Pemphigus Vulgaris Treatment Market
Pemphigus Vulgaris Treatment Market Size and Share Forecast Outlook 2025 to 2035
Pemphigus vulgaris treatment market is projected to grow from USD 0.5 billion in 2025 to USD 1.2 billion by 2035, at a CAGR of 8.3%. Anti-CD20 Monoclonal Antibodies (Rituximab) will dominate with a 42.3% market share, while hospital pharmacies will lead the distribution channel segment with a 81.6% share.
Pemphigus Vulgaris Treatment Market Forecast and Outlook 2025 to 2035
The global pemphigus vulgaris treatment market is projected to reach USD 1.15 billion by 2035, recording an absolute increase of USD 0.63 billion over the forecast period. The market is valued at USD 0.52 billion in 2025 and is set to rise at a CAGR of 8.3% during the assessment period.
Quick Stats for Pemphigus Vulgaris Treatment Market
- Pemphigus Vulgaris Treatment Market Value (2025): USD 0.52 billion
- Pemphigus Vulgaris Treatment Market Forecast Value (2035): USD 1.15 billion
- Pemphigus Vulgaris Treatment Market Forecast CAGR: 8.3%
- Leading Therapy Type in Pemphigus Vulgaris Treatment Market: Anti-CD20 Monoclonal Antibodies
- Key Growth Regions in Pemphigus Vulgaris Treatment Market: Asia Pacific, North America, and Europe
- Top Players in Pemphigus Vulgaris Treatment Market: Genentech, Inc. (Roche), Pfizer Inc., Amgen Inc., CELLTRION INC., Zenyaku Kogyo Co., Ltd., Scinai Immunotherapeutics Ltd., Novartis AG, Johnson & Johnson (Janssen Biotech), Sanofi S.A., Takeda Pharmaceutical Company Limited
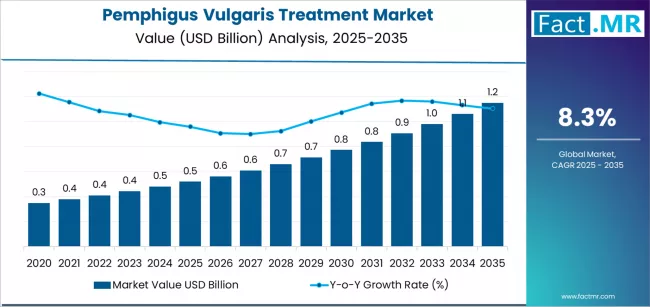
The market is expected to grow 2.2X during the same period, supported by increasing diagnosis rates of autoimmune blistering disorders and improved access to biologic therapies driving demand for specialized treatment formulations and increasing investments in targeted immunotherapy development and rare disease infrastructure globally.
The dermatology and immunology sectors face mounting pressure to deliver superior disease management solutions while meeting evolving clinical requirements for refractory pemphigus vulgaris cases and long-term remission maintenance, with modern anti-CD20 monoclonal antibody therapies providing documented clinical efficacy and disease control capabilities compared to traditional corticosteroid treatment alternatives.
Rising awareness of autoimmune disorders and expanding specialist healthcare access across emerging economies create substantial opportunities for pharmaceutical manufacturers and healthcare providers. However, high treatment costs and limited reimbursement coverage may pose obstacles to therapy accessibility expansion.
The anti-CD20 monoclonal antibodies segment dominates market activity with approximately 42.3% share in 2025, driven by the extensive clinical evidence base supporting rituximab efficacy with proven B-cell depletion properties across moderate-to-severe pemphigus vulgaris applications worldwide.
Clinicians increasingly recognize the transformative benefits of rituximab therapy, with typical treatment protocols providing effective disease remission and corticosteroid-sparing capabilities at specialized dermatology centers through established biologic infusion networks.
The systemic corticosteroids segment demonstrates continued utilization with 27.6% share, supported by first-line treatment guidelines and immediate disease control requirements driving continued prescription in acute pemphigus vulgaris management.
Anti-CD20 monoclonal antibodies emerge as the critical therapy category with highest market share, reflecting dermatologist emphasis on achieving durable remission and reducing long-term corticosteroid complications.
Hospital pharmacies represent the dominant distribution channel segment with 81.6% share, driven by infusion therapy requirements and specialty medication management needs for biologic administration across hospital-based dermatology and immunology departments.
Regional dynamics show North America and Europe maintaining market leadership with strong presence in 2025, supported by advanced biologic therapy adoption and comprehensive rare disease treatment infrastructure across USA and Germany markets.
Asia Pacific demonstrates accelerating growth trends driven by improving diagnosis capabilities and biosimilar rituximab availability, while emerging markets emphasize healthcare infrastructure development and specialist training programs.
India leads country-level growth at 9.5% CAGR through expanding diagnosis rates and biologic therapy access improvement, followed by China at 9.2% supported by healthcare infrastructure expansion and biosimilar market development.
The competitive landscape features moderate concentration with Genentech, Inc. (Roche) holding an 18.2% market share, while established players including Pfizer Inc., Amgen Inc., and CELLTRION INC. compete through comprehensive biologic product portfolios and biosimilar development capabilities across diverse therapeutic applications.
Pemphigus Vulgaris Treatment Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the pemphigus vulgaris treatment is projected to expand from USD 0.52 billion to USD 0.71 billion, resulting in a value increase of USD 0.19 billion, which represents 31.2% of the total forecast growth for the period. This phase of development will be shaped by rising adoption of rituximab and anti-CD20 therapies in refractory pemphigus vulgaris cases, expanded clinical guidelines incorporating biologic first-line treatment protocols, as well as increasing integration with dermatology specialty centers and improved diagnostic capabilities for early disease identification. Companies are establishing competitive positions through investment in clinical trial programs, biosimilar development initiatives, and strategic market expansion across hospital pharmacy networks, specialty distribution channels, and patient access programs.
From 2029 to 2035, the market is forecast to grow from USD 0.71 billion to USD 1.15 billion, adding another USD 0.44 billion, which constitutes 68.8% of the overall expansion. This period is expected to be characterized by the expansion of novel therapeutic approaches, including next-generation anti-CD20 antibodies and targeted B-cell therapies tailored for specific autoimmune mechanisms, strategic collaborations between pharmaceutical companies and academic medical centers, and an enhanced focus on long-term remission maintenance protocols and steroid-free treatment regimens. The growing emphasis on precision medicine in autoimmune dermatology and rising demand for improved quality-of-life outcomes will drive comprehensive pemphigus vulgaris treatment solutions across diverse patient populations.
Pemphigus Vulgaris Treatment Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 0.52 billion |
| Market Forecast Value (2035) | USD 1.15 billion |
| Forecast CAGR (2025-2035) | 8.3% |
Why is the Pemphigus Vulgaris Treatment Market Growing?
The pemphigus vulgaris treatment grows by enabling dermatologists and immunologists to achieve superior disease control and long-term remission while accessing targeted biologic therapies without substantial treatment protocol complexity requirements.
Physicians and healthcare systems face mounting pressure to reduce corticosteroid dependence and prevent treatment complications while managing severe autoimmune blistering disease across diverse patient populations and clinical presentations, with modern anti-CD20 monoclonal antibody therapies typically providing superior B-cell depletion and sustained remission benefits compared to conventional immunosuppressive treatment alternatives, making biologic adoption essential for evidence-based dermatology positioning.
The autoimmune disease treatment landscape's need for durable disease control and corticosteroid-sparing therapeutic capabilities creates demand for comprehensive pemphigus vulgaris solutions that can provide superior clinical outcomes, maintain long-term remission, and ensure improved quality of life without compromising patient safety or treatment tolerability standards.
Growing clinical evidence supporting rituximab efficacy drives adoption in dermatology centers, academic medical institutions, and specialized autoimmune disease clinics, where treatment performance has a direct impact on disease remission rates and patient functional outcomes. The increasing recognition of pemphigus vulgaris as a treatable autoimmune condition has created lasting changes in diagnostic protocols and specialist referral patterns, supporting sustained demand for effective biologic therapies across all healthcare systems.
Expanding healthcare insurance coverage for rare diseases in developed markets enables greater access to expensive biologic treatments with proven clinical benefit and long-term cost-effectiveness profiles. However, treatment cost constraints and limited specialist availability may restrict accessibility of anti-CD20 therapies among developing regions with insufficient healthcare infrastructure for complex autoimmune disease management.
Segmental Analysis
The market is segmented by therapy type, distribution channel, and region. By therapy type, the market is divided into anti-CD20 monoclonal antibodies (rituximab), systemic corticosteroids, conventional steroid-sparing immunosuppressants, intravenous immunoglobulin (IVIG - adjunct), and topical therapies.
Based on distribution channel, the market is categorized into hospital pharmacies, retail pharmacies, and others (online/infusion pharmacies). Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Therapy Type, Which Segment Accounts for the Dominant Market Share?
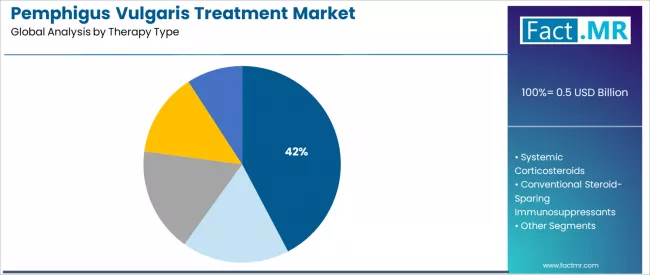
The anti-CD20 monoclonal antibodies (rituximab) segment represents the dominant force in the pemphigus vulgaris treatment, capturing 42.3% of the total market share in 2025. This established therapy category encompasses solutions featuring proven clinical efficacy and targeted immunotherapy mechanisms, including advanced B-cell depletion capabilities and sustained remission induction properties that enable superior disease control and corticosteroid reduction across all moderate-to-severe pemphigus vulgaris treatment applications worldwide.
The anti-CD20 monoclonal antibodies segment's market leadership stems from rituximab's transformative therapeutic advantages, with protocols capable of achieving durable complete remission while maintaining favorable safety profiles and comprehensive clinical evidence supporting first-line utilization across diverse patient populations.
The systemic corticosteroids segment maintains a substantial 27.6% market share, serving acute disease management requirements and initial treatment protocols where rapid disease control is essential for preventing disease progression and mucocutaneous complications.
These therapies offer immediate anti-inflammatory effects for patients experiencing severe blistering while providing sufficient disease suppression to meet urgent clinical demands. The systemic corticosteroids segment demonstrates continued clinical utilization despite long-term complication risks, driven by established treatment guidelines and accessibility considerations.
Within the anti-CD20 monoclonal antibodies segment, rituximab induction protocols command dominant share, driven by clinical guideline recommendations for first-line biologic therapy in moderate-to-severe pemphigus vulgaris cases. This application benefits from randomized controlled trial evidence and proven superiority over conventional immunosuppressive regimens in achieving complete remission across refractory and newly diagnosed patient populations.
Key therapeutic advantages driving the anti-CD20 monoclonal antibodies segment include:
- Advanced B-cell depletion mechanisms with targeted autoantibody suppression that enhance disease remission and ensure durable clinical response
- Established clinical protocols enabling corticosteroid-sparing treatment approaches across different disease severity categories without compromising efficacy
- Enhanced long-term outcomes featuring sustained remission maintenance while reducing treatment-related adverse events and corticosteroid complications
- Superior clinical evidence providing optimal therapeutic benefit for various pemphigus vulgaris phenotypes and refractory disease management requirements
By Distribution Channel, Which Segment Accounts for the Largest Market Share?
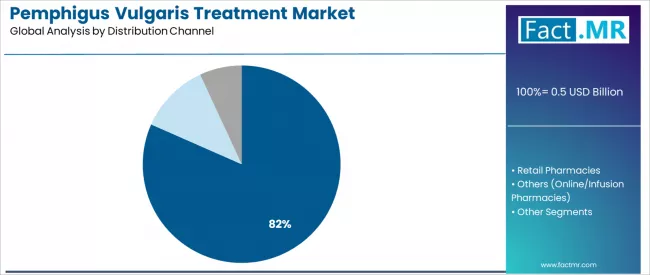
Hospital pharmacies dominate the pemphigus vulgaris distribution landscape with an 81.6% market share in 2025, reflecting the critical role of hospital-based specialty pharmacy services in supporting intravenous biologic administration and complex immunotherapy management across tertiary care dermatology departments worldwide.
The hospital pharmacies segment's market leadership is reinforced by rituximab infusion requirements, specialist supervision protocols, and adverse event monitoring capabilities combined with integrated pharmacy services in academic medical centers and university hospitals.
Within this segment, academic medical center hospital pharmacies represent significant share, driven by specialized pemphigus vulgaris treatment programs offering comprehensive disease management including biologic infusion facilities, dermatology consultation services, and clinical trial access. This sub-segment benefits from established referral networks and multidisciplinary care models integrating dermatologists, immunologists, and pharmacy specialists in coordinated treatment delivery.
The retail pharmacies segment represents a smaller distribution category, demonstrating presence through specialized requirements for oral immunosuppressant dispensing, corticosteroid prescriptions, and adjunct therapy access in community pharmacy settings. This segment serves patients requiring maintenance immunosuppression and supportive care medications following hospital-based biologic induction therapy.
The others category maintains presence through infusion centers, specialty pharmacies, and emerging online pharmacy platforms serving home infusion programs and mail-order specialty medication distribution, supporting convenient access for maintenance rituximab infusions and chronic immunosuppressive therapy refills.
Key market dynamics supporting distribution channel growth include:
- Hospital pharmacy expansion driven by biologic therapy adoption and specialty medication management, requiring comprehensive infusion capabilities
- Distribution infrastructure supporting cold-chain logistics and proper biologic handling protocols across hospital pharmacy networks
- Integration of specialty pharmacy services enabling coordinated care delivery and patient access program administration
- Growing emphasis on patient support services driving comprehensive medication management and adherence monitoring programs
What are the Drivers, Restraints, and Key Trends of the Pemphigus Vulgaris Treatment Market?
The market is driven by three concrete demand factors tied to clinical outcomes and therapeutic innovation. First, increasing pemphigus vulgaris diagnosis rates through improved dermatopathology capabilities and autoantibody testing create expanding treatment populations requiring advanced therapeutic interventions, with early diagnosis representing a critical factor for preventing severe disease complications worldwide, demanding comprehensive specialty care access. Second, expanding clinical evidence supporting rituximab first-line utilization and regulatory approvals for pemphigus vulgaris indications drive increased biologic therapy adoption, with clinical guidelines incorporating anti-CD20 therapies as preferred treatment options for achieving complete remission by 2030. Third, biosimilar rituximab development and market entry enable improved treatment affordability and accessibility that enhance therapy uptake while reducing healthcare system costs and expanding patient access in resource-limited settings.
Market restraints include high treatment costs for rituximab therapy and biologic alternatives that can challenge patient access in maintaining affordable disease management capabilities, particularly in healthcare systems where insurance coverage for rare autoimmune diseases remains limited and out-of-pocket expenses prove prohibitive. Limited specialist availability and dermatology expertise for pemphigus vulgaris management pose another significant challenge, as optimal treatment delivery depends on experienced clinicians and multidisciplinary care teams with autoimmune blistering disease expertise, potentially affecting treatment outcomes and geographic access disparities. Infusion infrastructure requirements for rituximab administration create additional complexity for healthcare systems, demanding specialized pharmacy facilities and trained nursing staff to deliver safe biologic therapy across hospital and infusion center environments.
Key trends indicate accelerated biosimilar adoption in emerging markets, particularly Asia Pacific and Latin America, where cost-effective rituximab alternatives demonstrate potential to expand treatment access for previously undertreated patient populations. Novel therapeutic development trends toward next-generation anti-CD20 antibodies and alternative B-cell targeting agents with improved efficacy profiles and subcutaneous administration routes enable convenient treatment approaches that optimize patient adherence while maintaining clinical effectiveness. However, the market thesis could face disruption if significant advances in alternative autoimmune therapies or major shifts toward non-depleting immunomodulatory approaches reduce reliance on traditional B-cell depletion strategies for pemphigus vulgaris management.
Analysis of the Pemphigus Vulgaris Treatment Market by Key Countries
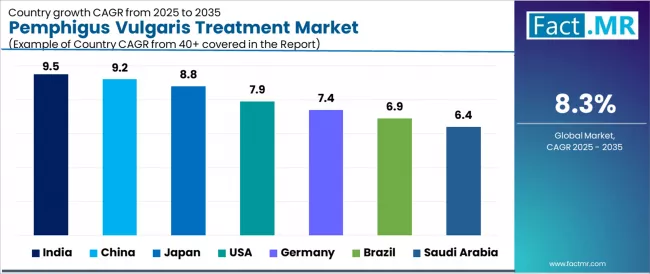
| Country | CAGR (2025-2035) |
|---|---|
| India | 9.5% |
| China | 9.2% |
| Japan | 8.8% |
| USA | 7.9% |
| Germany | 7.4% |
| Brazil | 6.9% |
| Saudi Arabia | 6.4% |
The global pemphigus vulgaris treatment is expanding robustly, with India leading at a 9.5% CAGR through 2035, driven by growing diagnosis rates, expanding access to biologic therapies, and improving healthcare infrastructure supporting specialized dermatology care. China follows at 9.2%, supported by healthcare system modernization, biosimilar rituximab availability, and expanding specialty hospital networks. Japan records 8.8%, reflecting early biologic approvals, strong government support for rare diseases, and established academic dermatology programs.
The USA grows at 7.9%, anchored by high rituximab adoption rates and comprehensive clinical trial infrastructure. Germany advances at 7.4%, leveraging increased disease awareness and rapid targeted biologic uptake. Brazil posts 6.9%, focusing on emerging treatment centers and growing healthcare expenditure, while Saudi Arabia grows steadily at 6.4%, emphasizing improved rare disease recognition and government-funded therapy programs.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the pemphigus vulgaris treatment with a CAGR of 9.5% through 2035. The country's leadership position stems from expanding diagnosis capabilities through improved dermatopathology services, growing awareness of autoimmune blistering disorders, and increasing access to rituximab therapy driving treatment uptake.
Growth is concentrated in major metropolitan medical centers and academic dermatology institutions, including Mumbai, Delhi, Bangalore, and Chennai, where specialist dermatologists are adopting biologic treatment protocols and establishing pemphigus vulgaris referral programs for comprehensive disease management.
Healthcare delivery through tertiary care hospitals, medical college dermatology departments, and emerging specialty clinics expands treatment access across previously underserved patient populations. The country's improving healthcare insurance coverage and government rare disease initiatives provide strong momentum for pemphigus vulgaris therapy adoption, including comprehensive integration across public and private healthcare sectors.
Key market factors:
- Dermatology specialty expansion concentrated in metropolitan teaching hospitals with established autoimmune disease expertise
- Biosimilar rituximab availability through domestic manufacturers enabling cost-effective biologic therapy access
- Comprehensive medical education ecosystem, including dermatology residency programs developing pemphigus vulgaris management capabilities
- Patient advocacy development featuring support organizations and awareness campaigns improving disease recognition and treatment seeking
Why is China Emerging as a High Growth Market?
In major urban centers including Beijing, Shanghai, Guangzhou, and Chengdu, the adoption of rituximab therapy for pemphigus vulgaris is accelerating across tertiary hospitals and academic dermatology departments, driven by healthcare infrastructure modernization and expanding specialty medication access. The market demonstrates strong growth momentum with a CAGR of 9.2% through 2035, linked to comprehensive healthcare system development and increasing focus on rare disease treatment capabilities for autoimmune disorders.
Dermatologists are implementing evidence-based treatment protocols and establishing specialized pemphigus vulgaris clinics to provide comprehensive disease management while meeting growing patient expectations for advanced therapeutic options. The country's expanding biosimilar market creates ongoing opportunities for affordable rituximab access, while increasing government emphasis on rare disease policies drives healthcare system support for expensive biologic therapies.
Key development areas:
- Academic hospitals and specialty dermatology centers leading rituximab adoption with emphasis on clinical protocol standardization
- Biosimilar market development enabling cost-effective biologic therapy alternatives through domestic pharmaceutical manufacturers
- Healthcare insurance expansion providing improved coverage for expensive autoimmune disease treatments
- Growing clinical research participation driving awareness and adoption of international treatment guidelines
What is Japan’s Outlook in the Pemphigus Vulgaris Treatment Landscape?
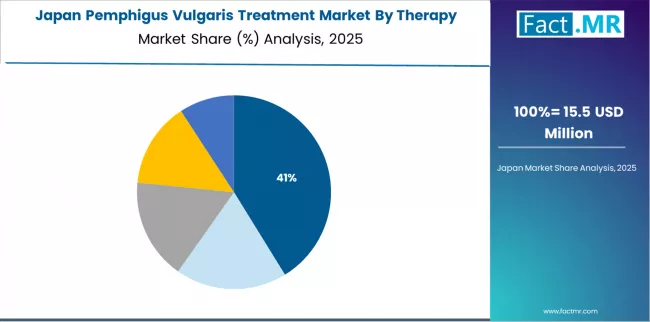
Japan’s market leadership is established through early rituximab approval for pemphigus vulgaris indications, including comprehensive regulatory pathways supporting biologic therapy development and strong government commitment to rare disease treatment access.
The country demonstrates solid growth potential with a CAGR of 8.8% through 2035, supported by established academic dermatology programs from leading universities and comprehensive national health insurance coverage.
Healthcare providers demonstrate advanced clinical expertise in pemphigus vulgaris management and rituximab administration protocols, enabling optimal therapeutic outcomes and evidence generation through clinical registries.
However, comprehensive healthcare infrastructure and established specialty pharmacy systems create substantial baseline treatment capacity for pemphigus vulgaris therapy delivery, particularly in university hospital settings where multidisciplinary care and clinical research drive optimal disease management.
Market characteristics:
- University hospital dermatology departments showing robust rituximab utilization across pemphigus vulgaris patient populations
- National health insurance providing comprehensive coverage for approved biologic therapies
- Future projections indicate continued clinical innovation with emphasis on long-term remission maintenance protocols
- Growing emphasis on quality-of-life outcomes and patient-reported measures supporting treatment optimization
How does USA Demonstrate Innovation Leadership in the Pemphigus Vulgaris Treatment Market?
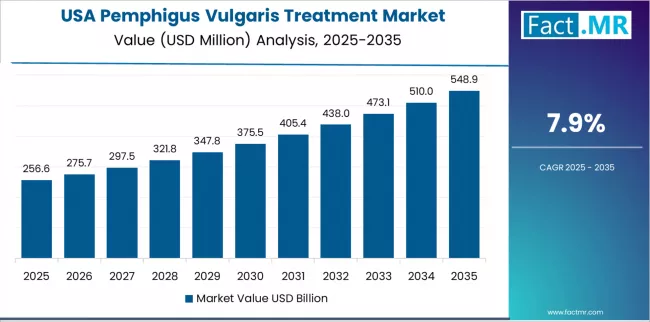
The USA’s market demonstrates advanced therapeutic adoption based on comprehensive clinical trial infrastructure and established biologic therapy utilization for autoimmune dermatology applications. The country shows strong potential with a CAGR of 7.9% through 2035, driven by high rituximab penetration rates and academic medical center leadership in major metropolitan regions, including institutions in Boston, New York, Philadelphia, and San Francisco.
American dermatologists are advancing treatment protocols and maintenance therapy strategies for sustained pemphigus vulgaris remission, particularly in tertiary care centers demanding comprehensive evidence-based care credentials. Specialty pharmacy networks through hospital-based infusion centers and academic medical institutions expand coverage across patient populations requiring ongoing biologic therapy administration.
Leading market segments:
- Academic medical centers in major cities implementing comprehensive pemphigus vulgaris treatment programs
- Clinical research collaborations with pharmaceutical manufacturers achieving high-quality evidence generation rates
- Strategic patient access programs expanding biologic therapy affordability through manufacturer support initiatives
- Focus on precision medicine approaches and biomarker development addressing treatment response prediction challenges
What Positions Germany for Market Development?
In major university medical centers and dermatology clinics, specialist physicians are implementing comprehensive rituximab treatment protocols featuring evidence-based administration schedules and patient monitoring systems, with documented clinical outcomes showing substantial remission rates through biologic therapy adoption and guideline adherence. The market shows steady growth potential with a CAGR of 7.4% through 2035, linked to ongoing awareness campaigns, rapid targeted biologic uptake, and established rare disease support infrastructure in major healthcare regions.
German healthcare providers are optimizing pemphigus vulgaris treatment pathways and implementing long-term follow-up protocols to achieve sustained remission while maintaining treatment safety standards demanded by rigorous healthcare quality requirements. The country's comprehensive health insurance system creates ongoing opportunities for innovative therapy access that differentiates through clinical evidence and improved patient outcomes.
Market development factors:
- University dermatology departments and specialty clinics leading rituximab adoption across Germany
- Health insurance coverage providing reliable reimbursement for approved pemphigus vulgaris treatments
- Clinical guideline implementation driving standardized treatment approaches and quality optimization
- Emphasis on patient registries and real-world evidence generation addressing long-term safety monitoring
How does Brazil Show Growth amid Healthcare Industry Development?
Brazil's pemphigus vulgaris treatment demonstrates expanding treatment capabilities focused on specialty center development and improved therapeutic access, with documented healthcare system evolution showing substantial progress in autoimmune disease recognition across dermatology specialty networks.
The country maintains growth momentum with a CAGR of 6.9% through 2035, driven by emerging treatment centers emphasizing specialized pemphigus vulgaris care and growing healthcare expenditure allocation supporting rare disease programs that align with national health priorities applied to autoimmune disorders.
Major metropolitan regions, including São Paulo, Rio de Janeiro, Brasília, and Porto Alegre, showcase advancing rituximab utilization where academic hospitals integrate specialty pharmacy services with dermatology consultation programs and comprehensive patient monitoring protocols.
Key market characteristics:
- Teaching hospitals and specialty dermatology centers driving biologic therapy adoption with emphasis on clinical training
- Healthcare financing expansion enabling improved access to expensive immunotherapy treatments
- Academic collaboration supporting clinical research and international guideline adaptation for local practice
- Emphasis on patient advocacy and disease awareness addressing underdiagnosis challenges in underserved regions
What Characterizes Saudi Arabia's Market Evolution?
In major healthcare centers including Riyadh, Jeddah, Dammam, and Medina, the recognition of pemphigus vulgaris and treatment with advanced therapies is expanding across tertiary hospitals and specialty dermatology services, driven by healthcare system modernization and rare disease program development. The market demonstrates growth potential with a CAGR of 6.4% through 2035, linked to improved diagnostic capabilities and increasing focus on autoimmune disorder management in comprehensive healthcare delivery systems.
Saudi Arabia’s healthcare providers are implementing international treatment guidelines and establishing specialty clinics to enhance pemphigus vulgaris diagnosis and treatment while meeting growing expectations for advanced medical care standards. The country's government-funded rare disease initiatives create opportunities for expanded rituximab access, while increasing medical tourism and international collaboration drive adoption of evidence-based therapeutic approaches.
Key development areas:
- Tertiary hospitals in major cities establishing specialized autoimmune dermatology programs
- Government rare disease funding providing financial support for expensive biologic therapy access
- International partnership development enabling knowledge transfer and clinical protocol standardization
- Integration of advanced diagnostic capabilities supporting accurate pemphigus vulgaris identification and treatment initiation
Europe Market Split by Country
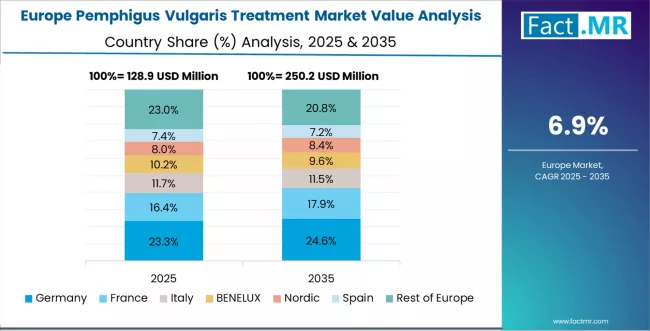
The pemphigus vulgaris treatment in Europe is projected to grow from USD 142.5 million in 2025 to USD 315.8 million by 2035, registering a CAGR of 8.3% over the forecast period. Germany is expected to maintain its leadership position with a 28.8% market share in 2025, adjusting slightly to 28.5% by 2035, supported by its comprehensive university hospital dermatology networks, advanced rare disease infrastructure, and established clinical guideline implementation serving major European pemphigus vulgaris patient populations.
France follows with a 22.5% share in 2025, projected to reach 22.8% by 2035, driven by comprehensive academic dermatology programs in major medical centers implementing rituximab treatment protocols. UK holds a 19.0% share in 2025, expected to maintain 19.2% by 2035 through ongoing development of National Health Service specialty dermatology services and clinical commissioning support.
Italy commands a 14.5% share, while Spain accounts for 11.0% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 4.2% to 4.7% by 2035, attributed to increasing pemphigus vulgaris awareness in Nordic countries and emerging Eastern European specialty dermatology centers implementing biologic therapy protocols.
Competitive Landscape of the Pemphigus Vulgaris Treatment Market
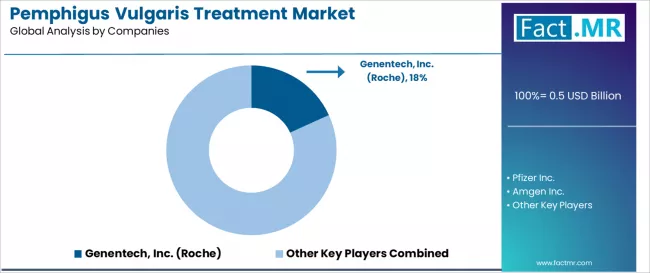
The pemphigus vulgaris treatment features approximately 10-12 meaningful players with moderate concentration, where the top three companies control roughly 45-50% of global market share through established rituximab franchises and comprehensive dermatology portfolios. Competition centers on clinical evidence generation, biosimilar development, and specialty distribution capabilities rather than differentiation through formulation alone.
Market leaders include Genentech, Inc. (Roche), Pfizer Inc., and Amgen Inc., which maintain competitive advantages through comprehensive anti-CD20 antibody portfolios, extensive clinical development programs, and deep expertise in the autoimmune disease sector, creating strong relationships among dermatologists and immunologists. These companies leverage established specialty pharmacy networks and ongoing real-world evidence initiatives to defend market positions while expanding into adjacent autoimmune dermatology indications and next-generation biologic development.
Challengers encompass CELLTRION INC. and biosimilar manufacturers, which compete through cost-effective rituximab alternatives and strong biosimilar development capabilities in key therapeutic markets. Specialty pharmaceutical companies, including Zenyaku Kogyo Co., Ltd., Scinai Immunotherapeutics Ltd., and regional players, focus on specific geographic markets or novel mechanism approaches, offering differentiated capabilities in local market access, pipeline development, and specialized patient support programs.
Established pharmaceutical companies including Novartis AG, Johnson & Johnson (Janssen Biotech), Sanofi S.A., and Takeda Pharmaceutical Company Limited create competitive presence through diversified immunology portfolios and potential pemphigus vulgaris pipeline candidates, particularly in markets with established dermatology franchises.
Market dynamics favor companies that combine strong clinical evidence with comprehensive patient access programs that address the complete treatment journey from diagnosis through long-term remission maintenance and specialty pharmacy coordination. Strategic emphasis on biosimilar development, patient support services, and real-world evidence generation enables differentiation in increasingly value-conscious healthcare systems across developed and emerging markets.
Global Pemphigus Vulgaris Treatment Market-- Stakeholder Contribution Framework
Pemphigus vulgaris treatment solutions represent a critical therapeutic category that enables dermatologists, immunologists, and healthcare systems to achieve superior disease control and long-term remission without extensive corticosteroid exposure, typically providing transformative B-cell depletion and autoantibody suppression capabilities compared to conventional immunosuppressive alternatives while ensuring improved quality of life and reduced treatment complications.
With the market projected to grow from USD 0.52 billion in 2025 to USD 1.15 billion by 2035 at an 8.3% CAGR, these solutions offer compelling advantages - durable clinical remission, corticosteroid-sparing efficacy, and improved safety profiles - making them essential for anti-CD20 monoclonal antibody applications (42.3% therapy share), hospital pharmacy distribution dominance (81.6% channel share), and diverse patient populations requiring evidence-based autoimmune disease management. Scaling treatment access and clinical outcomes requires coordinated action across rare disease policy, healthcare infrastructure, pharmaceutical manufacturers, specialty pharmacies, and patient advocacy organizations.
How Could Governments Spur Local Development and Adoption?
- Rare Disease Policy Development: Include pemphigus vulgaris in national rare disease registries, providing targeted support for specialist training programs and diagnostic infrastructure, and supporting treatment centers through center-of-excellence designation and funding assistance.
- Reimbursement Policy & Access Support: Implement comprehensive coverage for rituximab and biologic therapies in pemphigus vulgaris indications, provide financial assistance programs for high-cost treatments reducing patient out-of-pocket burden, and establish expedited approval pathways that encourage biosimilar development and market entry.
- Healthcare Infrastructure Development: Create specialized autoimmune dermatology centers within tertiary hospitals, establish clear referral pathways and diagnostic protocols for early pemphigus vulgaris identification, and develop telemedicine consultation networks that facilitate specialist access in underserved regions.
- Medical Education & Training: Fund fellowship programs for dermatologists specializing in autoimmune blistering diseases, invest in continuing medical education initiatives that disseminate evidence-based treatment protocols, and support knowledge transfer programs connecting academic centers with community practice settings.
- Patient Support & Advocacy: Establish patient assistance programs providing financial support for treatment access, support patient advocacy organizations expanding disease awareness and education, and create compassionate use frameworks that enable early access to investigational therapies for severe cases.
How Could Industry Bodies Support Market Development?
- Clinical Guidelines & Standards: Define evidence-based treatment algorithms for pemphigus vulgaris across different disease severity categories, establish standardized outcome measures and remission definitions, and create quality indicators for specialty center certification that physicians can reference.
- Disease Awareness & Education: Lead campaigns demonstrating early diagnosis importance, emphasizing improved clinical outcomes with biologic therapy, and reducing treatment delays compared to traditional management approaches.
- Treatment Pathway Optimization: Develop protocols for rituximab administration schedules, maintenance therapy strategies, and long-term monitoring requirements, ensuring optimal therapeutic outcomes across specialty practice settings.
- Professional Development: Run certification programs for dermatologists, infusion nurses, and specialty pharmacists on pemphigus vulgaris management, biologic administration techniques, and adverse event monitoring in specialized autoimmune disease treatment.
How Could Pharmaceutical Manufacturers and Biotechnology Companies Strengthen the Ecosystem?
- Clinical Development Programs: Conduct robust clinical trials demonstrating long-term efficacy, develop predictive biomarkers for treatment response, and generate real-world evidence supporting optimal rituximab utilization that enhances clinical decision-making while addressing regulatory requirements.
- Biosimilar Development: Provide cost-effective rituximab alternatives through biosimilar development, implement rigorous comparability studies demonstrating therapeutic equivalence, and establish competitive pricing strategies that expand treatment accessibility.
- Patient Support Programs: Offer comprehensive patient assistance including financial support, nurse educator services, and adherence monitoring programs that help patients navigate complex treatment pathways aligned with optimal outcomes.
- Medical Affairs & Education: Build comprehensive medical education capabilities, provide specialist training on evidence-based protocols, and support clinical research networks that advance pemphigus vulgaris treatment knowledge across academic and community practice settings.
How Could Healthcare Systems and Specialty Pharmacies Navigate the Market?
- Treatment Center Development: Establish specialized pemphigus vulgaris clinics within dermatology departments offering comprehensive care including diagnosis, treatment, and long-term monitoring, with particular focus on multidisciplinary team approaches integrating dermatologists, immunologists, and specialty pharmacists.
- Specialty Pharmacy Integration: Implement hospital-based specialty pharmacy services supporting rituximab infusion programs, cold-chain management, and adverse event monitoring, while strengthening coordination across academic medical centers and community infusion facilities.
- Care Coordination Programs: Develop patient navigation services combining specialty pharmacy support with nursing coordination, treatment adherence monitoring, and quality-of-life assessment that optimize therapeutic outcomes and patient satisfaction.
- Evidence Generation: Participate in disease registries and outcomes research initiatives documenting real-world effectiveness, treatment patterns, and long-term safety data that inform clinical practice improvement.
How Could Investors and Financial Enablers Unlock Value?
- Innovation Financing: Provide growth capital for companies developing next-generation anti-CD20 antibodies, alternative B-cell targeting therapies, and novel autoimmune disease treatments addressing unmet pemphigus vulgaris management needs.
- Biosimilar Investment: Back biosimilar developers creating cost-effective rituximab alternatives, particularly companies with emerging market distribution capabilities and comprehensive development expertise.
- Specialty Pharmacy Expansion: Finance specialty pharmacy network development supporting biologic therapy administration, home infusion services, and patient support programs in underserved markets.
- Digital Health Integration: Support technology platforms enabling telemedicine consultation, treatment monitoring applications, and patient education tools that enhance care delivery and treatment adherence across diverse patient populations.
Key Players in the Pemphigus Vulgaris Treatment Market
- Genentech, Inc. (Roche)
- Pfizer Inc.
- Amgen Inc.
- CELLTRION INC.
- Zenyaku Kogyo Co., Ltd.
- Scinai Immunotherapeutics Ltd.
- Novartis AG
- Johnson & Johnson (Janssen Biotech)
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 0.52 billion |
| Therapy Type | Anti-CD20 Monoclonal Antibodies (Rituximab), Systemic Corticosteroids, Conventional Steroid-Sparing Immunosuppressants, Intravenous Immunoglobulin (IVIG - Adjunct), Topical Therapies |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Others (Online/Infusion Pharmacies) |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | India, China, Japan, USA, Germany, Brazil, Saudi Arabia, and 40+ countries |
| Key Companies Profiled | Genentech, Inc. (Roche), Pfizer Inc., Amgen Inc., CELLTRION INC., Zenyaku Kogyo Co., Ltd., Scinai Immunotherapeutics Ltd., Novartis AG, Johnson & Johnson (Janssen Biotech), Sanofi S.A., Takeda Pharmaceutical Company Limited |
| Additional Attributes | Dollar sales by therapy type and distribution channel categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with biologic manufacturers and biosimilar developers, treatment protocol specifications and administration requirements, integration with specialty pharmacy networks and patient access programs, innovations in biologic therapy development and B-cell targeting mechanisms, and development of specialized applications with long-term remission maintenance and corticosteroid-sparing capabilities. |
Pemphigus Vulgaris Treatment Market by Segments
-
Therapy Type :
- Anti-CD20 Monoclonal Antibodies (Rituximab)
- Systemic Corticosteroids
- Conventional Steroid-Sparing Immunosuppressants
- Intravenous Immunoglobulin (IVIG - Adjunct)
- Topical Therapies
-
Distribution Channel :
- Hospital Pharmacies
- Retail Pharmacies
- Others (Online/Infusion Pharmacies)
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Union
- Rest of Middle East & Africa
- Asia Pacific
Pemphigus Vulgaris Treatment Market: Segmentation
Tentatively, the global pemphigus vulgaris treatment market can be segmented on the basis of types of treatment, route of administration and distribution channel.
Based on Types of Treatment, the global pemphigus vulgaris treatment market is segmented as:
- Non-biological treatment
- Corticosteroids
- Immunosuppressants (Mycophenolate mofetis, Azathioprin)
- Antibiotics, antifungal or anti-viral medication
- Other medications (Dapsone)
- Biological therapies (Mabs or monoclonal antibodies such as, Rituximab)
Based on the Route of Administration (RoA), the global pemphigus vulgaris treatment market is segmented as:
- Subcutaneous
- Peroeral
- Intravenous
Based on the Distribution Channel, the global pemphigus vulgaris treatment market is segmented as:
- Retailers
- Pharmacy
- E-Commerce
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Therapy Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Therapy Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Therapy Type, 2025 to 2035
- Anti-CD20 Monoclonal Antibodies (Rituximab)
- Systemic Corticosteroids
- Conventional Steroid-Sparing Immunosuppressants
- Intravenous Immunoglobulin (IVIG - Adjunct)
- Topical Therapies
- Y to o to Y Growth Trend Analysis By Therapy Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Therapy Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Distribution Channel
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Distribution Channel, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Distribution Channel, 2025 to 2035
- Hospital Pharmacies
- Retail Pharmacies
- Others (Online/Infusion Pharmacies)
- Y to o to Y Growth Trend Analysis By Distribution Channel, 2020 to 2024
- Absolute $ Opportunity Analysis By Distribution Channel, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Therapy Type
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Distribution Channel
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Therapy Type
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Distribution Channel
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Therapy Type
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Distribution Channel
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Therapy Type
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Distribution Channel
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Therapy Type
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Distribution Channel
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Therapy Type
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Distribution Channel
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Therapy Type
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy Type
- By Distribution Channel
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Therapy Type
- By Distribution Channel
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Therapy Type
- By Distribution Channel
- Competition Analysis
- Competition Deep Dive
- Genentech, Inc. (Roche)
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Pfizer Inc.
- Amgen Inc.
- CELLTRION INC.
- Zenyaku Kogyo Co., Ltd.
- Scinai Immunotherapeutics Ltd.
- Novartis AG
- Johnson & Johnson (Janssen Biotech)
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- Genentech, Inc. (Roche)
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Therapy Type, 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Therapy Type
- Figure 6: Global Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Distribution Channel
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 22: North America Market Attractiveness Analysis by Therapy Type
- Figure 23: North America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Distribution Channel
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 29: Latin America Market Attractiveness Analysis by Therapy Type
- Figure 30: Latin America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 32: Latin America Market Attractiveness Analysis by Distribution Channel
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 36: Western Europe Market Attractiveness Analysis by Therapy Type
- Figure 37: Western Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 39: Western Europe Market Attractiveness Analysis by Distribution Channel
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Therapy Type
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by Distribution Channel
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 50: East Asia Market Attractiveness Analysis by Therapy Type
- Figure 51: East Asia Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 53: East Asia Market Attractiveness Analysis by Distribution Channel
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Therapy Type
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by Distribution Channel
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Therapy Type, 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Therapy Type, 2025 to 2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Therapy Type
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by Distribution Channel
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the pemphigus vulgaris treatment market in 2025?
The global pemphigus vulgaris treatment market is estimated to be valued at USD 0.5 billion in 2025.
What will be the size of pemphigus vulgaris treatment market in 2035?
The market size for the pemphigus vulgaris treatment market is projected to reach USD 1.2 billion by 2035.
How much will be the pemphigus vulgaris treatment market growth between 2025 and 2035?
The pemphigus vulgaris treatment market is expected to grow at a 8.3% CAGR between 2025 and 2035.
What are the key product types in the pemphigus vulgaris treatment market?
The key product types in pemphigus vulgaris treatment market are anti-cd20 monoclonal antibodies (rituximab), systemic corticosteroids, conventional steroid-sparing immunosuppressants, intravenous immunoglobulin (ivig - adjunct) and topical therapies.
Which distribution channel segment to contribute significant share in the pemphigus vulgaris treatment market in 2025?
In terms of distribution channel, hospital pharmacies segment to command 81.6% share in the pemphigus vulgaris treatment market in 2025.