist device in USA is estimated to be valued at USD 2.3 billion in 2025.
What will be the size of ventricular assist device in USA in 2035?
The market size for the ventricular assist device in USA is projected to reach USD 5.2 billion by 2035.
How much will be the demand for ventricular assist device in USA growth between 2025 and 2035?
The demand for ventricular assist device in USA is expected to grow at a 8.6% CAGR between 2025 and 2035.
What are the key product types in the ventricular assist device in USA?
The key product types in ventricular assist device in USA are left ventricular assist device, right ventricular assist device, bi-ventricular assist device and total artificial heart.
Which application segment is expected to contribute significant share in the ventricular assist device in USA in 2025?
In terms of application, destination therapy segment is expected to command 41.6% share in the ventricular assist device in USA in 2025.