Cell and Gene Therapy CDMO Market
Cell and Gene Therapy CDMO Market Size and Share Forecast Outlook 2026 to 2036
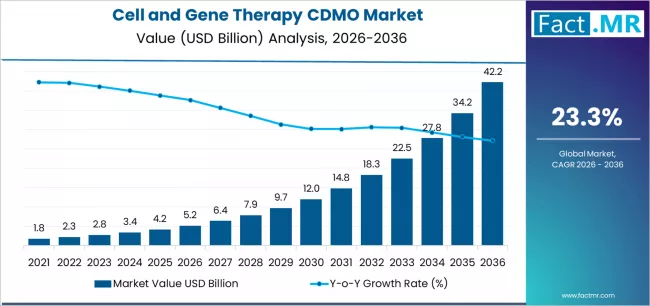
Cell and gene therapy CDMO market is projected to grow from USD 5.2 billion in 2026 to USD 42.2 billion by 2036, at a CAGR of 23.3%. Pre-clinical will dominate with a 64.9% market share, while cell therapy will lead the product segment with a 42.8% share.
Cell and Gene Therapy CDMO Market Forecast and Outlook 2026 to 2036
The global cell and gene therapy CDMO market is set to grow from USD 5.2 billion in 2026 to USD 42.2 billion by 2036, adding USD 37.0 billion in new revenue and advancing at a CAGR of 23.3%. Growth is driven by escalating demand for advanced therapeutic manufacturing validation, expanding regenerative medicine infrastructure across regulated markets, and accelerating regulatory approval requirements among pharmaceutical and biotechnology organizations seeking specialized production solutions.
Key Takeaways from Cell and Gene Therapy CDMO Market
- Cell and Gene Therapy CDMO Market Value (2026): USD 5.2 billion
- Cell and Gene Therapy CDMO Market Forecast Value (2036): USD 42.2 billion
- Cell and Gene Therapy CDMO Market Forecast CAGR: 23.3%
- Leading Phase in Cell and Gene Therapy CDMO Market: Pre-clinical (64.9%)
- Key Growth Regions in Cell and Gene Therapy CDMO Market: Asia Pacific, North America, and Europe
- Top Players in Cell and Gene Therapy CDMO Market: Lonza, Catalent Inc., Cytiva, Samsung Biologics, Thermo Fisher Scientific Inc.
Cell and gene therapy CDMO technologies are increasingly recognized as essential services for biopharmaceutical practitioners, offering precise viral vector production capabilities, regulatory compliance assurance, and comprehensive manufacturing characteristics compared to traditional in-house production approaches.
Pre-clinical phase services dominate the market, favored in research and development environments for their established validation properties, providing early-stage development mechanisms, scalability assessment capabilities, and regulatory pathway acceptance across diverse therapeutic applications and development demographics.
Cell Therapy remains fundamental in manufacturing protocols where routine process development and clinical material production match operational requirements and quality assurance confidence standards. Pre-clinical phase users are advancing among development categories as specialized CDMO facility networks expand and early-stage development infrastructure increases accessibility in research-convenient locations with stringent quality structures.
Cell and Gene Therapy CDMO Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2026) | USD 5.2 billion |
| Market Forecast (2036) | USD 42.2 billion |
| Growth Rate | 23.3% CAGR |
| Leading Phase | Pre-clinical |
| Primary Product | Cell Therapy |
Category
| Category | Segments |
|---|---|
| Phase | Pre-clinical; Clinical |
| Product | Gene Therapy (Ex-vivo; In-vivo); Gene-Modified Cell Therapy (CAR T-cell Therapies; CAR-NK Cell Therapy; TCR-T Cell Therapy; Others); Cell Therapy |
| Indication | Oncology; Infectious Diseases; Neurological Disorders; Rare Diseases; Others |
| Region | North America; Europe; Asia Pacific; Latin America; Middle East & Africa |
Segmental Analysis
What Makes Pre-clinical Phase Command the Largest Share in the Cell and Gene Therapy CDMO Market?
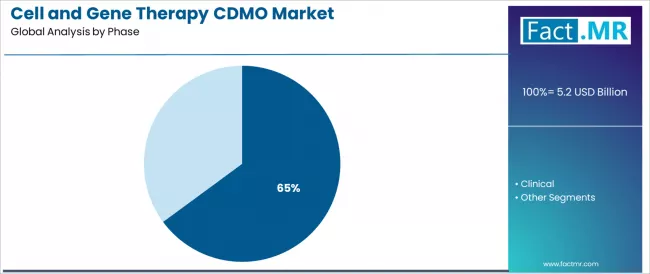
Pre-clinical phase commands the leading position in the cell and gene therapy CDMO market with a 64.9% market share through superior early-stage development characteristics, including established research partnerships, extensive process development documentation, and proven scalability pathways that enable biotechnology companies to achieve predictable manufacturing outcomes across varied therapeutic categories and diverse development demographics.
How does Cell Therapy Dominance Shape Product Preferences in CDMO Services?
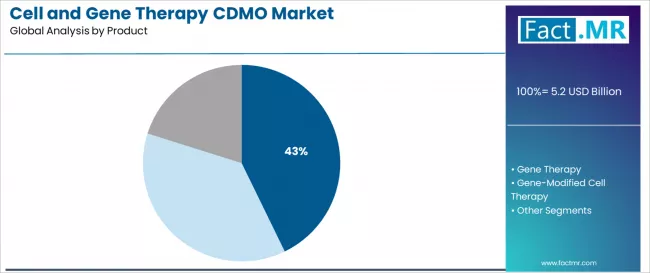
Cell therapy demonstrates product leadership in the cell and gene therapy CDMO market with a 42.8% share due to widespread therapeutic application requirements and established focus on autologous production, allogeneic manufacturing, and process optimization that maximizes clinical efficacy while maintaining consistent quality manufacturing characteristics.
What establishes Oncology's Market Leadership in Cell and Gene Therapy CDMO?
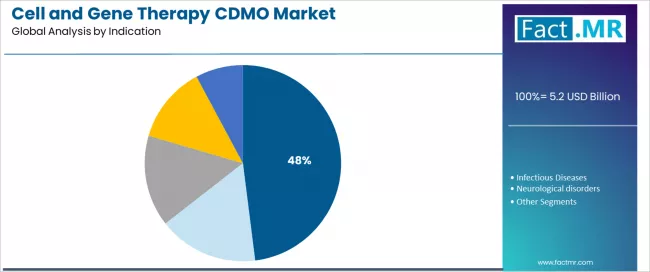
Oncology establishes market leadership in the cell and gene therapy CDMO sector with commanding 48.0% share due to comprehensive CAR-T development requirements and sustained focus on hematological malignancy treatment, solid tumor applications, and immunotherapy manufacturing that maximizes therapeutic outcomes while maintaining appropriate regulatory standards.
Pharmaceutical companies and clinical teams prioritize oncology applications for CAR-T commercialization, comprehensive clinical trial programs, and integration with precision medicine requirements that enables coordinated development experiences across multiple cancer categories.
What are the Drivers, Restraints, and Key Trends of the Cell and Gene Therapy CDMO Market?
The cell and gene therapy CDMO market is driven primarily by rising CAR-T therapy approvals and commercialization, which are expanding demand for GMP-compliant manufacturing at commercial scale. Rapid growth in gene therapy pipelines, particularly for rare and genetic diseases, is further increasing requirements for viral vector production, validated processes, and specialized facilities. Pharmaceutical outsourcing trends also support market expansion, as developers seek capital efficiency, flexible capacity, and access to specialized manufacturing expertise.
Market restraints include high manufacturing and facility investment costs associated with GMP infrastructure, cleanrooms, and advanced equipment, which limit adoption among early-stage and resource-constrained biotechnology firms. Technical complexity, scale-up challenges, and process transfer risks further constrain utilization, particularly for novel modalities.
Key trends include accelerating adoption of automated and closed manufacturing systems to improve scalability, reduce contamination risk, and lower costs. CDMOs are also expanding regional manufacturing footprints to strengthen supply chain resilience, improve regulatory access, and support geographically distributed clinical and commercial demand.
Analysis of the Cell and Gene Therapy CDMO Market by Key Countries
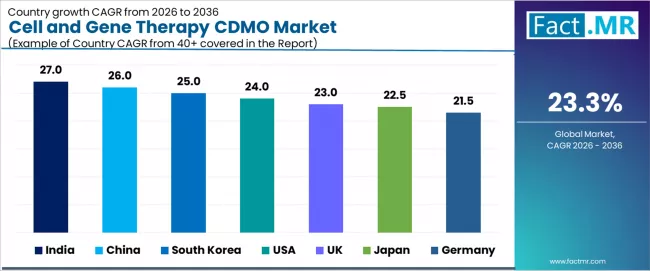
| Country | CAGR 2026-2036 |
|---|---|
| India | 27.0% |
| China | 26.0% |
| South Korea | 25.0% |
| USA | 24.0% |
| UK | 23.0% |
| Japan | 22.5% |
| Germany | 21.5% |
Why does India Drive the Fastest Growth in Cell and Gene Therapy CDMO Services?
India records the fastest growth rate at 27.0%, driven by rapid biotechnology manufacturing expansion and strengthening CDMO infrastructure. Growth is concentrated in hubs such as Hyderabad, Bangalore, and Pune, where facilities are scaling capacity to meet international GMP standards.
Rising biotechnology investment and expanding clinical trial activity are increasing demand for cost-efficient manufacturing services across early-stage and commercial therapies. Indian CDMOs are standardizing production protocols, integrating global technology platforms, and leveraging skilled workforce availability to support process development and regulatory requirements.
How does China Establish Leadership Through Large-Scale Biomanufacturing Capacity?
China grows at a 26.0% rate, supported by strong government backing for biotechnology and extensive manufacturing infrastructure investment. CDMO adoption is accelerating in Shanghai, Beijing, and Suzhou, where integrated service platforms combine process development with commercial-scale production. Biotech park development and expanding clinical trial infrastructure support both domestic and international demand.
Chinese CDMOs emphasize capacity flexibility, cost efficiency, and regulatory documentation to attract global clients. Quality system development and compliance alignment are strengthening confidence in large-scale cell and gene therapy manufacturing partnerships.
Why does the USA Lead CAR-T and Advanced Therapy Manufacturing?
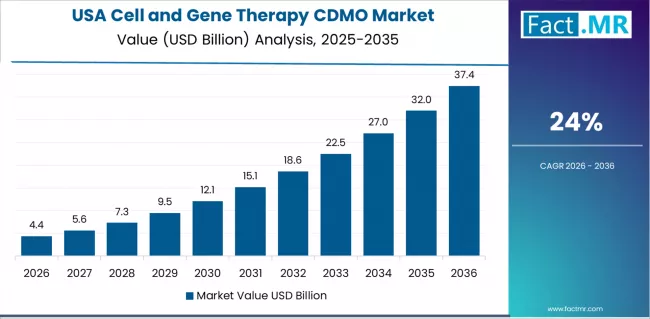
The USA maintains a 24.0% growth rate, anchored in CAR-T commercialization leadership and a mature FDA regulatory ecosystem. Growth centers in Massachusetts, California, and New Jersey, where CDMOs support clinical and commercial manufacturing for biotech firms and academic centers. Established quality systems, inspection readiness, and regulatory documentation remain critical differentiators.
Strong demand for premium CDMO services reflects expanding gene therapy approvals and ongoing investment in process optimization. The US market benefits from deep integration between innovation, regulation, and manufacturing execution.
How does South Korea Strengthen CDMO Adoption through Integrated Manufacturing Models?
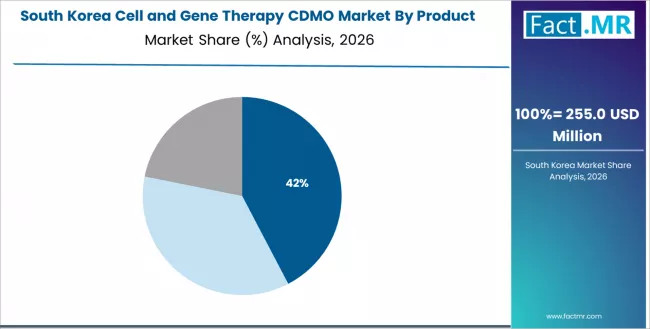
South Korea records 25.0% growth, driven by government-backed biotechnology initiatives and integrated CDMO development. Hubs such as Incheon, Seoul, and Songdo emphasize automation, quality systems, and regulatory support within advanced therapy manufacturing.
CDMOs focus on platform-based services that align with global quality expectations and efficient commercialization pathways. Strong biopharmaceutical cluster development and national bioeconomy strategies continue to support scalable cell and gene therapy manufacturing capabilities.
Why is the UK Recognized for ATMP Manufacturing and Regulatory Precision?
The UK grows at 23.0%, supported by strong ATMP infrastructure and regulatory rigor. CDMO activity is concentrated in the Oxford-Cambridge corridor, London, and Manchester, where GMP compliance and validation depth guide manufacturing selection.
UK CDMOs emphasize MHRA inspection readiness, comparative process studies, and detailed technical documentation. Established cell and gene therapy development pipelines and regulatory maturity support sustained demand for specialized, compliance-driven manufacturing services.
How does Japan Integrate Regenerative Medicine Expertise into CDMO Growth?
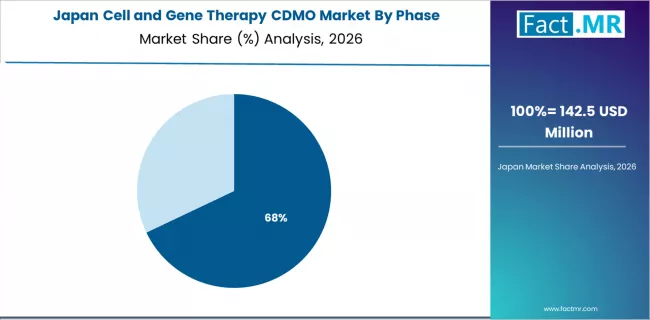
Japan achieves 22.5% growth, driven by regenerative medicine leadership and accelerated approval pathways. CDMO adoption is supported by strong pharmaceutical participation and specialized facilities with iPSC manufacturing expertise.
Japanese partners prioritize quality assurance, validation depth, and regulatory alignment with PMDA requirements. Integration of advanced manufacturing technologies and disciplined process control supports premium positioning in cell and gene therapy production.
Why does Germany Maintain Excellence in Biopharmaceutical Manufacturing Services?
Germany records 21.5% growth, leveraging its pharmaceutical engineering heritage and stringent regulatory standards. CDMO activity in Bavaria, North Rhine-Westphalia, and Hesse emphasizes process robustness, quality by design, and comprehensive documentation.
German CDMOs focus on precision manufacturing, analytical integration, and EMA compliance. Strong technical expertise and quality-driven production culture continue to attract global partners seeking reliable and validated advanced therapy manufacturing services.
Competitive Landscape of the Cell and Gene Therapy CDMO Market
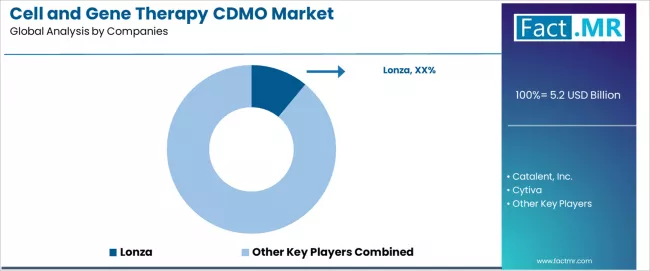
The cell and gene therapy CDMO market is moderately consolidated, with 40 to 70 active participants spanning global biomanufacturing networks and regional service providers. Competition reflects the maturation of advanced therapy manufacturing, where scale, regulatory credibility, and technical depth increasingly determine positioning. Large CDMOs benefit from established client relationships, validated manufacturing records of accomplishment, and broad service portfolios covering multiple cell and gene therapy modalities.
Lonza holds a leading position, supported by a diversified global manufacturing footprint, extensive process development capabilities, and long-standing relationships with biotechnology and pharmaceutical developers. Other established CDMOs compete by leveraging GMP-compliant facilities, technology transfer expertise, and regulatory support services that reduce development and commercialization risk for clients.
Specialized and regional CDMOs create competitive pressure through focused therapeutic expertise, cost advantages, and localized client support. Differentiation is increasingly visible in gene-modified cell therapies, automation, and closed-system manufacturing, where innovation-driven providers offer efficiency and contamination control benefits. Overall, competitiveness favors CDMOs combining global regulatory readiness, manufacturing efficiency, and integrated service offerings that extend beyond transactional production toward long-term development partnerships.
Key Players in the Cell and Gene Therapy CDMO Market
- Lonza
- Catalent, Inc.
- Cytiva
- Samsung Biologics
- Thermo Fisher Scientific Inc.
- Novartis AG
- WuXi AppTec
- AGC Biologics
- OmniaBio
- Rentschler Biopharma SE
- Charles River Laboratories
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2026) | USD 5.2 billion |
| Phase | Pre-clinical, Clinical |
| Product | Gene Therapy (Ex-vivo, In-vivo), Gene-Modified Cell Therapy (CAR T-cell therapies, CAR-NK cell therapy, TCR-T cell therapy, Other), Cell Therapy |
| Indication | Oncology, Infectious Diseases, Neurological disorders, Rare Diseases, Others |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, MEA |
| Countries Covered | USA, China, India, Germany, UK, France, Japan, South Korea, and 15+ additional countries |
| Key Companies Profiled | Lonza, Catalent Inc., Cytiva, Samsung Biologics, Thermo Fisher Scientific Inc., Novartis AG |
| Additional Attributes | Dollar sales by phase and product categories, regional adoption trends across Asia Pacific, North America, and Europe, competitive landscape with established CDMO corporations and specialized biomanufacturing companies, pharmaceutical preferences for Pre-clinical phase services and manufacturing flexibility, integration with biotechnology development facilities and pharmaceutical commercialization organizations, innovations in automated manufacturing technologies and closed-system platforms, and development of sophisticated GMP systems with enhanced scalability profiles and comprehensive regulatory documentation frameworks. |
Cell and Gene Therapy CDMO Market by Segments
-
Phase :
- Pre-clinical
- Clinical
-
Product :
- Gene Therapy
- Ex-vivo
- In-vivo
- Gene-Modified Cell Therapy
- CAR T-cell therapies
- CAR-NK cell therapy
- TCR-T cell therapy
- Other
- Cell Therapy
- Gene Therapy
-
Indication :
- Oncology
- Infectious Diseases
- Neurological disorders
- Rare Diseases
- Others
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- India
- China
- Japan
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- MEA
- GCC Countries
- South Africa
- Rest of MEA
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2021 to 2025 and Forecast, 2026 to 2036
- Historical Market Size Value (USD Million) Analysis, 2021 to 2025
- Current and Future Market Size Value (USD Million) Projections, 2026 to 2036
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2021 to 2025 and Forecast 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Phase
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Phase, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Phase, 2026 to 2036
- Pre-clinical
- Clinical
- Y to o to Y Growth Trend Analysis By Phase, 2021 to 2025
- Absolute $ Opportunity Analysis By Phase, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2026 to 2036
- Cell Therapy
- Gene Therapy
- Gene-Modified Cell Therapy
- Y to o to Y Growth Trend Analysis By Product, 2021 to 2025
- Absolute $ Opportunity Analysis By Product, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Indication
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Indication, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Indication, 2026 to 2036
- Oncology
- Infectious Diseases
- Neurological disorders
- Rare Diseases
- Others
- Y to o to Y Growth Trend Analysis By Indication, 2021 to 2025
- Absolute $ Opportunity Analysis By Indication, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2021 to 2025
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2026 to 2036
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- USA
- Canada
- Mexico
- By Phase
- By Product
- By Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- By Indication
- Key Takeaways
- Latin America Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Phase
- By Product
- By Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- By Indication
- Key Takeaways
- Western Europe Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Phase
- By Product
- By Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- By Indication
- Key Takeaways
- Eastern Europe Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Phase
- By Product
- By Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- By Indication
- Key Takeaways
- East Asia Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- China
- Japan
- South Korea
- By Phase
- By Product
- By Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- By Indication
- Key Takeaways
- South Asia and Pacific Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Phase
- By Product
- By Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- By Indication
- Key Takeaways
- Middle East & Africa Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Phase
- By Product
- By Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- By Indication
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Canada
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Mexico
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Brazil
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Chile
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Germany
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- UK
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Italy
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Spain
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- France
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- India
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- China
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Japan
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- South Korea
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Russia
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Poland
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Hungary
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- South Africa
- Pricing Analysis
- Market Share Analysis, 2025
- By Phase
- By Product
- By Indication
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Phase
- By Product
- By Indication
- Competition Analysis
- Competition Deep Dive
- Lonza
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Catalent, Inc.
- Cytiva
- Samsung Biologics
- Thermo Fisher Scientific Inc.
- Novartis AG
- WuXi AppTec
- AGC Biologics
- OmniaBio
- Rentschler Biopharma SE
- Charles River Laboratories
- Lonza
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2021 to 2036
- Table 2: Global Market Value (USD Million) Forecast by Phase, 2021 to 2036
- Table 3: Global Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 4: Global Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 5: North America Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 6: North America Market Value (USD Million) Forecast by Phase, 2021 to 2036
- Table 7: North America Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 8: North America Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 10: Latin America Market Value (USD Million) Forecast by Phase, 2021 to 2036
- Table 11: Latin America Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 12: Latin America Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 14: Western Europe Market Value (USD Million) Forecast by Phase, 2021 to 2036
- Table 15: Western Europe Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 16: Western Europe Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Phase, 2021 to 2036
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 20: Eastern Europe Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 22: East Asia Market Value (USD Million) Forecast by Phase, 2021 to 2036
- Table 23: East Asia Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 24: East Asia Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Phase, 2021 to 2036
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Phase, 2021 to 2036
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by Indication, 2021 to 2036
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2021 to 2036
- Figure 3: Global Market Value Share and BPS Analysis by Phase, 2026 and 2036
- Figure 4: Global Market Y to o to Y Growth Comparison by Phase, 2026 to 2036
- Figure 5: Global Market Attractiveness Analysis by Phase
- Figure 6: Global Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 7: Global Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 8: Global Market Attractiveness Analysis by Product
- Figure 9: Global Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 10: Global Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 11: Global Market Attractiveness Analysis by Indication
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2026 and 2036
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2026 to 2036
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 23: North America Market Value Share and BPS Analysis by Phase, 2026 and 2036
- Figure 24: North America Market Y to o to Y Growth Comparison by Phase, 2026 to 2036
- Figure 25: North America Market Attractiveness Analysis by Phase
- Figure 26: North America Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 27: North America Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 28: North America Market Attractiveness Analysis by Product
- Figure 29: North America Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 30: North America Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 31: North America Market Attractiveness Analysis by Indication
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 33: Latin America Market Value Share and BPS Analysis by Phase, 2026 and 2036
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Phase, 2026 to 2036
- Figure 35: Latin America Market Attractiveness Analysis by Phase
- Figure 36: Latin America Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 38: Latin America Market Attractiveness Analysis by Product
- Figure 39: Latin America Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 41: Latin America Market Attractiveness Analysis by Indication
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 43: Western Europe Market Value Share and BPS Analysis by Phase, 2026 and 2036
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Phase, 2026 to 2036
- Figure 45: Western Europe Market Attractiveness Analysis by Phase
- Figure 46: Western Europe Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 48: Western Europe Market Attractiveness Analysis by Product
- Figure 49: Western Europe Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 51: Western Europe Market Attractiveness Analysis by Indication
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Phase, 2026 and 2036
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Phase, 2026 to 2036
- Figure 55: Eastern Europe Market Attractiveness Analysis by Phase
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 58: Eastern Europe Market Attractiveness Analysis by Product
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 61: Eastern Europe Market Attractiveness Analysis by Indication
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 63: East Asia Market Value Share and BPS Analysis by Phase, 2026 and 2036
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Phase, 2026 to 2036
- Figure 65: East Asia Market Attractiveness Analysis by Phase
- Figure 66: East Asia Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 68: East Asia Market Attractiveness Analysis by Product
- Figure 69: East Asia Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 70: East Asia Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 71: East Asia Market Attractiveness Analysis by Indication
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Phase, 2026 and 2036
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Phase, 2026 to 2036
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Phase
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Product
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by Indication
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Phase, 2026 and 2036
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Phase, 2026 to 2036
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Phase
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Product
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 91: Middle East & Africa Market Attractiveness Analysis by Indication
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the cell and gene therapy CDMO market in 2026?
The global cell and gene therapy CDMO market is estimated to be valued at USD 5.2 billion in 2026.
What will be the size of cell and gene therapy CDMO market in 2036?
The market size for the cell and gene therapy CDMO market is projected to reach USD 42.2 billion by 2036.
How much will be the cell and gene therapy CDMO market growth between 2026 and 2036?
The cell and gene therapy CDMO market is expected to grow at a 23.3% CAGR between 2026 and 2036.
What are the key product types in the cell and gene therapy CDMO market?
The key product types in cell and gene therapy CDMO market are pre-clinical and clinical.
Which product segment to contribute significant share in the cell and gene therapy CDMO market in 2026?
In terms of product, cell therapy segment to command 42.8% share in the cell and gene therapy CDMO market in 2026.