Endoluminal Device Systems Market
Endoluminal Device Systems Market Forecast By indication (Brain Aneurysm ,Abdominal Aortic Aneurysm ,Thoracic aortic Aneurysm) By end user (Hospitals ,Ambulatory Surgical Centers ,Specialty Clinics ,Catheterization labs) - Global Market Insights 2025 to 2035
Analysis of Endoluminal Device Systems market covering 30+ countries including analysis of US, Canada, UK, Germany, France, Nordics, GCC countries, Japan, Korea and many more
Endoluminal Device Systems Market Outlook 2025 to 2035
The endoluminal device systems market is projected to grow from USD 2.4 billion in 2025 to USD 4.2 billion by 2035, reflecting a CAGR of 5.8%. Minimally invasive gastrointestinal procedures, obesity management, and early-stage oncology interventions fuel demand. Advancements in endoscopic technologies and reimbursement expansion support broader clinical adoption worldwide.
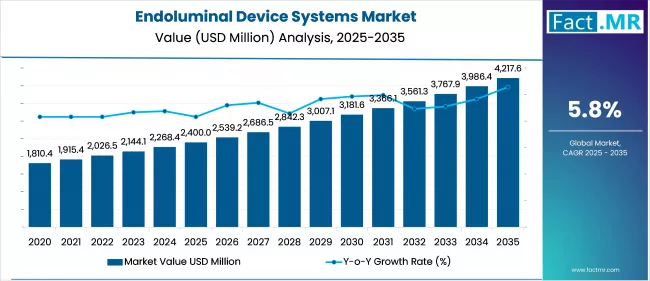
| Metric | Value |
|---|---|
| Endoluminal Device Systems Market Estimated Value in (2025 E) | USD 2400.0 million |
| Endoluminal Device Systems Market Forecast Value in (2035 F) | USD 4217.6 million |
| Forecast CAGR (2025 to 2035) | 5.8% |
Endoluminal device systems is a type of medical device that majorly use to treat different type aneurysms in the vessel of brain and other arteries. Endoluminal device systems uses a permanent metal wire that provide support in the aneurysm area of the blood vessel to reduce or stop the blood flow into the aneurysm.
Endoluminal device systems consist of self-expanding stent of different diameter that releases from the delivery system once it is placed in the point of aneurysm. The implant have dual layer integrated coverage that is designed to divert the blood flow away from the aneurysmal area in the artery.
Endoluminal device systems is available in different diameters ranging from 2.5 mm to 5.5 mm and in different implant length ranging from 13 mm to 50 mm depending upon the size and diameter of the vessel.
| Countries | CAGR |
|---|---|
| U.S. | 3.8% |
| China | 4.8% |
| Germany | 5.6% |
| Italy | 4.0% |
Endoluminal Device Systems Market: Drivers and Restraints
Rising awareness and adoption of minimally invasive procedure, increase in number of successful implants throughout the globe is the major growth driver of endoluminal device systems market. Moreover, the rapid increase in the prevalence of asymptomatic vascular diseases among people also contribute to progress the endoluminal device systems market.
However the availability of treatment substitute, device failure and recalls are the major factor that hinders the growth of this market. Moreover, rising price regulations on stent implants by key regulatory authorities may also hamper endoluminal device systems market during the forecast period.
Endoluminal Device Systems Market: Overview
The endoluminal device systems market is expected to grow exponentially over the forecast period due to rising prevalence of asymptomatic aneurysm among the population and shift of people towards minimally invasive procedures.
WHO has estimated the global crude incidence for aneurysmal subarachnoid hemorrhage from 0.71 to 12.38 per 100,000 people. Flow re-direction endoluminal device has been recently added in clinical practice for the treatment of brain aneurysm.
This device is a stent-like structure that divert the flow away from the aneurysm may hold the major share over the forecast period. Also, every year around 30,000 patients in U.S. suffers from ruptured cerebral aneurysm, and around 6 percent of population have an un-ruptured cerebral aneurysm as per the data revealed by American Association of Neurological Surgeons.
Endoluminal Device Systems Market: Regional Overview
Endoluminal device systems market is dominated by U.S. and Europe due to availability of favorable reimbursement in these region, acceptance of technologically advanced devices and well developed infrastructure. After U.S. and Europe global endoluminal device systems market is then followed by Japan and China due to rising number of patients suffering from aneurysmal disease due to sedentary life style.
Countries like India, South Africa and Australia are the emerging countries for endoluminal device systems market. The endoluminal device systems market for these countries is expected to show exponential growth during the forecast period. Lack of funding by government and slow growth for healthcare sector in MEA and Latin America may impede the growth of endoluminal device systems market in these countries.
Endoluminal Device Systems Market: Key Players
Currently only Terumo Corporation is having FDA and EMA approval for manufacturing endoluminal device system. Other manufacturer such as Cardinal Health, Medtronic plc, Cardiatis SA, Endologix, Cook Medical LLC, Boston Scientific Corporation, biFlow Medical Ltd, Endoluminal Science Pvt Ltd, Braile Biomedica, Getinge AB, Lemaitre Vascular Inc. are developing endoluminal device system.
The research report on endoluminal device systems Market presents a comprehensive assessment of the market and contains thoughtful insights, facts, historical data, and statistically supported and industry-validated market data. It also contains projections using a suitable set of assumptions and methodologies.
The research report on Surgical Table Accessories market provides analysis and information according to market segments such as geographies, application, and industry.
The report covers exhaust analysis on Endoluminal Device Systems Market
- Market Segments
- Market Dynamics
- Market Size
- Supply & Demand
- Current Trends/Issues/Challenges
- Competition & Companies involved
- Technology
- Value Chain
Report on Endoluminal Device Systems market includes regional analysis
- North America (U.S., Canada)
- Latin America (Mexico. Brazil)
- Europe (Germany, Italy, France, U.K, Spain, , Russia)
- East Asia (China, Japan, South Korea)
- South Asia (India, ASEAN)
- Oceania (Australia, New Zealand)
- Middle East and Africa (GCC Countries, South Africa, Northern Africa)
The report on Surgical Table Accessories market is a compilation of first-hand information, qualitative and quantitative assessment by industry analysts, inputs from industry experts and industry participants across the value chain. The report provides in-depth analysis of parent market trends, macro-economic indicators and governing factors along with market attractiveness as per segments. The report also maps the qualitative impact of various market factors on market segments and geographies.
Report on Endoluminal Device Systems market highlights
- Detailed overview of parent market
- Changing market dynamics in the industry
- In-depth market segmentation
- Historical, current, and projected market size in terms of volume and value
- Recent industry trends and developments
- Competitive landscape
- Strategies of key players and products offered
- Potential and niche segments, geographical regions exhibiting promising growth
- A neutral perspective on market performance
- Must-have information for market players to sustain and enhance their market footprint
Endoluminal Device Systems Market: Segmentation
The global endoluminal device systems market is classified on the basis of indication, end and region
Based on indication, endoluminal device systems market is segmented into following:
- Brain Aneurysm
- Abdominal Aortic Aneurysm
- Thoracic aortic Aneurysm
Based on end user, endoluminal device systems market is segmented into following
- Hospitals
- Ambulatory Surgical Centers
- Specialty Clinics
- Catheterization labs
Table of Content
- Executive Summary
- Global Market Outlook
- Demand-side Trends
- Supply-side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Inclusion and Exclusion
- Value Added Insights
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Supply Side Participants and their Roles
- Producers
- Mid-Level Participants (Traders/ Agents/ Brokers)
- Wholesalers and Distributors
- Value Added and Value Created at Node in the Supply Chain
- List of Raw Material Suppliers
- List of Existing and Potential Buyers
- Supply Side Participants and their Roles
- Pricing Analysis of Market
- Pricing Analysis By Region
- Pricing Analysis By Country
- Pricing Analysis By End Use
- Pricing Analysis By Distributor
- Pricing Analysis By Application
- Value Chain Analysis
- Profit Margin Analysis
- Wholesalers and Distributors
- Retailers
- Product Portfolio Analysis
- Regulatory Landscape
- By Key Regions
- By Key Countries
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Investment Feasibility Matrix
- PESTLE Analysis
- Political Factors
- Government stability and change
- Taxation policy and incentives
- Trade tariffs and import/export regulations
- Political risk and corruption levels
- Economic Factors
- GDP growth and economic cycles
- Inflation and interest rates
- Exchange rate fluctuations
- Unemployment and consumer spending power
- Social Factors
- Demographic trends (age, population growth)
- Cultural attitudes and lifestyle changes
- Education levels and workforce skills
- Health consciousness and social values
- Technological Factors
- Rate of technological innovation
- R&D activity and automation trends
- Digital infrastructure and connectivity
- Intellectual property protection
- Legal Factors
- Employment and labor laws
- Health & safety regulations
- Consumer protection laws
- Data privacy and antitrust legislation
- Environmental Factors
- Environmental regulations and compliance
- Climate change impacts and carbon footprint
- Resource availability and sustainability practices
- Waste management and recycling initiatives
- Political Factors
- Market Background and Dynamics
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- SWOT Analysis of Global Market
- Strengths
- Weaknesses
- Opportunities
- Threats
- Market Dynamics
- Global Market Analysis 2020-2024 and Forecast, 2025-2035
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Projections, 2025-2035
- Y-o-Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Revenue Forecast Scenario, 2020-2035
- Conservative Scenario
- Likely Scenario
- Optimistic Scenario
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis By Application, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By Application, 2025-2035
- Y-o-Y Growth Trend Analysis By Application, 2020-2024
- Absolute $ Opportunity Analysis By Application, 2025-2035
- Market Attractiveness Analysis By Application, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis By End Use, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By End Use, 2025-2035
- Y-o-Y Growth Trend Analysis By End Use, 2020-2024
- Absolute $ Opportunity Analysis By End Use, 2025-2035
- Market Attractiveness Analysis By End Use, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Region
- Introduction
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis By Region, 2020-2024
- Current Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By Region, 2025-2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific & Pacific
- MEA
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020-2024 and Forecast 2025-2035
- Introduction
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By Market Taxonomy, 2025-2035
- By Country
- USA
- Canada
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- USA Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Canada Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Mexico Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- USA Market
- Latin America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Brazil
- Argentina
- Chile
- Rest of Latin America
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Brazil Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Argentina Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Chile Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of Latin America Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Brazil Market
- Western Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Germany Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- UK Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Italy Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Spain Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- France Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Nordic Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- BENELUX Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of Western Europe Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Germany Market
- Eastern Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Russia Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Poland Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Hungary Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Balkan & Baltic Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of Eastern Europe Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Russia Market
- East Asia Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- China
- Japan
- South Korea
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- China Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Japan Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- South Korea Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- China Market
- South Asia and Pacific Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- India Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- ASEAN Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Australia & New Zealand Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of South Asia and Pacific Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- India Market
- MEA Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Kingdom of Saudi Arabia Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Other GCC Countries Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Turkiye Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- South Africa Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- South Africa Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of MEA Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of MEA Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Kingdom of Saudi Arabia Market
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Region
- By Application
- By End Use
- Competition Analysis
- Competition Deep Dive
- Global Key Players
- Key Players by Region
- Key Players by Country
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (US$ Mn) Forecast by Region, 2020 to 2035
- Table 2: Global Market Volume (Units) Forecast by Region, 2020 to 2035
- Table 3: Global Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 4: Global Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 5: Global Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 6: Global Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 7: North America Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 8: North America Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 9: North America Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 10: North America Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 11: North America Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 12: North America Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 13: Latin America Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 14: Latin America Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 15: Latin America Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 16: Latin America Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 17: Latin America Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 18: Latin America Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 19: Europe Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 20: Europe Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 21: Europe Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 22: Europe Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 23: Europe Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 24: Europe Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 25: East Asia Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 26: East Asia Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 27: East Asia Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 28: East Asia Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 29: East Asia Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 30: East Asia Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 31: South Asia Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 32: South Asia Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 33: South Asia Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 34: South Asia Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 35: South Asia Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 36: South Asia Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 37: Oceania Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 38: Oceania Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 39: Oceania Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 40: Oceania Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 41: Oceania Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 42: Oceania Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 43: MEA Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 44: MEA Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 45: MEA Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 46: MEA Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 47: MEA Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 48: MEA Market Volume (Units) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 2: Global Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 3: Global Market Value (US$ Mn) by Region, 2020 to 2035
- Figure 4: Global Market Value (US$ Mn) Analysis by Region, 2020 to 2035
- Figure 5: Global Market Volume (Units) Analysis by Region, 2020 to 2035
- Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2020 to 2035
- Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2020 to 2035
- Figure 8: Global Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 9: Global Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 10: Global Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 11: Global Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 12: Global Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 13: Global Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 14: Global Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 15: Global Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 16: Global Market Attractiveness by Application, 2020 to 2035
- Figure 17: Global Market Attractiveness by End Use, 2020 to 2035
- Figure 18: Global Market Attractiveness by Region, 2020 to 2035
- Figure 19: North America Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 20: North America Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 21: North America Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 22: North America Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 23: North America Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 24: North America Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 25: North America Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 26: North America Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 27: North America Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 28: North America Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 29: North America Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 30: North America Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 31: North America Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 32: North America Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 33: North America Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 34: North America Market Attractiveness by Application, 2020 to 2035
- Figure 35: North America Market Attractiveness by End Use, 2020 to 2035
- Figure 36: North America Market Attractiveness by Country, 2020 to 2035
- Figure 37: Latin America Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 38: Latin America Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 39: Latin America Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 40: Latin America Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 41: Latin America Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 42: Latin America Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 43: Latin America Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 44: Latin America Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 45: Latin America Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 46: Latin America Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 48: Latin America Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 49: Latin America Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 50: Latin America Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 51: Latin America Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 52: Latin America Market Attractiveness by Application, 2020 to 2035
- Figure 53: Latin America Market Attractiveness by End Use, 2020 to 2035
- Figure 54: Latin America Market Attractiveness by Country, 2020 to 2035
- Figure 55: Europe Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 56: Europe Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 57: Europe Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 58: Europe Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 59: Europe Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 60: Europe Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 61: Europe Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 62: Europe Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 63: Europe Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 64: Europe Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 65: Europe Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 66: Europe Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 67: Europe Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 68: Europe Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 69: Europe Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 70: Europe Market Attractiveness by Application, 2020 to 2035
- Figure 71: Europe Market Attractiveness by End Use, 2020 to 2035
- Figure 72: Europe Market Attractiveness by Country, 2020 to 2035
- Figure 73: East Asia Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 74: East Asia Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 75: East Asia Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 76: East Asia Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 77: East Asia Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 78: East Asia Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 79: East Asia Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 80: East Asia Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 81: East Asia Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 82: East Asia Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 83: East Asia Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 84: East Asia Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 85: East Asia Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 86: East Asia Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 87: East Asia Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 88: East Asia Market Attractiveness by Application, 2020 to 2035
- Figure 89: East Asia Market Attractiveness by End Use, 2020 to 2035
- Figure 90: East Asia Market Attractiveness by Country, 2020 to 2035
- Figure 91: South Asia Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 92: South Asia Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 93: South Asia Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 94: South Asia Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 95: South Asia Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 96: South Asia Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 97: South Asia Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 98: South Asia Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 99: South Asia Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 100: South Asia Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 101: South Asia Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 102: South Asia Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 103: South Asia Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 104: South Asia Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 105: South Asia Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 106: South Asia Market Attractiveness by Application, 2020 to 2035
- Figure 107: South Asia Market Attractiveness by End Use, 2020 to 2035
- Figure 108: South Asia Market Attractiveness by Country, 2020 to 2035
- Figure 109: Oceania Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 110: Oceania Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 111: Oceania Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 112: Oceania Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 113: Oceania Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 114: Oceania Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 115: Oceania Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 116: Oceania Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 117: Oceania Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 118: Oceania Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 119: Oceania Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 120: Oceania Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 121: Oceania Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 122: Oceania Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 123: Oceania Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 124: Oceania Market Attractiveness by Application, 2020 to 2035
- Figure 125: Oceania Market Attractiveness by End Use, 2020 to 2035
- Figure 126: Oceania Market Attractiveness by Country, 2020 to 2035
- Figure 127: MEA Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 128: MEA Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 129: MEA Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 130: MEA Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 131: MEA Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 132: MEA Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 133: MEA Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 134: MEA Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 135: MEA Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 136: MEA Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 137: MEA Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 138: MEA Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 139: MEA Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 140: MEA Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 141: MEA Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 142: MEA Market Attractiveness by Application, 2020 to 2035
- Figure 143: MEA Market Attractiveness by End Use, 2020 to 2035
- Figure 144: MEA Market Attractiveness by Country, 2020 to 2035