Lewy Body Dementia Treatment Market
Lewy Body Dementia Treatment Market Size and Share Forecast Outlook 2025 to 2035
Lewy body dementia treatment market is projected to grow from USD 5.2 billion in 2025 to USD 9.0 billion by 2035, at a CAGR of 5.6%. Cholinesterase inhibitors will dominate with a 44.0% market share, while hospitals will lead the end user segment with a 61.0% share.
Lewy Body Dementia Treatment Market Forecast and Outlook 2025 to 2035
The Lewy body dementia treatment industry stands at the threshold of a decade-long expansion trajectory that promises to reshape neurological care solutions, pharmaceutical development, and therapeutic intervention systems worldwide.
The market's journey from USD 5.2 billion in 2025 to USD 9.0 billion by 2035 represents substantial growth, the market will rise at a CAGR of 5.6% which demonstrating the accelerating adoption of specialized neurological treatments and advanced therapeutic protocols across hospitals, specialty clinics, and healthcare distribution networks.
The first half of the decade (2025-2030) will witness the market climbing from USD 5.2 billion to approximately USD 6.8 billion, adding USD 1.6 billion in value, which constitutes 42% of the total forecast growth period.
This phase will be characterized by the rapid adoption of cholinesterase inhibitor-based therapeutic systems, driven by increasing disease prevalence and the growing need for effective neurological treatment solutions worldwide. Advanced pharmaceutical capabilities and flexible healthcare distribution systems will become standard expectations rather than premium options.
The latter half (2030-2035) will witness sustained growth from USD 6.8 billion to USD 9.0 billion, representing an addition of USD 2.2 billion or 58% of the decade's expansion. This period will be defined by mass market penetration of specialized dementia therapies, integration with comprehensive healthcare platforms, and seamless compatibility with existing pharmaceutical distribution infrastructure.
The market trajectory signals fundamental shifts in how healthcare providers approach neurological treatment and therapeutic optimization, with participants positioned to benefit from sustained demand across multiple therapy types and distribution channels.
Quick Stats for Lewy Body Dementia Treatment Market
- Lewy Body Dementia Treatment Market Value (2025): USD 5.2 billion
- Lewy Body Dementia Treatment Market Forecast Value (2035): USD 9.0 billion
- Lewy Body Dementia Treatment Market Forecast CAGR: 5.6%
- Leading Therapy in Lewy Body Dementia Treatment Market: Cholinesterase inhibitors
- Key Growth Regions in Lewy Body Dementia Treatment Market: North America, Europe, and Asia Pacific
- Top Key Players in Lewy Body Dementia Treatment Market: Eisai, Novartis, Teva, Biogen, Roche
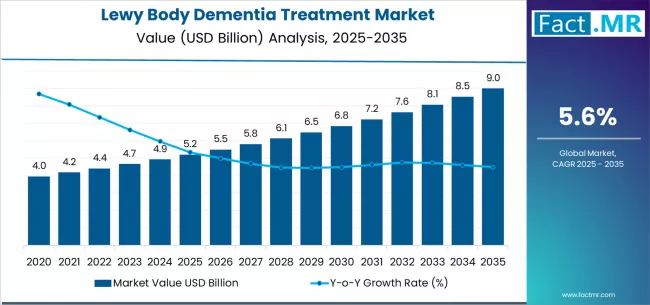
The Lewy Body Dementia Treatment market demonstrates distinct growth phases with varying market characteristics and competitive dynamics. Between 2025 and 2030, the market progresses through its therapeutic adoption phase, expanding from USD 5.2 billion to USD 6.8 billion with steady annual increments averaging 5.6% growth. This period showcases the transition from basic neurological treatments to advanced cholinesterase inhibitor systems with enhanced efficacy capabilities and integrated patient monitoring systems becoming mainstream features.
The 2025-2030 phase adds USD 1.6 billion to market value, representing 42% of total decade expansion. Market maturation factors include standardization of neurological and healthcare protocols, declining component costs for specialized pharmaceutical formulations, and increasing physician awareness of treatment benefits reaching 95-98% therapeutic effectiveness in neurological and dementia care applications.
Competitive landscape evolution during this period features established pharmaceutical companies like Eisai and Novartis expanding their dementia treatment portfolios while specialty manufacturers focus on advanced drug development and enhanced therapeutic capabilities.
From 2030 to 2035, market dynamics shift toward advanced therapeutic integration and global healthcare expansion, with growth continuing from USD 6.8 billion to USD 9.0 billion, adding USD 2.2 billion or 58% of total expansion.
This phase transition centers on specialized dementia treatment systems, integration with automated healthcare networks, and deployment across diverse hospital and clinical scenarios, becoming standard rather than specialized therapies.
The competitive environment matures with focus shifting from basic treatment capability to comprehensive patient care optimization systems and integration with healthcare monitoring platforms.
Lewy Body Dementia Treatment Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 5.2 billion |
| Market Forecast (2035) ↑ | USD 9.0 billion |
| Growth Rate ★ | 5.6% CAGR |
| Leading Therapy → | Cholinesterase inhibitors Therapy |
| Primary End User → | Hospitals Segment |
The market demonstrates strong fundamentals with cholinesterase inhibitor therapies capturing a dominant share through advanced neurological treatment and symptom management optimization capabilities. Hospital end users drive primary demand, supported by increasing dementia prevalence and specialized healthcare requirements.
Geographic expansion remains concentrated in developed markets with established neurological care infrastructure, while emerging economies show accelerating adoption rates driven by healthcare awareness and rising neurological treatment standards.
Why is the Lewy Body Dementia Treatment Market Growing?
An increase in the aging population demographic creates compelling therapeutic advantages through Lewy body dementia treatments that provide immediate symptom management without complex procedures, enabling healthcare providers to achieve optimal patient outcomes while maintaining treatment efficacy and reducing disease progression.
Neurological care modernization accelerates as healthcare facilities worldwide seek advanced therapeutic systems that complement traditional dementia treatments, enabling precise symptom control and quality management that align with medical standards and patient care regulations.
Pharmaceutical innovation enhancement drives adoption from neurological departments and specialty medical centers requiring effective treatment solutions that minimize cognitive decline while maintaining patient productivity during therapy administration and care integration operations.
However, growth faces headwinds from drug development complexity challenges that vary across pharmaceutical manufacturers regarding the development of dementia treatments and specialty efficacy requirements, which may limit adoption in cost-sensitive healthcare environments.
Technical limitations also persist regarding treatment personalization and side effect management that may reduce effectiveness in complex patient populations, which affect therapeutic performance and treatment consistency.
Opportunity Pathways - Lewy Body Dementia Treatment Market
The Lewy body dementia treatment market represents a specialized pharmaceutical opportunity driven by expanding aging demographics, neurological care modernization, and the need for superior therapeutic effectiveness in diverse dementia care applications. As healthcare providers worldwide seek to achieve optimal patient outcomes, reduce disease progression, and integrate advanced treatment systems with comprehensive care plans, dementia treatments are evolving from basic symptom management to sophisticated therapeutic solutions ensuring patient welfare and care optimization.
The market's growth trajectory from USD 5.2 billion in 2025 to USD 9.0 billion by 2035 at a 5.6% CAGR reflects fundamental shifts in neurological care requirements and dementia treatment optimization. Geographic expansion opportunities are particularly pronounced in North America and European markets, while the dominance of cholinesterase inhibitor therapies (44.0% market share) and hospital end users (61.0% share) provides clear strategic focus areas.
Pathway A - Cholinesterase Inhibitor Leadership & Advanced Therapeutic OptimizationStrengthening the dominant cholinesterase inhibitor segment (44.0% market share) through enhanced drug formulations, superior efficacy features, and automated treatment monitoring systems. This pathway focuses on optimizing cognitive benefits, improving symptom management, extending therapeutic effectiveness to 95-98% success rates, and developing specialized formulations for diverse patient populations. Market leadership consolidation through advanced pharmaceutical engineering and automated care integration enables premium positioning while defending competitive advantages against alternative therapeutic approaches. Expected revenue pool: USD 540-720 million
Pathway B - North America Market Expansion & Treatment LocalizationRapid neurological care and aging population growth across North America creates substantial expansion opportunities through local pharmaceutical capabilities and technology transfer partnerships. Growing dementia awareness and government healthcare initiatives drive sustained demand for advanced therapeutic systems. Localization strategies reduce distribution costs, enable faster medical support, and position companies advantageously for healthcare programs while accessing growing domestic markets. Expected revenue pool: USD 415-555 million
Pathway C - Hospital Market Dominance & Quality IntegrationExpansion within the dominant hospital segment (61.0% market share) through specialized treatments addressing medical quality standards and high-volume patient care requirements. This pathway encompasses automated hospital systems, quality control integration, and compatibility with diverse neurological processes. Premium positioning reflects superior therapeutic performance and comprehensive medical compliance supporting modern hospital operations. Expected revenue pool: USD 350-470 million
Pathway D - Clinic Application DiversificationStrategic expansion into clinic applications (28.0% market share) requires enhanced treatment capabilities and specialized therapies addressing outpatient operational requirements. This pathway addresses specialty neurological clinics, ambulatory care centers, and healthcare integration with advanced treatments for demanding patient care conditions. Premium pricing reflects specialized performance requirements and extended treatment standards. Expected revenue pool: USD 295-395 million
Pathway E - Antipsychotic Therapy InnovationDevelopment of specialized antipsychotic treatments for dementia applications (29.0% share), addressing specific behavioral requirements and psychiatric symptom demands. This pathway encompasses behavioral management products, psychiatric-optimized formulations, and cost-effective alternatives for complex symptom management markets. Technology differentiation through proprietary formulations enables diversified revenue streams while reducing dependency on single therapeutic platforms. Expected revenue pool: USD 245-330 million
Pathway F - Retail Pharmacy Distribution Development & Healthcare IntegrationExpansion of retail pharmacy distribution segment (48.0% market share) through enhanced accessibility properties, patient convenience applications, and specialized community healthcare requirements. This pathway encompasses community pharmacy development, patient access applications, and specialty distribution products requiring consistency characteristics. Market development through advanced distribution engineering enables differentiated positioning while accessing healthcare markets requiring community-based solutions. Expected revenue pool: USD 205-275 million
Pathway G - Regulatory Compliance & Advanced Therapeutic DevelopmentDevelopment of medically superior dementia treatments addressing regulatory compliance and safety requirements across hospital and clinical applications. This pathway encompasses clinical trial processes, FDA approval pathways, and comprehensive medical documentation. Premium positioning reflects regulatory leadership and medical expertise while enabling access to regulated healthcare programs and safety-driven partnerships. Expected revenue pool: USD 175-235 million
Segmental Analysis
The market segments by therapy into cholinesterase inhibitors, antipsychotics, and other categories, representing the evolution from traditional neurological treatments to specialized therapeutic solutions for comprehensive dementia care optimization.
End user segmentation of the Lewy body dementia treatment includes hospitals, clinics, and other sectors, reflecting distinct requirements for treatment performance, patient capacity, and healthcare quality standards.
By distribution, the market is segmented into retail pharmacies, hospitals, and online categories, with retail pharmacy systems leading adoption while hospital distribution shows specialized growth patterns driven by healthcare accessibility expansion programs.
The segmentation structure reveals technology progression from standard neurological treatments toward specialized therapeutic systems with enhanced efficacy and patient monitoring capabilities, while application diversity spans from hospital neurological care to specialized clinical and community healthcare applications requiring precise treatment solutions.
By Therapy, the Cholinesterase inhibitors Segment Accounts for Dominant Market Share
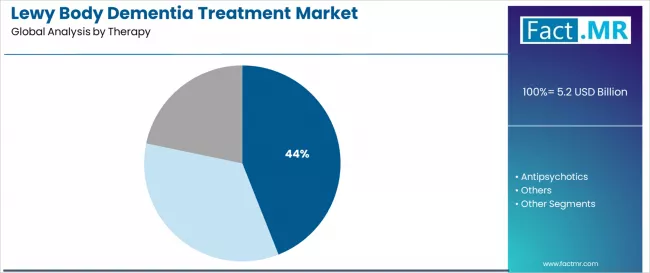
Cholinesterase inhibitor systems command the leading position in the Lewy body dementia treatment market with approximately 44.0% market share through advanced therapeutic features, including superior cognitive enhancement, neurotransmitter optimization capability, and patient care optimization that enable healthcare providers to achieve optimal treatment results across diverse neurological and dementia environments.
The segment benefits from physician preference for evidence-based therapeutic systems that provide consistent cognitive performance, reduced disease progression, and treatment efficiency optimization without requiring significant care protocol modifications. Advanced inhibitor features enable standardized treatment systems, therapeutic consistency, and integration with existing neurological care equipment, where treatment performance and patient safety represent critical medical requirements.
Cholinesterase inhibitor dementia treatment systems differentiate through proven therapeutic stability, consistent cognitive characteristics, and integration with advanced neurological care systems that enhance treatment effectiveness while maintaining optimal quality suitable for diverse dementia and neurological applications.
Key market characteristics:
- Advanced pharmaceutical formulations with optimized neurotransmitter effects and cognitive capabilities
- Extended therapeutic effectiveness, enabling 95-98% treatment success with consistent patient care quality
- Medical compatibility, including evidence-based treatment systems, efficacy monitoring, and care integration for neurological and dementia operations
Antipsychotic Treatments Show Strong Market Growth
Antipsychotic treatments maintain a significant 29.0% market share in the Lewy body dementia treatment market due to their specialized behavioral management properties and versatile application advantages.
Antipsychotic treatments appeal to healthcare providers requiring behavioral symptom control products with consistent characteristics for psychiatric management, agitation control, and specialty applications. Growth is driven by complex symptom management expansion, emphasizing reliable therapeutic solutions and operational efficiency through optimized pharmaceutical systems.
By End User, the Hospitals Segment Accounts for the Largest Market Share
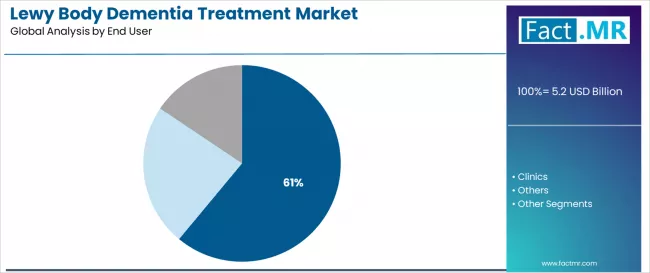
Hospital applications dominate the Lewy body dementia treatment market with approximately 61.0% market share due to widespread adoption of neurological care systems and increasing focus on dementia treatment, specialized patient care, and hospital management applications that minimize treatment complications while maintaining healthcare industry standards.
Hospital administrators prioritize treatment reliability, therapeutic consistency, and integration with existing neurological infrastructure that enables coordinated dementia care across multiple medical departments. The segment benefits from substantial healthcare investment and modernization programs that emphasize the acquisition of therapeutic systems for quality control and patient care efficiency applications.
Healthcare expansion programs incorporate dementia treatments as standard therapies for neurological operations, while dementia care initiatives increase demand for advanced therapeutic capabilities that comply with medical standards and minimize treatment defects.
Application dynamics include:
- Strong growth in neurological departments and dementia care centers requiring precise therapeutic capabilities
- Increasing adoption in geriatric care applications for age-related cognitive requirements
- Rising integration with automated healthcare systems for treatment optimization and quality assurance
Clinics Demonstrate Steady Growth
Clinic applications capture approximately 28.0% market share through specialized treatment requirements in outpatient neurological facilities, specialty dementia clinics, and ambulatory care optimization applications. These facilities demand robust therapeutic systems capable of operating in diverse clinical conditions while providing effective patient treatment and care management capabilities.
Other End User Applications Show Specialized Demand
Other end user applications account for approximately 11.0% market share, including long-term care facilities, home healthcare services, and specialized neurological requiring dementia treatments for comprehensive patient care and treatment optimization.
What are the Drivers, Restraints, and Key Trends of the Lewy Body Dementia Treatment Market?
Aging population expansion drives primary adoption as Lewy body dementia treatments provide therapeutic capabilities that enable healthcare providers to meet specific patient care requirements without complex invasive procedures, supporting quality healthcare lifestyles and medical missions that require effective neurological treatment applications.
Neurological care innovation demand accelerates market expansion as healthcare facilities seek effective therapeutic systems that minimize cognitive decline failures while maintaining treatment effectiveness during patient care and medication management scenarios.
Pharmaceutical spending increases worldwide, creating sustained demand for therapeutic systems that complement traditional neurological treatments and provide care flexibility in complex medical environments.
Drug development complexity challenges vary across pharmaceutical manufacturers regarding the development of dementia treatments and specialty efficacy requirements, which may limit operational flexibility and market penetration in regions with stringent regulatory environments or cost-sensitive healthcare systems.
Treatment personalization performance limitations persist regarding patient response variability and side effect management that may reduce therapeutic effectiveness in complex patient populations, medication interaction, or comorbidity conditions, affecting treatment quality and patient care consistency. Market fragmentation across multiple medical specifications and healthcare standards creates compatibility concerns between different suppliers and existing healthcare infrastructure.
Adoption accelerates in neurological care and premium healthcare sectors where patient care requirements justify treatment costs, with geographic concentration in developed markets transitioning toward mainstream adoption in emerging economies driven by healthcare awareness expansion and neurological care development.
Technology development focuses on enhanced therapeutic capabilities, improved patient monitoring features, and integration with automated healthcare systems that optimize treatment effectiveness and operational reliability.
The market could face disruption if alternative neurological treatment technologies or medical regulations significantly limit the deployment of dementia-based therapeutic systems in hospital or clinical applications, though the treatments' unique combination of cognitive benefits, symptom management, and patient care effectiveness continues to make them valuable in specialized neurological care applications.
Analysis of the Lewy Body Dementia Treatment Market by Key Country
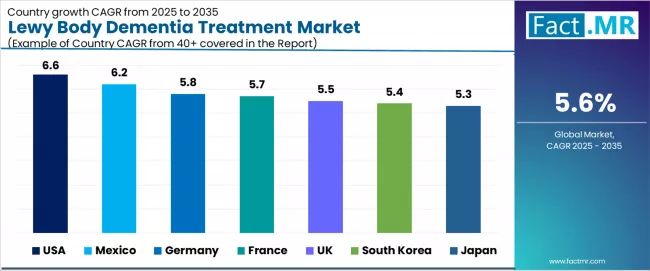
The Lewy body dementia treatment market demonstrates varied regional dynamics with Growth Leaders including the United States (6.6% CAGR) and Mexico (6.2% CAGR) driving expansion through healthcare capacity additions and neurological care development programs.
Steady performers encompass Germany (5.8% CAGR), France (5.7% CAGR), and South Korea (5.5% CAGR), benefiting from established healthcare industries and advanced neurological treatment adoption. Mature markets feature United Kingdom (5.5% CAGR) and Japan (5.3% CAGR), where specialized dementia applications and healthcare technology integration support consistent growth patterns.
| Country | CAGR (2025-2035) |
|---|---|
| USA | 6.6% |
| Mexico | 6.2% |
| Germany | 5.8% |
| France | 5.7% |
| UK | 5.5% |
| South Korea | 5.4% |
| Japan | 5.3% |
Regional synthesis reveals North American markets leading adoption through neurological care innovation and healthcare infrastructure development, while European countries maintain steady expansion supported by pharmaceutical technology advancement and healthcare standardization requirements. Asian markets show moderate growth driven by aging population applications and healthcare technology integration trends.
Growth in the US Market Propelled by Technological Advancements in Healthcare Endeavors
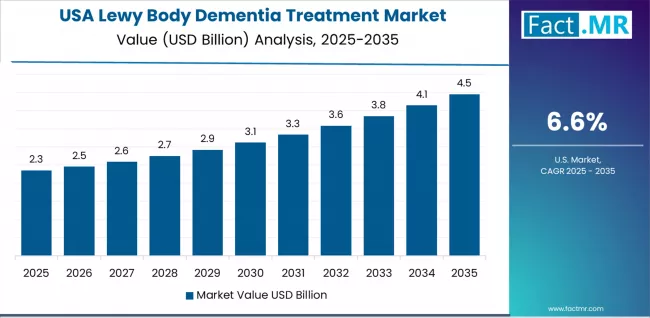
The US market emphasizes advanced therapeutic features, including precision neurological control and integration with comprehensive healthcare platforms that manage treatment quality, patient care optimization, and medical control applications through unified monitoring systems.
The country demonstrates strong growth at 6.6% CAGR, driven by healthcare modernization, pharmaceutical innovation projects, and emerging geriatric care applications that support therapeutic system integration. American neurologists prioritize operational effectiveness with dementia treatments delivering consistent patient care performance through advanced pharmaceutical algorithms and healthcare adaptation capabilities.
Technology deployment channels include major medical centers, specialized neurological suppliers, and pharmaceutical industry procurement programs that support professional applications for complex dementia treatment and patient care applications. Healthcare platform integration capabilities with established neurological systems expand market appeal across diverse operational requirements seeking precision and reliability benefits. The resilient healthcare sector and expanding neurological capacity additions create sustained demand, while innovative applications in geriatric care open new growth avenues.
Performance Metrics:
- Neurological care facilities in California, New York, and Florida leading adoption for dementia treatment operations
- Pharmaceutical contractor channels maintaining 78% market share for complex healthcare integration applications
- Hospital programs supporting 49% of treatment acquisitions across healthcare and neurological facilities
- Healthcare platform compatibility with major neurological systems driving procurement selection criteria
Germany Maintains Technology Leadership
Germany's advanced healthcare market demonstrates sophisticated dementia treatment deployment with documented operational effectiveness in neurological applications and medical facilities through integration with existing healthcare systems and pharmaceutical infrastructure.
The country leverages expertise in pharmaceutical development and healthcare systems integration to maintain strong growth at 5.8% CAGR. Medical centers, including Munich, Berlin, and Hamburg, showcase premium installations where therapeutic systems integrate with comprehensive neurological platforms and patient care management systems to optimize dementia treatment and therapeutic effectiveness.
German pharmaceutical companies prioritize system reliability and EU compliance in treatment development, creating demand for premium therapeutic systems with advanced features, including efficacy monitoring integration and automated treatment systems. The market benefits from established healthcare infrastructure and a willingness to invest in advanced neurological technologies that provide long-term patient benefits and compliance with international medical and pharmaceutical standards. Premium dementia applications, specialty therapeutic systems, and healthcare programs drive diversified demand across multiple end-use segments.
Market Intelligence Brief:
- Engineering focuses on EU standardization and medical compliance, driving premium segment growth
- Healthcare partnerships providing 53% faster development cycles
- Technology collaboration between German pharmaceutical companies and international neurological companies
- Medical training programs expanding system integration in healthcare and neurological scenarios
France Shows Balanced Healthcare Growth
France maintains steady expansion at 5.7% CAGR through diversified demand from healthcare programs, hospital modernization activities, and neurological development projects. Major medical regions in Paris, Lyon, and Marseille drive dementia treatment adoption for commercial and clinical neurological applications.
Medical research and development programs create sustained treatment demand, while premium neurological applications provide additional growth opportunities. Government support for healthcare innovation and pharmaceutical technology initiatives supports consistent market development.
Market Characteristics:
- Advanced medical research capabilities and pharmaceutical regulations are creating demand for innovative therapeutic technologies supporting sustainable neurological development and patient care optimization
- Strong healthcare tradition and medical excellence leadership are driving the adoption of precision therapeutic technologies and pharmaceutical materials throughout hospital and healthcare facilities
United Kingdom Drives Healthcare and Neurological Integration
The U.K. market holds steady growth at 5.5% CAGR, driven by healthcare modernization activities, neurological care programs, and pharmaceutical adoption trends. British healthcare facilities and medical companies are implementing advanced dementia treatment systems to enhance therapeutic capabilities and support healthcare operations that align with medical regulations and quality standards.
Market expansion benefits from government healthcare innovation programs that mandate neurological capabilities in healthcare and medical specifications, creating sustained demand where operational flexibility and medical compliance represent critical requirements.
Strategic Market Indicators:
- Healthcare and neurological facilities leading adoption with treatment modernization programs requiring advanced therapeutic systems
- Government healthcare innovation programs providing regulatory support for advanced pharmaceutical acquisition
- Medical compliance requirements driving demand for standardized systems with international operational compatibility
- Specialized geriatric care and sustainable healthcare segments adopting comprehensive therapeutic solutions for patient care optimization
Japan Emphasizes Precision and Therapeutic Excellence
Japan demonstrates steady market development with a 5.3% CAGR, distinguished by healthcare and neurological producers' preference for high-quality dementia treatment systems that integrate seamlessly with existing medical systems and provide reliable long-term operation in specialized neurological applications.
The market prioritizes advanced features, including precision therapeutic control, medical durability, and integration with comprehensive healthcare platforms that reflect Japanese industry expectations for technological sophistication and operational excellence.
High-specification neurological and specialty medical applications drive demand, supported by advanced dementia research and development initiatives. Japanese healthcare providers emphasize treatment precision, consistent performance characteristics, and comprehensive quality documentation that aligns with stringent medical standards. The focus on premium applications and technical excellence supports stable growth despite mature market conditions.
Market Characteristics:
- Premium focus on cholinesterase inhibitor systems with advanced pharmaceutical algorithms and precision therapeutic capabilities
- Integration requirements with existing neurological and healthcare platforms
- Emphasis on operational reliability and long-term durability in medical and specialty applications
South Korea Emphasizes Advanced Therapeutic Integration
South Korea demonstrates robust market development with a 5.4% CAGR, distinguished by healthcare and neurological producers' preference for high-quality dementia treatment systems that integrate seamlessly with existing medical systems and provide reliable long-term operation in specialized neurological applications.
The market prioritizes advanced features, including precision therapeutic control, medical durability, and integration with comprehensive healthcare platforms that reflect Korean industry expectations for technological sophistication and operational excellence.
Growth drivers encompass medical technology applications, expanding healthcare modernization requirements, and advanced therapeutic system integration. Korean healthcare providers emphasize quality control systems and comprehensive technical support that align with domestic medical standards.
The convergence of high-tech healthcare processing, neurological innovation, and growing specialty medical production creates diversified demand across multiple application segments.
Market Characteristics:
- Premium focus on cholinesterase inhibitor systems with advanced pharmaceutical formulation algorithms and precision therapeutic capabilities
- Integration requirements with existing neurological and healthcare platforms
- Emphasis on operational reliability and long-term durability in medical and neurological applications
Mexico Emerges as High-Growth Market
Mexico leads growth momentum with a 6.2% CAGR, driven by rapid healthcare modernization, expanding neurological applications, and pharmaceutical development adoption across major urban regions including Mexico City, Guadalajara, and Monterrey.
Healthcare development and neurological care accessibility requirements drive primary demand, while growing pharmaceutical industry and healthcare sectors create diversified application opportunities.
Government health initiatives and pharmaceutical industry programs support sustained expansion. The convergence of healthcare modernization, medical standards improvement, and neurological capacity expansion positions Mexico as a key emerging market for dementia treatment systems.
Strategic Market Indicators:
- Government support for healthcare development and pharmaceutical industry expansion is driving demand for specialty neurological treatments throughout major urban regions and healthcare clusters across hospital facilities, medical centers, and pharmaceutical manufacturing centers
- Strong healthcare sector growth and an expanding network of medical facilities are supporting the rapid adoption of dementia treatment technologies among healthcare operators seeking enhanced neurological efficiency and innovative patient care offerings
Europe Market Split by Country
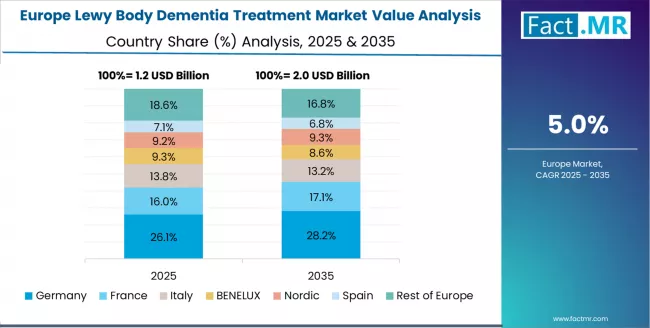
The Lewy body dementia treatment market in Europe is projected to grow from USD 1.14 billion in 2025 to USD 1.98 billion by 2035, registering a CAGR of 5.7% over the forecast period. Germany is expected to maintain its leadership position with a 31.6% market share in 2025, declining slightly to 31.1% by 2035, supported by its advanced healthcare infrastructure and major neurological centers in Munich and Berlin.
France follows with a 19.3% share in 2025, projected to reach 19.7% by 2035, driven by comprehensive healthcare modernization programs and neurological initiatives. The United Kingdom holds a 17.5% share in 2025, expected to moderate to 17.1% by 2035 through specialized healthcare activities and neurological applications. Italy commands a 12.3% share in 2025, rising to 12.6% by 2035 through strong healthcare and medical projects.
Spain accounts for 8.8% in 2025, reaching 9.0% by 2035 aided by healthcare modernization and neurological applications. The Netherlands maintains a 4.4% share in 2025, increasing to 4.5% by 2035 driven by specialty healthcare and neurological innovation demand. Rest of Europe is anticipated to hold 6.1% in 2025, increasing to 5.6% by 2035, reflecting steady adoption in Nordic countries and Central & Eastern European healthcare upgrades.
Cholinesterase Inhibitors Dominate Treatment Demand in Japan
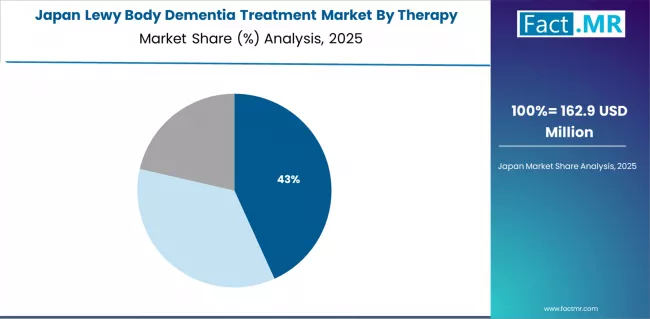
In Japan, the Lewy body dementia treatment market prioritizes cholinesterase inhibitor systems, which capture the dominant share of neurological and specialty medical installations due to their advanced features, including precision therapeutic optimization and seamless integration with existing neurological infrastructure.
Healthcare providers in Japan emphasize reliability, precision, and long-term operational excellence, creating demand for cholinesterase inhibitor systems that provide consistent therapeutic capabilities and adaptive treatment performance based on neurological requirements and patient care conditions.
Antipsychotic systems maintain secondary positions primarily in specialized behavioral applications and psychiatric installations where comprehensive symptom management functionality meets operational requirements without compromising therapeutic efficiency.
Strategic Market Indicators:
- Premium focus on cholinesterase inhibitor systems with advanced pharmaceutical formulation algorithms and precision therapeutic capabilities
- Integration requirements with existing neurological platforms and specialty medical systems
- Emphasis on operational reliability and long-term durability in healthcare and neurological applications
Pharmaceutical Companies Lead Treatment Services in South Korea
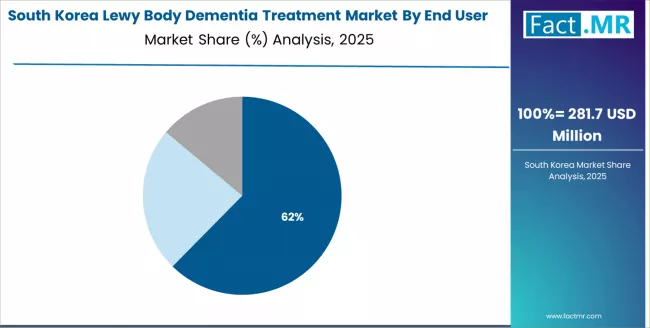
In South Korea, the market structure favors international pharmaceutical companies, including Eisai, Novartis, and Teva, which maintain dominant positions through comprehensive product portfolios and established healthcare networks supporting both hospital and clinical neurological installations.
These providers offer integrated solutions combining advanced dementia treatment systems with professional medical services and ongoing healthcare support that appeal to Korean healthcare providers seeking reliable specialty therapeutic systems.
Local pharmaceutical contractors and system integrators capture a moderate market share by providing localized service capabilities and competitive pricing for standard hospital installations, while domestic manufacturers focus on specialized applications and cost-effective solutions tailored to Korean healthcare characteristics.
Channel Insights:
- International pharmaceutical brands maintaining premium market positioning through advanced therapeutic offerings
- Local healthcare networks expanding to support growing demand for professional treatment distribution and maintenance
- System integration capabilities becoming a key differentiator for facility-wide and neurological treatment applications
Competitive Landscape of the Lewy Body Dementia Treatment Market
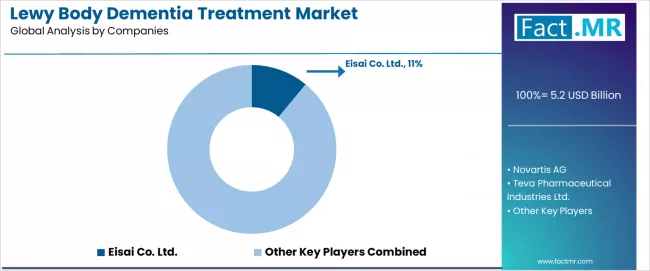
The Lewy body dementia treatment market operates with moderate concentration, featuring approximately 10-12 meaningful participants, where leading companies control roughly 38-43% of the global market share through established healthcare relationships and comprehensive pharmaceutical portfolios.
Competition emphasizes advanced therapeutic capabilities, drug reliability, and healthcare integration rather than price-based rivalry. The leading company, Eisai, commands approximately 11.0% market share through its premium dementia treatment products and extensive neurological and healthcare industry presence.
Market leaders encompass Eisai, Novartis, and Teva, which maintain competitive advantages through extensive pharmaceutical expertise, global healthcare contractor networks, and comprehensive therapeutic integration capabilities that create customer switching costs and support premium pricing.
These companies leverage decades of pharmaceutical experience and ongoing research investments to develop advanced dementia treatment systems with precision therapeutic control and efficacy monitoring features.
Technology Innovators include Biogen, Roche, and AbbVie, which compete through specialized pharmaceutical technology focus and innovative therapeutic interfaces that appeal to healthcare providers seeking advanced treatment capabilities and operational flexibility. These companies differentiate through rapid drug development cycles and specialized neurological and hospital application focus.
Regional Specialists feature companies like Lundbeck, Otsuka, Sun Pharma, and Acadia, which focus on specific geographic markets and specialized applications, including dementia treatment-based systems and integrated healthcare solutions.
Market dynamics favor participants that combine reliable pharmaceutical capabilities with advanced therapeutic software, including precision treatment control and automatic performance optimization capabilities.
Competitive pressure intensifies as traditional pharmaceutical contractors expand into dementia treatment systems, while specialized neurological companies challenge established players through innovative therapeutic solutions and cost-effective platforms targeting specialized healthcare and neurological segments.
Key Players in the Lewy Body Dementia Treatment Market
- Eisai Co. Ltd.
- Novartis AG
- Teva Pharmaceutical Industries Ltd.
- Biogen Inc.
- F. Hoffmann-La Roche AG
- AbbVie Inc.
- H. Lundbeck A/S
- Otsuka Pharmaceutical Co. Ltd.
- Sun Pharmaceutical Industries Ltd.
- Acadia Pharmaceuticals Inc.
Scope of the Report
| Item | Value |
|---|---|
| Quantitative Units | USD 5.2 Billion |
| Therapy | Cholinesterase inhibitors, Antipsychotics, Others |
| End User | Hospitals, Clinics, Others |
| Distribution | Retail pharmacies, Hospitals, Online |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | United States, Germany, France, United Kingdom, Japan, South Korea, Mexico, and 12+ additional countries |
| Key Companies Profiled | Eisai, Novartis, Teva, Biogen, Roche, AbbVie, Lundbeck |
| Additional Attributes | Dollar sales by therapy and end user categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with pharmaceutical manufacturers and healthcare suppliers, physician preferences for therapeutic efficacy and treatment reliability, integration with healthcare platforms and neurological quality monitoring systems, innovations in cholinesterase inhibitor formulations and medical compliance, and development of automated treatment solutions with enhanced performance and healthcare optimization capabilities. |
Lewy Body Dementia Treatment Market by Segments
-
Therapy :
- Cholinesterase inhibitors
- Antipsychotics
- Others
-
End User :
- Hospitals
- Clinics
- Others
-
Distribution :
- Retail pharmacies
- Hospitals
- Online
-
Region :
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Netherlands
- Rest of Europe
- Asia Pacific
- Japan
- South Korea
- China
- India
- Australia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Therapy
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Therapy , 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Therapy , 2025 to 2035
- Cholinesterase inhibitors
- Antipsychotics
- Others
- Y to o to Y Growth Trend Analysis By Therapy , 2020 to 2024
- Absolute $ Opportunity Analysis By Therapy , 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End User
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End User, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End User, 2025 to 2035
- Hospitals
- Clinics
- Others
- Y to o to Y Growth Trend Analysis By End User, 2020 to 2024
- Absolute $ Opportunity Analysis By End User, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Therapy
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By End User
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Therapy
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By End User
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Therapy
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By End User
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Therapy
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By End User
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Therapy
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By End User
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Therapy
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By End User
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Therapy
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By End User
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy
- By End User
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Therapy
- By End User
- Competition Analysis
- Competition Deep Dive
- Eisai Co. Ltd.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Novartis AG
- Teva Pharmaceutical Industries Ltd.
- Biogen Inc.
- F. Hoffmann-La Roche AG
- AbbVie Inc.
- H. Lundbeck A/S
- Otsuka Pharmaceutical Co. Ltd.
- Sun Pharmaceutical Industries Ltd.
- Acadia Pharmaceuticals Inc.
- Eisai Co. Ltd.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Therapy , 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Therapy , 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Therapy , 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Therapy , 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Therapy , 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Therapy , 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Therapy , 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Therapy , 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by End User, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Therapy , 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Therapy , 2025-2035
- Figure 5: Global Market Attractiveness Analysis by Therapy
- Figure 6: Global Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by End User, 2025-2035
- Figure 8: Global Market Attractiveness Analysis by End User
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025-2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025-2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025-2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025-2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025-2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Therapy , 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Therapy , 2025-2035
- Figure 22: North America Market Attractiveness Analysis by Therapy
- Figure 23: North America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by End User, 2025-2035
- Figure 25: North America Market Attractiveness Analysis by End User
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Therapy , 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Therapy , 2025-2035
- Figure 29: Latin America Market Attractiveness Analysis by Therapy
- Figure 30: Latin America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by End User, 2025-2035
- Figure 32: Latin America Market Attractiveness Analysis by End User
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Therapy , 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Therapy , 2025-2035
- Figure 36: Western Europe Market Attractiveness Analysis by Therapy
- Figure 37: Western Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by End User, 2025-2035
- Figure 39: Western Europe Market Attractiveness Analysis by End User
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Therapy , 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Therapy , 2025-2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Therapy
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by End User, 2025-2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by End User
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Therapy , 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Therapy , 2025-2035
- Figure 50: East Asia Market Attractiveness Analysis by Therapy
- Figure 51: East Asia Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by End User, 2025-2035
- Figure 53: East Asia Market Attractiveness Analysis by End User
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Therapy , 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Therapy , 2025-2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Therapy
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by End User, 2025-2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by End User
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Therapy , 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Therapy , 2025-2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Therapy
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by End User, 2025-2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by End User
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the lewy body dementia treatment market in 2025?
The global lewy body dementia treatment market is estimated to be valued at USD 5.2 billion in 2025.
What will be the size of lewy body dementia treatment market in 2035?
The market size for the lewy body dementia treatment market is projected to reach USD 9.0 billion by 2035.
How much will be the lewy body dementia treatment market growth between 2025 and 2035?
The lewy body dementia treatment market is expected to grow at a 5.6% CAGR between 2025 and 2035.
What are the key product types in the lewy body dementia treatment market?
The key product types in lewy body dementia treatment market are cholinesterase inhibitors, antipsychotics and others.
Which end user segment to contribute significant share in the lewy body dementia treatment market in 2025?
In terms of end user, hospitals segment to command 61.0% share in the lewy body dementia treatment market in 2025.