Peanut Allergy Treatment Market
Peanut Allergy Treatment Market Size and Share Forecast Outlook 2025 to 2035
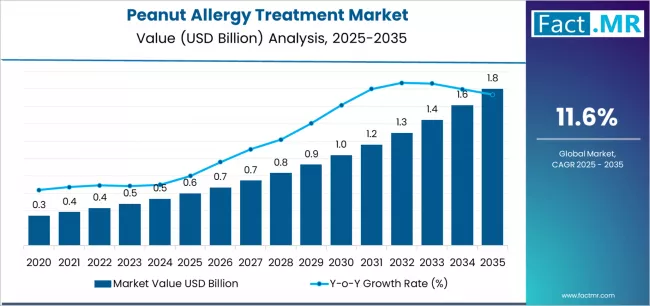
Peanut allergy treatment market is projected to grow from USD 0.6 billion in 2025 to USD 1.8 billion by 2035, at a CAGR of 11.6%. Epinephrine will dominate with a 46.6% market share, while injectable will lead the route of administration segment with a 61.7% share.
Peanut Allergy Treatment Market Forecast and Outlook 2025 to 2035
The global peanut allergy treatment market is set to grow from USD 0.6 billion in 2025 to USD 1.8 billion by 2035, adding USD 1.2 billion in new revenue and advancing at a CAGR of 11.6%. Growth is driven by escalating prevalence of food allergies, expanding oral immunotherapy adoption infrastructure across healthcare markets, and accelerating emergency preparedness requirements among families and educational institutions seeking anaphylaxis management solutions.
Peanut allergy treatment technologies are increasingly recognized as essential interventions for allergists, offering superior emergency response capabilities, desensitization therapy advantages, and comprehensive safety management characteristics compared to traditional allergen avoidance approaches.
Quick Stats for Peanut Allergy Treatment Market
- Peanut Allergy Treatment Market Value (2025): USD 0.6 billion
- Peanut Allergy Treatment Market Forecast Value (2035): USD 1.8 billion
- Peanut Allergy Treatment Market Forecast CAGR: 11.6%
- Leading Drug Class in Peanut Allergy Treatment Market: Epinephrine (46.6%)
- Key Growth Regions in Peanut Allergy Treatment Market: North America, Asia Pacific, and Europe
- Top Players in Peanut Allergy Treatment Market: Aimmune Therapeutics, DBV Technologies, Sanofi, ALK-Abelló, Prota Therapeutics
Epinephrine formulations dominate the market, favored in emergency and home care environments for their established life-saving properties, providing rapid anaphylaxis reversal mechanisms, auto-injector convenience capabilities, and immediate symptom control across diverse allergic reaction severities and patient demographics.
Injectable routes remain fundamental in administration protocols where immediate systemic delivery and emergency response match clinical requirements and anaphylaxis management confidence standards. Oral formulations are advancing among administration categories as specialized immunotherapy facility networks expand and desensitization protocol infrastructure increases accessibility in outpatient-convenient locations with gradual dose escalation structures.
Geographic concentration demonstrates dynamic growth patterns with the USA and China leading expansion, supported by rising food allergy prevalence capacity, anaphylaxis awareness expansion among healthcare populations, and allergy specialty clinic establishment programs in urban centers.
Japan, Germany, India, Brazil, and UK demonstrate robust development through established allergy care ecosystems, regulatory framework maturity for biologic therapy standards, and continued acceptance of oral immunotherapy procedures. Competitive advantage is consolidating around safety profile documentation, desensitization efficacy compatibility, patient adherence support, and integrated allergy management portfolios rather than standalone epinephrine formulations alone.
The first half of the decade will witness the market climbing from USD 0.6 billion to approximately USD 1.1 billion, adding USD 0.5 billion in value, which constitutes 42% of the total forecast growth period. This phase will be characterized by the continued dominance of epinephrine auto-injector methodologies in emergency settings, combined with accelerating adoption of oral immunotherapy technologies in controlled desensitization applications where tolerance induction and quality of life improvement create favorable patient outcome scenarios.
The latter half will witness sustained expansion from USD 1.1 billion to USD 1.8 billion, representing an addition of USD 0.7 billion or 58% of the decade's growth, defined by broadening acceptance of biologics-based therapies and integration of preventative intervention platforms across mainstream allergy practice facilities.
Where revenue comes from - Now Vs Next (industry-level view)
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | Epinephrine | 46.6% | Emergency dominance |
| Injectable Route | 61.7% | Auto-injector delivery | |
| Hospital Pharmacy | 55.5% | Clinical distribution | |
| Antihistamines | 30.0% | Symptomatic relief | |
| Immunotherapies | 18.0% | Desensitization segment | |
| Future (3-5 yrs) | Oral Immunotherapy | 38-44% | Tolerance induction |
| Biologics (Anti-IgE) | 32-38% | Targeted therapy | |
| Epicutaneous Patches | 28-34% | Non-invasive delivery | |
| Preventative Interventions | 22-28% | Early exposure | |
| Combination Therapies | 24-30% | Multi-modal approach | |
| Retail Pharmacy Access | 35-41% | Patient convenience | |
| Emerging Markets | 35-41% | Awareness expansion |
Peanut Allergy Treatment Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 0.6 billion |
| Market Forecast (2035) ↑ | USD 1.8 billion |
| Growth Rate ★ | 11.6% CAGR |
| Leading Drug Class → | Epinephrine |
| Primary Route → | Injectable |
The market demonstrates exceptional fundamentals with Epinephrine capturing a commanding 46.6% share through superior emergency response characteristics, established anaphylaxis management advantages, and proven life-saving profiles across allergic reaction applications. Injectable routes drive primary administration demand at 61.7% share, supported by established auto-injector infrastructure and rapid systemic delivery requirements that maintain emergency preparedness across diverse patient segments.
Geographic concentration remains anchored in North America and Asia Pacific with emerging market leadership through food allergy prevalence expansion and allergy specialty care infrastructure development, while developed markets show accelerated adoption rates driven by immunotherapy demographics and preventative intervention procedure preferences.
Imperatives for Stakeholders in Peanut Allergy Treatment Market
Design for safety and adherence, not just symptom management
- Offer complete allergy solutions: treatment products + emergency action plans + patient education materials + adherence support systems + anaphylaxis training platforms.
- Preconfigured management packages: emergency kit specifications, desensitization protocol configurations, school safety programs, and travel preparation protocols for diverse patient requirements.
Regulatory readiness for allergy therapeutic applications
- Comprehensive safety documentation, FDA approval systems, and clinical infrastructure (phase III trial data, real-world evidence, pharmacovigilance protocols).
Patient education-focused market penetration
- Established allergy awareness workshops + comprehensive certification programs (auto-injector training, emergency response, allergen avoidance); direct patient and caregiver engagement for relationship development and treatment confidence building.
Access expansion-focused positioning
- Insurance coverage advocacy, patient assistance programs, school access initiatives, and transparent treatment cost documentation.
Segmental Analysis
The market segments by drug class into epinephrine, antihistamines, immunotherapies, and others, representing the evolution from emergency symptomatic treatment toward disease-modifying interventions with desensitization capabilities, tolerance induction, and long-term management characteristics.
By route of administration, the segmentation divides the market into injectable (61.7%), oral (30.0%), and others, reflecting distinct delivery objectives for emergency anaphylaxis management and rapid systemic action versus gradual desensitization and home-based therapy convenience.
The distribution channel segmentation shows hospital pharmacy's commanding 55.5% position, followed by retail pharmacy at 35.0% and others, demonstrating varied healthcare access specialization levels and prescription dispensing concentrations.
Which Drug Class is Preferred for Treating Peanut Allergies?
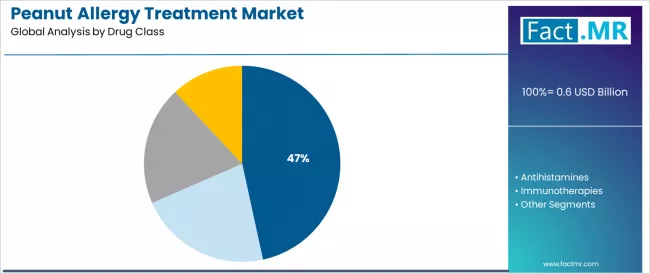
Epinephrine commands the leading position in the peanut allergy treatment market with a 46.6% market share through superior emergency response characteristics, including immediate vasoconstriction capacity, bronchodilation effectiveness, and anaphylaxis reversal pathways that enable patients and caregivers to achieve predictable life-saving outcomes across varied allergic reaction severities and diverse emergency situations.
The segment benefits from rapid action advantages through intramuscular injection, symptom reversal results without delay complications, and established emergency protocol documentation without requiring medical supervision for administration. Advanced device technology enables auto-injector design optimization, dose accuracy variation, and needle safety customization, where ease of use and reliability represent critical emergency preparedness requirements.
EpiPen and generic epinephrine auto-injectors hold significant share within the epinephrine segment, appealing to patients seeking reliable emergency anaphylaxis treatment capabilities for immediate response. Epinephrine products differentiate through proven life-saving profiles, patient familiarity advantages, and integration with established emergency action protocols that enhance preparedness confidence while maintaining cost-effective safety outcomes for diverse anaphylaxis risk applications.
Key market characteristics:
- Advanced pharmacological properties with rapid onset and systemic effects for immediate symptom reversal
- Superior delivery systems, enabling auto-injector convenience and emergency self-administration for patient empowerment
- Comprehensive safety profiles, including established use history, minimal contraindications, and clear administration guidelines for emergency protocols
Why do Antihistamines Represent a Symptomatic Relief Option?
Antihistamines maintain specialized market position through histamine blocking characteristics and mild reaction management capabilities. These products appeal to allergy patients and healthcare providers seeking symptom control with comprehensive tolerability, offering oral administration patterns and over-the-counter accessibility approaches through H1-receptor antagonism. Market adoption is driven by mild allergic reaction applications, emphasizing symptom relief and supportive care through first-generation and second-generation antihistamine formulations.
Which Category of Drugs are Preferred by Route of Administration?
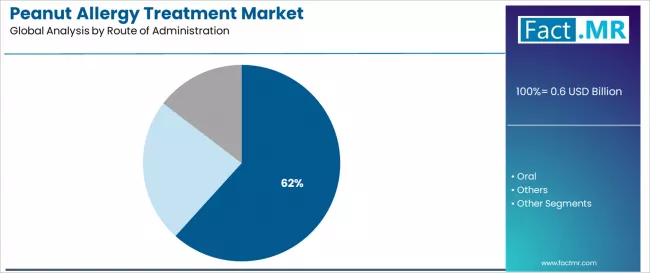
Injectable drugs are the most preferred, capturing a market share of 61.7%. Growth is attributed to widespread emergency response requirements and established focus on rapid systemic delivery, anaphylaxis management, and immediate bioavailability that maximizes survival outcomes while maintaining administration simplicity characteristics.
Patients and caregivers prioritize injectable administration for emergency preparedness, rapid symptom reversal, and integration with established anaphylaxis protocols that enables coordinated emergency experiences across multiple reaction scenarios. The segment benefits from substantial auto-injector technology maturity and emergency training standardization that emphasizes injectable platforms for life-threatening allergic reaction management across diverse age demographics.
Epinephrine auto-injectors capture significant share within the injectable route segment, demonstrating patient preference for pre-filled emergency devices. Food allergy prevalence expansion incorporates injectable epinephrine as essential emergency components for anaphylaxis preparedness, while school safety programs increase auto-injector availability that meets accessibility requirements and ensures rapid intervention capabilities.
Will Oral Drug Administration remain Prevalent?
Oral routes capture substantial administration share through comprehensive requirements in oral immunotherapy protocols, daily dosing convenience, and home-based desensitization optimization. Oral drugs demand sophisticated safety monitoring protocols capable of achieving tolerance induction while providing gradual dose escalation and adherence support, appealing to motivated patients and specialized allergists seeking evidence-based desensitization advantages beyond emergency management approaches.
Which Distribution Channel Accounts for Maximum Peanut Allergy Drug Sales?
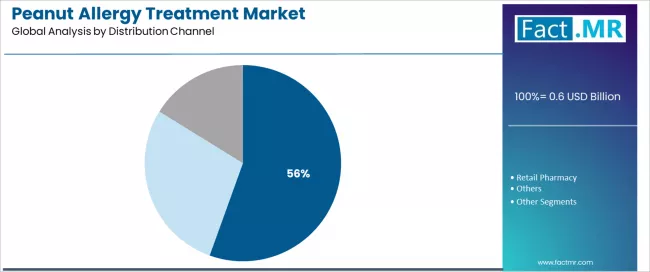
Hospital pharmacies constitute the maximum peanut allergy treatment drug sales, with a 55.5% market share. This is attributed to comprehensive prescription fulfillment requirements and sustained focus on specialty medication access, insurance coordination, and clinical support integration that maximizes patient safety while maintaining appropriate therapy monitoring standards.
Healthcare providers and patients prioritize hospital pharmacy for specialty product availability, insurance billing expertise, and integration with allergy clinic services that enables coordinated treatment experiences across multiple therapy phases. The sector benefits from substantial specialty pharmacy infrastructure maturity and prior authorization support campaigns that emphasize hospital-based platforms for novel immunotherapy dispensing across diverse patient populations.
Oral immunotherapy adoption expansion incorporates hospital pharmacy as essential dispensing components for specialty product access, while biologics approval increases specialty channel utilization that meets safety monitoring requirements and ensures appropriate patient selection capabilities.
Distribution dynamics include:
- Strong growth in specialty pharmacy networks requiring coordinated care and patient education service arrangements
- Increasing adoption in mail-order pharmacy for maintenance therapy convenience and adherence support positioning
- Rising integration with patient assistance programs for affordability enhancement and access barrier reduction
How are Retail Pharmacies Advancing Community Access Requirements?
Retail pharmacies capture growing distribution share through established community presence frameworks, complex prescription convenience capabilities, and integrated patient counseling protocols.
The segment demonstrates specialized accessibility expertise across diverse geographic markets, with advanced insurance processing applications and immediate product availability infrastructure gaining traction in emergency preparedness programs while cost transparency frameworks drive continued channel preference requiring careful balance between convenience optimization and specialty product handling assurance.
What are the Drivers, Restraints, and Key Trends of the Peanut Allergy Treatment Market?
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Rising food allergy prevalence & peanut allergy incidence (hygiene hypothesis, dietary changes) | ★★★★★ | Allergy epidemic enables treatment demand for management solutions; increasing diagnosis rates drive therapy adoption across pediatric markets and diverse patient segments. |
| Driver | Growth in oral immunotherapy approvals and desensitization adoption (clinical evidence, regulatory acceptance) | ★★★★★ | Drives demand for disease-modifying therapies and tolerance induction protocols; providers offering effective desensitization gain competitive advantage in allergy-focused healthcare segments. |
| Driver | Expanding anaphylaxis awareness and emergency preparedness (education campaigns, advocacy groups) | ★★★★☆ | Families demand accessible emergency treatments and safety planning systems; awareness visibility expanding addressable segments beyond diagnosed patient demographics and healthcare provider clientele. |
| Restraint | High treatment costs & insurance coverage limitations (specialty therapy pricing, reimbursement barriers) | ★★★★★ | Cost-conscious families face affordability barriers and therapy access constraints, restricting immunotherapy adoption and affecting treatment penetration in uninsured populations and developing market healthcare systems. |
| Restraint | Safety concerns & adverse reaction risks (anaphylaxis during therapy, adherence challenges) | ★★★☆☆ | Patients face treatment fear and dropout concerns; increases therapy hesitation and affects adoption confidence in risk-averse families and severe allergy populations. |
| Trend | Biologics integration & anti-IgE therapy development (omalizumab, targeted immunomodulation) | ★★★★★ | Growing clinical demand for enhanced safety profiles and improved desensitization success beyond immunotherapy alone; biologics combination becomes core differentiation strategy for severe allergy positioning. |
| Trend | Preventative intervention & early allergen introduction (infant exposure, tolerance development) | ★★★★☆ | Allergy prevention evolving beyond treatment-only approaches toward primary prevention strategies; early intervention positioning drives paradigm shift and population-level allergy reduction in progressive healthcare environments. |
Analysis of the Peanut Allergy Treatment Market by Key Countries
The peanut allergy treatment market demonstrates robust regional growth dynamics with emerging leaders including the USA (12.5% CAGR) and China (12.0% CAGR) driving expansion through food allergy awareness programs and allergy specialty care development.
Strong performers encompass Japan (11.4% CAGR), Germany (10.9% CAGR), and India (10.3% CAGR), benefiting from established allergy diagnosis infrastructure and immunotherapy adoption demographics. Developed markets feature Brazil (9.8% CAGR) and UK (9.1% CAGR), where food allergy recognition normalization and emergency preparedness expertise support consistent growth patterns.
Regional synthesis reveals North American markets leading adoption through comprehensive allergy specialty positioning and immunotherapy clinical trial infrastructure, while Asian countries demonstrate strong growth potential supported by westernization dietary pattern preferences and allergy awareness campaigns. European markets show solid development driven by healthcare system integration and specialty pharmacy expansion.
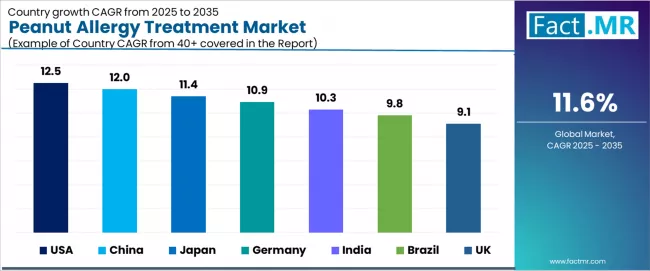
| Region/Country | 2025-2035 Growth | How to win | What to watch out |
|---|---|---|---|
| USA | 12.5% | Provide innovation-focused solutions | Highest prevalence rates and strong immunotherapy adoption |
| China | 12.0% | Lead with awareness-building positioning | Growing westernization and food allergy recognition |
| Japan | 11.4% | Deliver safety-focused technologies | Increasing diagnosis rates and specialty care access |
| Germany | 10.9% | Maintain evidence-based positioning | Strong allergy care infrastructure and reimbursement support |
| India | 10.3% | Offer accessible treatment strategies | Rising urbanization and allergy prevalence growth |
| Brazil | 9.8% | Push education-focused programs | Emerging allergy awareness and specialty care development |
| UK | 9.1% | Provide guideline-compliant solutions | NHS integration and allergy service expansion |
USA Drives Fastest Market Growth
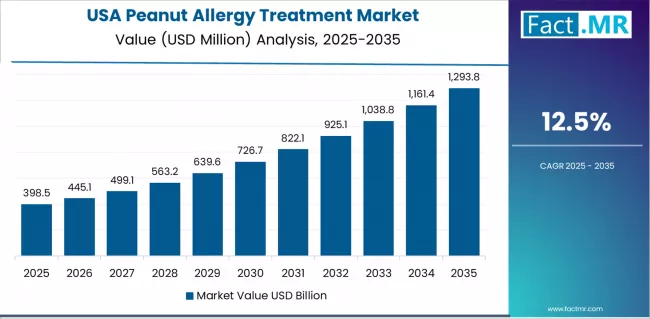
USA establishes fastest market growth through progressive food allergy awareness expansion and comprehensive oral immunotherapy infrastructure development, positioning peanut allergy treatment technologies as essential disease management solutions in allergy specialty practices and pediatric care facilities.
The country's 12.5% growth rate reflects highest prevalence rates combined with strong immunotherapy adoption supporting innovative therapy development that encourages the deployment of novel treatment products in diverse clinical settings. Growth concentrates in major allergy centers, including Boston, New York, and San Francisco, where allergists showcase increasing capacity for oral immunotherapy protocol implementation that appeals to evidence-focused practitioners demanding FDA-approved therapies and comprehensive safety monitoring.
American allergists are developing standardized desensitization protocols that combine approved oral immunotherapy products with clinical trial participation partnerships, including biologics combination therapy expansion and preventative intervention research growth. Distribution channels through specialty pharmacy networks and hospital allergy clinics expand market access, while patient advocacy organization initiatives support adoption across diverse family types and allergy severity specializations.
China Emerges as Food Allergy Awareness Leader
In Beijing, Shanghai, and Guangzhou regions, allergy clinics and pediatric hospitals are adopting peanut allergy treatment technologies as essential care tools for food allergy management operations, driven by growing westernization patterns combined with food allergy recognition supporting diagnostic capacity development that emphasizes the importance of emergency preparedness systems.
The market holds a 12.0% growth rate, supported by urbanization acceleration and dietary pattern changes that promote treatment adoption for safety-oriented applications. Chinese allergists are favoring epinephrine auto-injector systems that provide comprehensive patient training and emergency response guidance, particularly appealing in urban healthcare markets where food allergy awareness and international treatment standards represent critical care factors.
Market expansion benefits from substantial allergy education investment and specialty clinic establishment that enable emerging adoption of international standard allergy management for diverse pediatric applications. Healthcare system adoption follows patterns established in chronic disease management excellence, where patient education advantages and emergency preparedness documentation drive physician confidence and treatment protocol implementation.
Japan Shows Diagnostic Capacity Integration
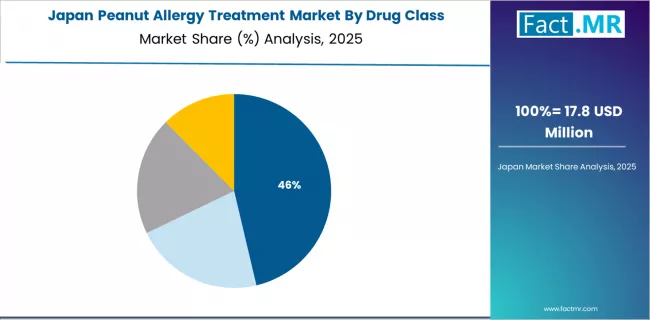
Japan's advancing healthcare market demonstrates progressive peanut allergy treatment adoption with documented diagnosis emphasis in allergy testing expansion and specialty care access through comprehensive pediatric allergy departments and university hospitals. The country maintains a 11.4% growth rate, driven by increasing diagnosis rates combined with specialty care access supporting clinical infrastructure development.
Major healthcare regions, including Tokyo, Osaka, and Nagoya, showcase diagnosis-driven treatment approaches where peanut allergy therapies integrate with established Japanese pediatric cultures and comprehensive allergy assessment programs to optimize patient safety and maintain care quality standards under growing food allergy recognition.
Japanese allergists prioritize comprehensive diagnostic validation and extensive safety monitoring in treatment selection, creating demand for proven products with validated characteristics, including clinical trial data, safety profiles, and emergency response documentation. The market benefits from established pediatric allergy sectors and increasing food allergy awareness that provide treatment positioning opportunities and maintain alignment with Japanese healthcare quality standards.
Germany Shows Evidence-Based Care Leadership
Germany's sophisticated healthcare market demonstrates established peanut allergy treatment adoption with documented evidence emphasis in clinical guideline adherence and allergy specialty care through comprehensive university hospital allergy departments and specialized pediatric practices. The country leverages strong allergy care infrastructure combined with reimbursement support approaches to maintain a 10.9% growth rate.
Premium allergy centers, including Bavaria, North Rhine-Westphalia, and Baden-Württemberg, showcase guideline-driven priorities where peanut allergy treatments integrate with established German allergy cultures and comprehensive safety practices to optimize patient outcomes and ensure appropriate therapy monitoring.
German allergists prioritize comprehensive clinical evidence and extensive safety documentation in therapy adoption, creating demand for approved products with thorough characteristics, including European regulatory clearance, clinical study publications, and long-term safety data. The market benefits from established allergy specialty segments and evidence-based medicine tradition that provide premium positioning opportunities and compliance with strict German healthcare regulations.
India Shows Urbanization-Driven Growth
India's expanding healthcare market demonstrates progressive peanut allergy treatment adoption with documented urbanization emphasis in lifestyle disease emergence and allergy recognition through emerging allergy clinics and pediatric specialty facilities. The country maintains a 10.3% growth rate, driven by rising urbanization combined with allergy prevalence growth supporting healthcare awareness development.
Major metropolitan regions, including Mumbai, Delhi, and Bangalore, showcase lifestyle-driven allergy approaches where peanut allergy treatments integrate with established westernization patterns and growing specialty care access to optimize emergency preparedness and maintain patient safety under dietary transition pressures.
Indian allergists prioritize comprehensive patient education and extensive emergency planning in treatment implementation, creating demand for accessible products with proven characteristics, including affordable pricing, clear instructions, and training support availability. The market benefits from established pediatric care sectors and growing middle-class health consciousness that provide treatment positioning opportunities and maintain alignment with Indian healthcare accessibility goals.
Brazil Shows Allergy Awareness Integration
Brazil's developing healthcare market demonstrates emerging peanut allergy treatment adoption with documented awareness emphasis in allergy recognition campaigns and specialty care establishment through comprehensive pediatric hospitals and emerging allergy practices. The country maintains a 9.8% growth rate, reflecting emerging allergy awareness combined with specialty care development supporting clinical capacity building.
Major healthcare regions, including São Paulo, Rio de Janeiro, and Brasília, showcase education-driven growth approaches where peanut allergy treatments integrate with established pediatric cultures and comprehensive family education programs to optimize safety preparedness and maintain healthcare quality standards under growing food allergy diagnosis.
Brazilian healthcare providers prioritize comprehensive family education and extensive emergency training in treatment delivery, creating demand for supportive products with validated characteristics, including Portuguese-language materials, training videos, and affordability documentation. The market benefits from established pediatric healthcare sectors and expanding allergy specialty recognition that provide treatment positioning opportunities and maintain alignment with Brazilian healthcare development standards.
UK Shows Healthcare Integration Leadership
UK's comprehensive healthcare market demonstrates established peanut allergy treatment adoption with documented NHS integration emphasis in allergy service provision and emergency preparedness through National Health Service allergy clinics and pediatric departments. The country maintains a 9.1% growth rate, reflecting NHS integration combined with allergy service expansion supporting standardized care delivery.
Key healthcare regions, including England, Scotland, and Wales, showcase system-driven priorities where peanut allergy treatments integrate with established NHS frameworks and comprehensive clinical pathways to optimize patient access and maintain care standards under healthcare service expansion initiatives.
The allergists prioritize comprehensive guideline adherence and extensive patient safety in treatment utilization, creating demand for approved products with validated characteristics, including NICE guidance compatibility, NHS formulary inclusion, and cost-effectiveness documentation. The market benefits from established NHS allergy services and patient advocacy influence that provide treatment positioning opportunities and maintain alignment with UK healthcare quality standards.
Europe Market Split by Country

The peanut allergy treatment market in Europe is projected to grow from USD 0.1 billion in 2025 to USD 0.4 billion by 2035, representing 24.0% of the global market in 2025 and expanding to 22.2% by 2035. Germany is expected to maintain its leadership position with USD 0.04 billion in 2025, accounting for 26.8% of the European market, supported by its advanced allergy care infrastructure and established specialty pharmacy networks.
France follows with USD 0.03 billion, representing 18.9% of the European market in 2025, driven by comprehensive pediatric allergy integration and specialty care concentration. UK holds USD 0.03 billion with 17.3% market share through established NHS allergy service acceptance and emergency preparedness density.
Italy commands USD 0.02 billion representing 14.2% share, while Spain accounts for USD 0.02 billion or 11.6% in 2025. The rest of Europe region maintains USD 0.02 billion, representing 11.2% of the European market, attributed to increasing peanut allergy treatment adoption in Nordic countries and emerging Eastern European allergy care sectors implementing food allergy management programs.
Competitive Landscape of the Peanut Allergy Treatment Market
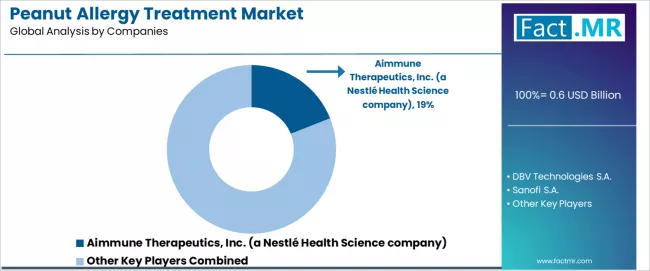
The peanut allergy treatment market exhibits a moderately consolidated competitive structure with approximately 15-25 active players operating across global pharmaceutical development networks and specialty therapy portfolios. Aimmune Therapeutics maintains market leadership at a 19.0% share, reflecting strong product positioning with oral immunotherapy innovation and sophisticated clinical development strategies.
This competitive landscape demonstrates the emerging maturation of peanut allergy therapeutics, where established players leverage regulatory approval advantages, extensive clinical trial documentation, and specialty pharmacy relationship programs to maintain positions, while emerging biotech companies and academic spinouts create innovation opportunities through novel mechanism offerings and biologics combination strategies.
Market leadership is maintained through several critical competitive advantages extending beyond development capabilities and product portfolios. Global regulatory expertise networks enable leading players to navigate complex FDA and EMA requirements and access varied customer segments including allergists, pediatricians, and specialty pharmacies.
Clinical development infrastructure and key opinion leader engagement program availability represent crucial differentiators in allergy therapeutic categories, where decades of immunotherapy expertise, safety monitoring protocols, and patient support frameworks create prescribing preference among evidence-focused allergists.
Manufacturing efficiency in oral immunotherapy production, supply chain patient kit management, and dose escalation packaging control separate major suppliers from smaller competitors, while comprehensive safety documentation addressing phase III trial results, real-world evidence, and REMS program compliance strengthen market position and physician confidence.
The market demonstrates emerging differentiation opportunities in biologics combination categories and epicutaneous patch technologies, where traditional oral immunotherapy faces competition from innovation-focused entrants offering enhanced safety advantages.
Significant competitive advantages persist in established epinephrine categories through comprehensive emergency preparedness portfolios and patient familiarity depth. Premium positioning strategies with FDA-approved oral immunotherapy and comprehensive patient support capabilities command margin premiums through superior clinical evidence and desensitization outcome assurance.
Specialized therapeutic portfolios combining oral immunotherapy with anti-IgE biologics create comprehensive positioning that justifies higher price points beyond generic epinephrine competition. Integrated allergy management solution offerings emphasizing patient education support, unified specialty pharmacy services, and cross-indication expertise programs generate physician loyalty and treatment preferences beyond transactional prescription writing.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Global pharmaceutical corporations | Comprehensive development resources; distribution networks; regulatory expertise | Market access; physician relationships; clinical trial capacity; manufacturing scale | Innovation agility; patient intimacy; niche populations; specialty focus |
| Biotech innovators | Novel mechanism development; IP protection; clinical innovation | Technology differentiation; scientific credibility; academic partnerships; FDA breakthrough status | Commercial infrastructure; specialty pharmacy; market access; profitability timeline |
| Generic manufacturers | Cost-competitive production; established distribution; volume capacity | Price positioning; market penetration; auto-injector manufacturing; formulary access | Innovation leadership; brand differentiation; premium positioning; clinical research |
| Specialty pharmacies | Patient dispensing; insurance coordination; adherence support | Service expertise; patient relationships; reimbursement navigation; therapy management | Product development; clinical research; manufacturing capacity; global expansion |
| Academic medical centers | Clinical research; protocol development; patient recruitment | Scientific credibility; KOL influence; investigator networks; publication record | Commercial operations; manufacturing capacity; market access; profit orientation |
Key Players in the Peanut Allergy Treatment Market
- Aimmune Therapeutics, Inc. (a Nestlé Health Science company)
- DBV Technologies S.A.
- Sanofi S.A.
- ALK-Abelló A/S
- Prota Therapeutics Pty Ltd
- Camallergy Ltd
- Aravax Pty Ltd
- Genentech, Inc. (a member of the Roche Group)
- Regeneron Pharmaceuticals, Inc.
- Stallergenes Greer Ltd.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 0.6 billion |
| Drug Class | Epinephrine, Antihistamines, Immunotherapies, Others |
| Route of Administration | Injectable, Oral, Others |
| Distribution Channel | Hospital Pharmacy, Retail Pharmacy, Others |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | USA, China, Japan, Germany, India, Brazil, UK, and 15+ additional countries |
| Key Companies Profiled | Aimmune Therapeutics, DBV Technologies, Sanofi, ALK-Abelló, Prota Therapeutics, Camallergy, Aravax, Genentech |
| Additional Attributes | Dollar sales by drug class, route, and distribution channel categories, regional adoption trends across North America, Asia Pacific, and Europe, competitive landscape with established pharmaceutical corporations and emerging biotech innovators, physician preferences for epinephrine methodologies and oral immunotherapy protocols, integration with specialty pharmacy operations and allergy clinic facilities, innovations in biologics combination therapies and epicutaneous delivery systems, and development of sophisticated desensitization platforms with enhanced safety profiles and comprehensive patient support frameworks. |
Peanut Allergy Treatment Market by Segments
-
Drug Class :
- Epinephrine
- Antihistamines
- Immunotherapies
- Others
-
Route of Administration :
- Injectable
- Oral
- Others
-
Distribution Channel :
- Hospital Pharmacy
- Retail Pharmacy
- Others
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Drug Class
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Drug Class, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Drug Class, 2025 to 2035
- Epinephrine
- Antihistamines
- Immunotherapies
- Others
- Y to o to Y Growth Trend Analysis By Drug Class, 2020 to 2024
- Absolute $ Opportunity Analysis By Drug Class, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Route of Administration
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Route of Administration, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Route of Administration, 2025 to 2035
- Injectable
- Oral
- Others
- Y to o to Y Growth Trend Analysis By Route of Administration, 2020 to 2024
- Absolute $ Opportunity Analysis By Route of Administration, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Distribution Channel
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Distribution Channel, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Distribution Channel, 2025 to 2035
- Hospital Pharmacy
- Retail Pharmacy
- Others
- Y to o to Y Growth Trend Analysis By Distribution Channel, 2020 to 2024
- Absolute $ Opportunity Analysis By Distribution Channel, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Drug Class
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Drug Class
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Drug Class
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Drug Class
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Drug Class
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Drug Class
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Drug Class
- By Route of Administration
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Route of Administration
- By Distribution Channel
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Drug Class
- By Route of Administration
- By Distribution Channel
- Competition Analysis
- Competition Deep Dive
- Aimmune Therapeutics, Inc. (a Nestlé Health Science company)
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- DBV Technologies S.A.
- Sanofi S.A.
- ALK-Abelló A/S
- Prota Therapeutics Pty Ltd
- Camallergy Ltd
- Aravax Pty Ltd
- Genentech, Inc. (a member of the Roche Group)
- Regeneron Pharmaceuticals, Inc.
- Stallergenes Greer Ltd.
- Aimmune Therapeutics, Inc. (a Nestlé Health Science company)
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Route of Administration, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Drug Class
- Figure 6: Global Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Route of Administration
- Figure 9: Global Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Distribution Channel
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Drug Class
- Figure 26: North America Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Route of Administration
- Figure 29: North America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Distribution Channel
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Drug Class
- Figure 36: Latin America Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Route of Administration
- Figure 39: Latin America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Distribution Channel
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Drug Class
- Figure 46: Western Europe Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Route of Administration
- Figure 49: Western Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by Distribution Channel
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Drug Class
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Route of Administration
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by Distribution Channel
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Drug Class
- Figure 66: East Asia Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Route of Administration
- Figure 69: East Asia Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by Distribution Channel
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Drug Class
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Route of Administration
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by Distribution Channel
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Drug Class
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Route of Administration, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Route of Administration, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Route of Administration
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by Distribution Channel
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the peanut allergy treatment market in 2025?
The global peanut allergy treatment market is estimated to be valued at USD 0.6 billion in 2025.
What will be the size of peanut allergy treatment market in 2035?
The market size for the peanut allergy treatment market is projected to reach USD 1.8 billion by 2035.
How much will be the peanut allergy treatment market growth between 2025 and 2035?
The peanut allergy treatment market is expected to grow at a 11.6% CAGR between 2025 and 2035.
What are the key product types in the peanut allergy treatment market?
The key product types in peanut allergy treatment market are epinephrine, antihistamines, immunotherapies and others.
Which route of administration segment to contribute significant share in the peanut allergy treatment market in 2025?
In terms of route of administration, injectable segment to command 61.7% share in the peanut allergy treatment market in 2025.