Biosimilar Market
Biosimilar Market Size and Share Forecast Outlook 2025 to 2035
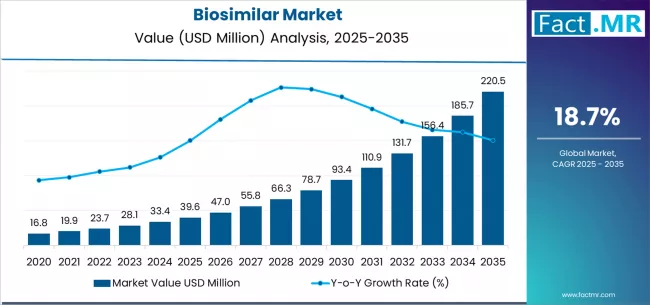
Biosimilar market is projected to grow from USD 39.6 million in 2025 to USD 220.5 million by 2035, at a CAGR of 18.7%. Monoclonal Antibodies will dominate with a 45.0% market share, while autoimmune disorders will lead the indication segment with a 40.0% share.
Biosimilar Market Forecast and Outlook 2025 to 2035
The global biosimilar market is projected to grow from USD 39.6 million in 2025 to approximately USD 220.5 million by 2035, expanding at a compound annual growth rate (CAGR) of 18.7% between 2025 and 2035.
The market is positioned for exceptional expansion, driven by accelerating patent expiry of originator biologics, expanding healthcare cost containment initiatives, and increasing regulatory approval of biosimilar monoclonal antibodies across hospital systems, specialty pharmacy networks, and healthcare payer organizations globally.
Quick Stats on Biosimilar Market
- Biosimilar Market Value (2025): USD 39.6 million
- Biosimilar Market Forecast Value (2035): USD 220.5 million
- Biosimilar Market Forecast CAGR (2025 to 2035): 18.7%
- Leading Drug Class in Biosimilar Market: Monoclonal Antibodies (45.0%)
- Leading Indication in Biosimilar Market: Autoimmune Disorders (40.0%)
- Leading End Use in Biosimilar Market: Hospitals
- Key Growth Regions in Biosimilar Market: North America, Europe, and Asia Pacific
- Key Players in Biosimilar Market: Amgen, Roche, Sandoz, Dr. Reddy's, Teva, Pfizer, Samsung Bioepis, Biocon, Viatris, Celltrion Healthcare
The market demonstrates extraordinary fundamentals supported by growing adoption of biosimilar therapies, healthcare providers' focus on treatment accessibility optimization and rising recognition of biosimilar as essential therapeutic alternatives in achieving cost-effective patient care, expanded treatment access, and sustainable healthcare system economics within modern pharmaceutical landscapes across diverse therapeutic applications.
Market growth is underpinned by regulatory framework maturation, particularly FDA biosimilar pathway establishment and EMA biosimilar guideline implementation, which provide validated approval mechanisms, comprehensive safety assessment protocols, and superior clinical interchangeability confidence compared to initial biosimilar generation prevalent in early adoption periods.
Healthcare systems and pharmaceutical payers increasingly prioritize biosimilar utilization that delivers optimal balance between clinical equivalence, cost savings potential, and patient access expansion while adhering to increasingly sophisticated biosimilar adoption policies and formulary management requirements across global healthcare markets.
The convergence of blockbuster biologic patent expiration in developed healthcare markets, biosimilar manufacturing capacity expansion in emerging production regions, and healthcare cost pressure intensification in public health systems creates multifaceted growth opportunities for biosimilar manufacturers and pharmaceutical distribution organizations.
Advanced biosimilar development technologies incorporating analytical characterization platforms, comparative immunogenicity assessment, and therapeutic equivalence demonstration protocols are improving regulatory approval success rates, physician adoption confidence, and patient acceptance across pharmaceutical applications.
The market's trajectory reflects the pharmaceutical industry's strategic pivot toward biosimilar portfolio development following originator biologic patent losses, the expanding role of specialty pharmacy channels in biosimilar distribution optimization, and the growing healthcare system integration of value-based procurement strategies requiring cost-effective biologic alternatives.
Chronic disease management programs and specialty medication access initiatives are establishing comprehensive frameworks for biosimilar adoption advancement, while interchangeability designation achievement enables seamless pharmacy-level substitution connectivity across global healthcare delivery operations.
Between 2020 and 2025, the biosimilar market expanded from USD 14.5 million to USD 39.6 million, demonstrating exceptional foundational growth driven by landmark regulatory approvals including adalimumab and trastuzumab biosimilar, increasing healthcare system adoption initiatives, and initial deployment of specialty pharmacy distribution infrastructure supporting biosimilar utilization across hospital-based infusion centers and specialty medication programs. This growth phase established biosimilar acceptance precedents, validated regulatory approval pathways, and created comprehensive stakeholder education networks supporting global pharmaceutical biosimilar integration.
From 2025 to 2030, the market is projected to accelerate substantially, reaching USD 93.2 million, representing a critical inflection point as multiple blockbuster biologic patents expire including major oncology and immunology franchises, as interchangeability designations become more prevalent enabling automatic substitution, and as value-based procurement mandates drive healthcare system biosimilar preference. This period will be characterized by expanded therapeutic area coverage, enhanced physician comfort with biosimilar prescribing, and widespread implementation of biosimilar-preferring formulary policies across major payer organizations.
From 2030 to 2035, the market is forecast to reach USD 220.5 million, driven by mature biosimilar market penetration in developed regions achieving substantial originator displacement, emerging market biosimilar manufacturing capacity enabling global supply expansion, and comprehensive healthcare system integration of biosimilar-first treatment protocols. The growing portfolio of interchangeable biosimilar, expansion into complex biologic mechanisms including novel monoclonal antibody targets, and integration of biosimilar options across all major therapeutic categories will drive demand for comprehensive biosimilar product portfolios with proven clinical equivalence and established real-world safety profiles.
Biosimilar Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value (2025E) | USD 39.6 million |
| Forecast Value (2035F) | USD 220.5 million |
| Forecast CAGR (2025 to 2035) | 18.7% |
Why is the Biosimilar Market Growing?
Market expansion is being supported by the unprecedented wave of biologic patent expiries and the corresponding healthcare system imperative for cost-effective therapeutic alternatives in specialty medication management across global pharmaceutical markets. Modern healthcare organizations face unsustainable specialty drug expenditure growth requiring biosimilar adoption to maintain treatment access while controlling pharmaceutical budgets.
The proven clinical equivalence of approved biosimilar through rigorous comparative studies and extensive post-marketing surveillance makes them essential components of comprehensive specialty pharmacy strategies and value-based care implementation programs. The growing emphasis on healthcare cost containment and treatment access expansion is driving demand for biosimilar products that deliver therapeutic equivalence at substantially reduced acquisition costs enabling expanded patient access.
Healthcare payers' requirement for cost-effective specialty medication alternatives that maintain clinical outcomes while reducing pharmaceutical spending creates opportunities for biosimilar manufacturers establishing competitive portfolios and specialty distribution capabilities. The rising influence of interchangeability designations and automatic substitution policies is accelerating biosimilar adoption rates across different therapeutic categories and healthcare settings requiring proven biologic alternatives.
Opportunity Pathways - Biosimilar Market
The biosimilar market represents a transformative pharmaceutical opportunity, expanding from USD 39.6 million in 2025 to USD 220.5 million by 2035 at an 18.7% CAGR. As healthcare systems confront specialty medication cost crises and as patent expiries expose blockbuster biologics to biosimilar competition, these therapeutic alternatives have evolved from regulatory experiments to essential pharmaceutical strategies enabling sustainable specialty drug access, substantial healthcare system savings, and competitive pharmaceutical market dynamics across oncology treatment programs and autoimmune disease management applications.
The convergence of patent cliff acceleration for major biologics, regulatory pathway maturation enabling efficient approvals, manufacturing capacity expansion supporting supply reliability, and healthcare policy evolution mandating biosimilar consideration creates explosive demand momentum.
High-value monoclonal antibody biosimilar targeting blockbuster originators established insulin biosimilar platforms addressing diabetes treatment economics, and emerging oncology biosimilar expanding cancer care access will capture market leadership, while geographic expansion into high-growth Asian manufacturing regions and therapeutic diversification into novel biologic classes will enable market participation. Healthcare cost pressure universality and treatment access imperatives provide unprecedented growth foundation.
- Pathway A - Monoclonal Antibodies Drug Class Dominance: Leading with 45.0% market share, monoclonal antibody biosimilar drive primary demand through adalimumab, trastuzumab, rituximab, and bevacizumab biosimilar addressing blockbuster originator patents. High-value mAb biosimilar targeting multi-billion dollar originator franchises in immunology and oncology command substantial market opportunity from healthcare systems seeking cost savings while maintaining therapeutic efficacy. Expected revenue pool: USD 17.8-99.2 million.
- Pathway B - Autoimmune Disorders Indication Leadership: Dominating with 40.0% market share through rheumatoid arthritis, inflammatory bowel disease, and psoriasis biosimilar treatments, autoimmune applications serve largest biosimilar utilization reflecting high originator costs and chronic treatment requirements. This indication addresses both treatment access imperatives and cost containment objectives, making it the primary driver for biosimilar adoption and healthcare system savings. Opportunity: USD 15.8-88.2 million.
- Pathway C - Asian Manufacturing Emergence: China (20.5% CAGR) and India (19.4% CAGR) lead global growth through domestic biosimilar manufacturing capacity expansion, government biosimilar promotion policies, and cost-competitive production advantages. Strategic manufacturing infrastructure development in Asian markets, leveraging technical expertise and economic advantages, enables market leadership supporting both domestic healthcare needs and global supply diversification. Geographic expansion upside: USD 12.7-71.2 million.
- Pathway D - Hospital End-use Dominance: Hospitals commanding majority market share serve primary biosimilar administration setting for infusion therapies requiring clinical oversight and specialty medication management. Hospital-based specialty pharmacy integration, infusion center biosimilar adoption, and institutional formulary biosimilar preference maintain essential distribution channel from pharmaceutical manufacturers to patient treatment delivery. End-use revenue potential: USD 20.6-114.8 million.
- Pathway E - Interchangeability Designation & Automatic Substitution: Companies achieving FDA interchangeability designation enabling pharmacy-level substitution without prescriber intervention gain competitive advantages through simplified adoption and enhanced market access. Interchangeable biosimilar status eliminating prescription switching barriers and enabling automatic substitution captures substantial market share advantages and accelerated adoption trajectories. Regulatory differentiation premium: USD 7.9-44.1 million.
- Pathway F - Specialty Pharmacy Networks & Distribution Excellence: Specialized biosimilar distribution infrastructure, patient support program integration, and healthcare provider education services create competitive differentiation in complex specialty medication markets. Companies offering comprehensive distribution solutions, reimbursement support services, and clinical education programs gain preferred position with healthcare systems implementing biosimilar adoption initiatives. Distribution service value: USD 5.9-33.1 million.
- Pathway G - Therapeutic Expansion & Novel Biologic Biosimilar: Beyond established adalimumab and trastuzumab biosimilar, emerging opportunities in novel monoclonal antibody mechanisms, complex biologic structures, and combination therapy biosimilar represent growth frontiers. Companies developing next-generation biosimilar portfolios, establishing manufacturing for complex biologics, and capturing emerging therapeutic categories secure first-mover advantages in expanding markets. Portfolio diversification opportunity: USD 4.8-26.5 million.
Segmental Analysis
The market is segmented by drug class, indication, end use, and region. By drug class, the market is divided into monoclonal antibodies, growth factors & hematopoietic agents, insulin & analogues, osteoporosis/bone agents, and others.
Based on indication, the market is categorized into autoimmune disorders, oncology, diabetes mellitus, ophthalmic disorders, hematologic/rare disorders, and others. By end use, the market is segmented into hospitals, specialty pharmacies, and online & retail. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
How do Monoclonal Antibodies Drive Drug Class Leadership?
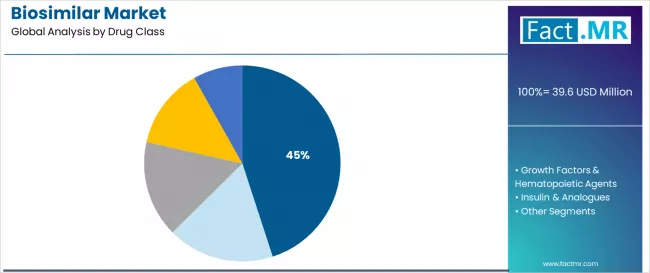
The monoclonal antibodies drug class is projected to account for 45.0% of the biosimilar market in 2025, establishing dominant position as the therapeutic category driving market growth. Healthcare systems and pharmaceutical manufacturers recognize that mAb biosimilar, targeting blockbuster originator products including Humira (adalimumab), Herceptin (trastuzumab), Rituxan (rituximab), and Avastin (bevacizumab), represent the highest-value biosimilar opportunities due to massive originator sales and substantial cost reduction potential.
This drug class addresses both healthcare affordability imperatives and treatment access expansion while providing clinically equivalent alternatives to originator biologics across immunology and oncology applications. This segment forms the foundation of biosimilar market economics and strategic pharmaceutical investment, as mAb biosimilar generate the most substantial healthcare system savings and pharmaceutical company revenues within the biosimilar landscape.
Regulatory approval precedents and extensive clinical switching studies continue strengthening healthcare provider confidence in mAb biosimilar among oncologists, rheumatologists, and gastroenterologists managing chronic autoimmune and cancer patients. With continuing patent expiries exposing additional blockbuster mAbs to biosimilar competition and growing real-world evidence supporting biosimilar safety and efficacy, monoclonal antibody biosimilar will maintain their central position driving overall market expansion throughout the forecast period.
Why do Autoimmune Disorders Command Majority Indication Share?
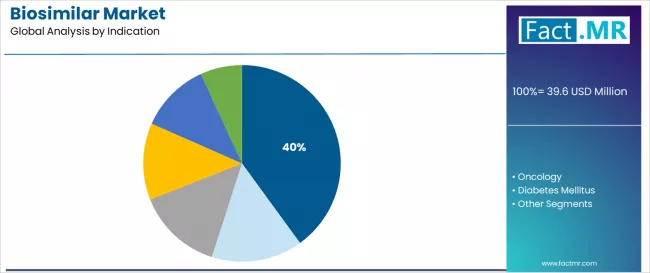
Autoimmune disorders indication is projected to represent 40.0% of biosimilar demand in 2025, underscoring its role as the primary therapeutic application driving biosimilar utilization. Healthcare providers recognize that autoimmune disease treatment requirements, encompassing rheumatoid arthritis, inflammatory bowel disease, psoriasis, and ankylosing spondylitis management, generate enormous specialty medication expenditures that biosimilar can substantially reduce while maintaining therapeutic efficacy.
Chronic autoimmune conditions requiring continuous long-term biologic therapy create sustained biosimilar demand addressing both patient access and healthcare system sustainability. The segment reflects the convergence of high originator biologic costs in rheumatology and gastroenterology, large patient populations requiring chronic treatment, and compelling healthcare economic arguments supporting biosimilar adoption in chronic disease management.
Clinical evidence demonstrating successful switching from originators to biosimilar in autoimmune populations and favorable real-world safety data continue supporting rheumatologist and gastroenterologist biosimilar prescribing confidence. As additional immunology biologics face patent expiration and as payer mandates increasingly require biosimilar utilization, autoimmune disorder applications will maintain their essential role driving biosimilar market growth within the global specialty pharmaceutical landscape.
What drives Hospitals End-use Segment Prominence?
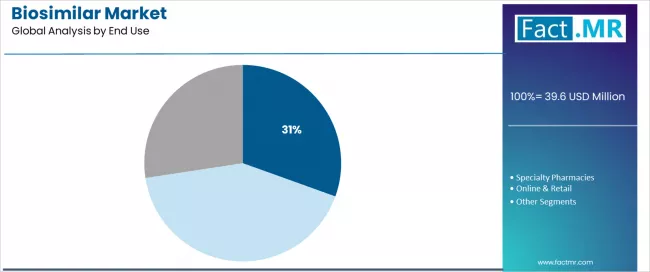
Hospitals account for about 30.5% of biosimilar distribution in 2025, reinforcing their position as the primary patient-care setting for administering these therapies. Healthcare delivery systems recognize that hospital environments, infusion centers, hospital pharmacies and inpatient units, remain the core locations for infused and injected biologics that require clinical supervision and specialized handling.
This channel supports the clinical oversight needed for specialty medications while also giving institutions stronger formulary control, which helps streamline biosimilar adoption. The segment highlights the central role hospital pharmacy departments play in product selection, formulary management and implementing therapeutic interchange protocols that shape institutional uptake.
Hospitals that prioritize biosimilars through formulary preferences and substitution policies continue to see meaningful reductions in pharmaceutical spending while maintaining clinical outcomes. As consolidation expands integrated delivery networks, and as value-based purchasing strengthens cost-containment priorities, hospital-based utilization will continue to function as the leading market channel within the global biosimilar distribution landscape.
What are the Drivers, Restraints, and Key Trends of the Biosimilar Market?
The biosimilar market is experiencing unprecedented growth due to fundamental pharmaceutical patent cycle dynamics and healthcare system economic imperatives requiring specialty medication cost management. However, the market faces challenges, including physician hesitancy regarding biosimilar prescribing in certain therapeutic areas despite regulatory approval, patient concerns about switching from established originator therapies to biosimilar alternatives, and originator manufacturer strategies including authorized generics and competitive pricing undermining biosimilar economic advantages. Continuous regulatory evolution and healthcare policy development continues influencing market dynamics and adoption trajectories.
Patent Cliff Acceleration and Blockbuster Biologic Exclusivity Loss
The accelerating pace of biologic patent expiries exposes multiple blockbuster franchises to biosimilar competition creating unprecedented market entry opportunities for biosimilar manufacturers.
Healthcare systems facing unsustainable specialty drug expenditure growth actively promote biosimilar adoption through formulary preference, prior authorization requirements favoring biosimilar, and value-based contracting arrangements incentivizing biosimilar utilization.
Patent expiry timing for major biologics including adalimumab, trastuzumab, bevacizumab, and rituximab creates concentrated biosimilar launch opportunities generating substantial healthcare system savings while establishing competitive biosimilar pharmaceutical markets.
Regulatory Pathway Maturation and Interchangeability Designation Achievement
Regulatory agencies' ongoing biosimilar pathway refinement including FDA interchangeability designation standards and EMA biosimilar guidance updates provide increasing clarity supporting pharmaceutical investment and healthcare system adoption confidence.
Interchangeability designation achievement enables pharmacy-level automatic substitution eliminating prescriber intervention requirements and substantially accelerating biosimilar adoption similarly to small molecule generic substitution practices.
Regulatory precedent establishment through approved biosimilar portfolios and post-marketing safety surveillance data demonstrating biosimilar safety profiles equivalent to originators continue reducing stakeholder concerns and accelerating market acceptance.
Analysis of the Biosimilar Market by Key Countries
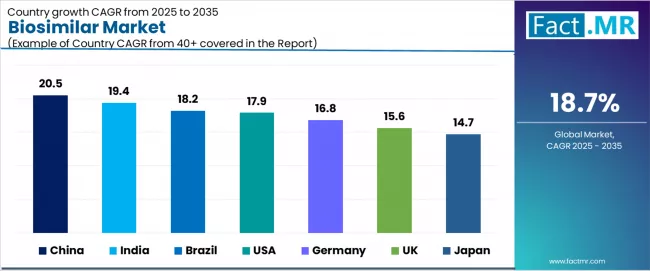
| Country | CAGR (2025-2035) |
|---|---|
| China | 20.5% |
| India | 19.4% |
| Brazil | 18.2% |
| usa | 17.9% |
| Germany | 16.8% |
| UK | 15.6% |
| Japan | 14.7% |
The biosimilar market is experiencing extraordinary growth globally, with China leading at a 20.5% CAGR through 2035, driven by government biosimilar promotion policies, domestic manufacturing capacity scaling, and healthcare cost containment imperatives across expanding public health insurance systems. India follows at 19.4%, supported by local biosimilar production capabilities, diabetes treatment burden growth, and pharmaceutical export market development.
Brazil records 18.2% growth, benefiting from hospital biosimilar adoption acceleration and oncology biosimilar market penetration. The USA demonstrates 17.9% growth, emphasizing new oncology biosimilar approvals and hospital system adoption expansion. Germany shows 16.8% growth, representing strong mAb biosimilar acceptance and favorable patent expiry pipeline. UK records 15.6% growth with NHS biosimilar adoption initiatives and broad clinical trial participation, while Japan shows 14.7% growth, representing rapid insulin biosimilar uptake and aging population demographics.
How does China Demonstrate Exceptional Market Potential with Manufacturing Scale?
The biosimilar market in China is projected to exhibit exceptional growth with a CAGR of 20.5% through 2035, driven by government strategic policies actively promoting biosimilar development and adoption combined with domestic pharmaceutical manufacturers' aggressive capacity expansion in biosimilar production infrastructure. The country's massive patient populations requiring chronic biologic therapy and government healthcare cost containment objectives create compelling biosimilar adoption imperatives supporting rapid market development.
Domestic biosimilar manufacturers including leading Chinese pharmaceutical companies are establishing comprehensive portfolios serving both domestic healthcare needs and international export opportunities across monoclonal antibody and insulin biosimilar categories. The Chinese government's healthcare reform initiatives and pharmaceutical procurement policies increasingly favor biosimilar utilization through preferential formulary placement, streamlined regulatory approval pathways, and public insurance reimbursement advantages.
This policy framework, combined with the country's established biomanufacturing capabilities and cost-competitive production advantages, creates exceptionally favorable environment for biosimilar market expansion. Chinese healthcare systems implementing biosimilar-first treatment protocols and pharmaceutical manufacturers establishing global biosimilar franchises position China as emerging biosimilar market leader.
- Government healthcare policies and pharmaceutical procurement reforms driving biosimilar preference through formulary positioning and reimbursement advantages
- Domestic manufacturing capacity expansion and technical capability development supporting comprehensive biosimilar portfolio establishment nationwide
- Healthcare cost containment imperatives and expanding public insurance coverage accelerating biosimilar adoption across therapeutic categories
- Export market development and international partnership establishment enabling Chinese biosimilar manufacturers to pursue global market opportunities
What Makes India Demonstrate Market Leadership with Production Capabilities?
The biosimilar market in India is expanding at a CAGR of 19.4%, supported by established biosimilar manufacturing expertise, diabetes treatment burden growth, and domestic pharmaceutical industry's pioneering role in global biosimilar development. The country's leading biosimilar manufacturers including major Indian pharmaceutical companies established early market positions in biosimilar development and commercialization serving both domestic and international markets. India's substantial diabetes population requiring insulin therapy and growing chronic disease burden create expanding biosimilar demand while domestic production capabilities ensure supply availability and cost competitiveness.
India's pharmaceutical industry benefits from established biomanufacturing infrastructure, regulatory expertise in biosimilar development, and cost-competitive production supporting both domestic market supply and pharmaceutical export opportunities. The country's biosimilar manufacturers pioneered early biosimilar commercialization establishing technical capabilities and regulatory track records supporting continued market leadership. This foundation enables sustained biosimilar market growth as therapeutic portfolios expand and international market access increases.
- Established biosimilar manufacturing capabilities and pharmaceutical industry expertise supporting comprehensive product portfolio development
- Diabetes burden growth and chronic disease prevalence expansion driving biosimilar insulin and therapeutic protein demand
- Cost-competitive production advantages and technical expertise enabling domestic market supply and international export market participation
- Regulatory experience and pharmaceutical industry maturity supporting continued biosimilar development and commercialization capabilities
Why Does USA Maintain Oncology Biosimilar and Hospital Expansion Leadership?
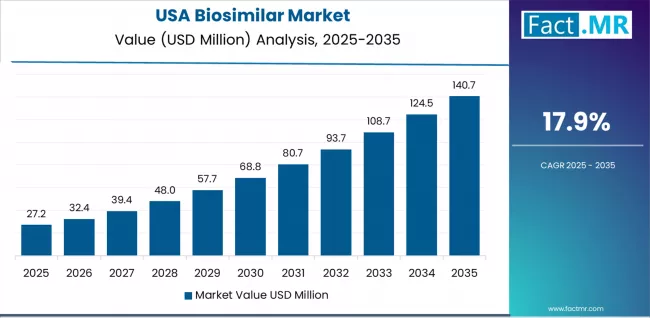
The biosimilar market in USA is projected to exhibit strong growth with a CAGR of 17.9% through 2035, driven by continuing FDA oncology biosimilar approvals and hospital system adoption initiatives. The country's sophisticated healthcare infrastructure and comprehensive specialty pharmacy networks enable effective biosimilar distribution while hospital system formulary management drives institutional adoption. American healthcare payers increasingly implement biosimilar-preferring policies through step therapy protocols, prior authorization requirements favoring biosimilar, and value-based contracting arrangements incentivizing biosimilar utilization across therapeutic categories.
The USA market benefits from regulatory pathway maturity following landmark biosimilar approvals, growing physician comfort with biosimilar prescribing supported by clinical evidence and real-world experience, and healthcare economic imperatives driving payer and provider biosimilar adoption. Hospital systems implementing biosimilar conversion programs and specialty pharmacies developing biosimilar distribution capabilities create infrastructure supporting accelerated market penetration.
Strategic Market Considerations:
- Oncology and immunology biosimilar launches driving growth with focus on blockbuster mAb originator competition and hospital formulary adoption
- Healthcare system initiatives and payer policies accelerating biosimilar utilization from originator-dominant to biosimilar-preferred treatment paradigms
- Interchangeability designation achievement and automatic substitution policies enabling pharmacy-level conversion supporting adoption acceleration
- Specialty pharmacy infrastructure and patient support program integration facilitating biosimilar access and adherence optimization
How Does Germany Maintain mAb Acceptance and Patent Expiry Benefits?
Germany's advanced healthcare market demonstrates sophisticated biosimilar utilization with documented adoption success in monoclonal antibody categories and established biosimilar prescribing patterns across rheumatology and gastroenterology specialties. The country maintains favorable biosimilar adoption environment supporting a 16.8% CAGR through 2035. German healthcare system including statutory health insurance and hospital sectors showcase biosimilar implementations where cost-effectiveness requirements integrate with clinical quality standards and physician prescribing practices to optimize pharmaceutical expenditure management.
German healthcare stakeholders demonstrate high biosimilar acceptance supported by comprehensive clinical evidence, favorable regulatory environment, and economic incentives supporting biosimilar preference. The market benefits from established biosimilar utilization patterns and healthcare system structures supporting systematic adoption across therapeutic categories.
Strategic Market Considerations:
- Rheumatology and gastroenterology specialties leading biosimilar adoption with focus on mAb biosimilar and chronic disease management
- Healthcare system cost containment objectives driving biosimilar preference through economic incentives and formulary management
- Physician acceptance and clinical evidence foundation supporting continued biosimilar prescribing growth across specialties
- Patent expiry pipeline and regulatory approval timing supporting sustained biosimilar market expansion
What Drives UK Market Growth with NHS Adoption Initiatives?
UK's established healthcare system demonstrates accelerating biosimilar adoption with a 15.6% CAGR through 2035 driven by NHS biosimilar promotion initiatives and comprehensive clinical trial participation establishing evidence foundation. The country's National Health Service implements systematic biosimilar adoption policies including prescribing targets, financial incentives for biosimilar utilization, and commissioning frameworks supporting cost-effective medication selection. UK healthcare providers participate extensively in biosimilar clinical trials and real-world evidence generation supporting clinical confidence and adoption momentum.
The healthcare system benefits from centralized pharmaceutical procurement and unified adoption policies enabling coordinated biosimilar implementation across NHS trusts. This healthcare structure supports efficient biosimilar adoption translating cost savings into expanded treatment access and healthcare system sustainability.
Strategic Market Considerations:
- NHS biosimilar initiatives and prescribing targets driving systematic adoption across therapeutic categories and healthcare settings
- Clinical evidence generation and real-world study participation supporting physician confidence and prescribing practice evolution
- Healthcare economic objectives and pharmaceutical budget management supporting biosimilar preference in formulary decisions
- Centralized procurement and unified policies enabling efficient market adoption and healthcare system savings realization
How Does Japan Demonstrate Insulin Uptake with Aging Demographics?
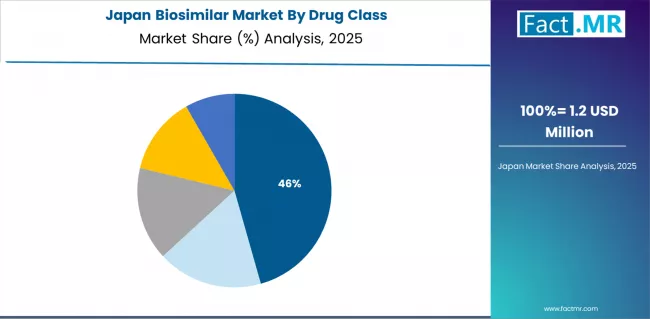
Japan's mature healthcare market demonstrates growing biosimilar adoption with a 14.7% CAGR through 2035, driven by rapid insulin biosimilar uptake and aging population demographics increasing chronic disease treatment requirements. The country's universal healthcare coverage and comprehensive pharmaceutical reimbursement system support biosimilar access while regulatory approval of insulin and mAb biosimilar expands available treatment options. Japanese healthcare providers increasingly adopt biosimilar supported by domestic clinical evidence and health authority guidance promoting cost-effective medication selection.
The Japanese market benefits from aging population creating expanding chronic disease burden, government healthcare cost management objectives, and pharmaceutical industry development of biosimilar portfolios serving domestic market requirements. This demographic and policy environment supports sustained biosimilar market growth as therapeutic options expand and adoption practices mature.
Strategic Market Considerations:
- Insulin biosimilar adoption and diabetes management requirements leading growth supported by aging population and disease prevalence
- Healthcare system cost containment and pharmaceutical budget management supporting biosimilar preference in reimbursement policies
- Regulatory approvals and domestic clinical evidence generation supporting physician adoption and patient acceptance
- Aging demographics and chronic disease burden expansion driving sustained biosimilar demand across therapeutic categories
Why Does Brazil Show Hospital Adoption and Oncology Penetration?
The biosimilar market in Brazil is projected to grow at a CAGR of 18.2% through 2035, driven by hospital-based biosimilar adoption acceleration and oncology biosimilar market penetration addressing cancer treatment access challenges. The country's public and private hospital systems increasingly adopt biosimilar through formulary inclusion and therapeutic substitution protocols supporting cost management while expanding patient access. Brazilian healthcare system faces specialty medication affordability challenges that biosimilar address through substantial cost reductions enabling expanded treatment availability.
The Brazilian market benefits from domestic biosimilar manufacturing development, healthcare system cost pressures driving biosimilar preference, and oncology treatment access imperatives supporting adoption in cancer care settings. This creates favorable environment for biosimilar market expansion as healthcare providers seek cost-effective treatment alternatives.
Strategic Market Considerations:
- Hospital formulary adoption and institutional biosimilar preference driving market growth across public and private healthcare systems
- Oncology biosimilar penetration addressing cancer treatment access and affordability challenges in specialty medication management
- Healthcare cost containment imperatives and budget constraints supporting biosimilar adoption across therapeutic categories
- Domestic manufacturing development and pharmaceutical industry investment supporting market supply and growth enablement
Europe Market Split by Country
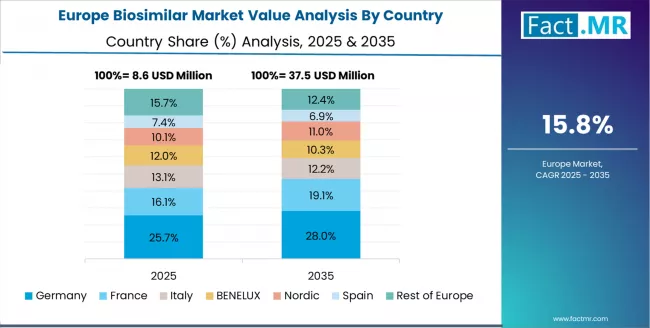
The biosimilar market in Europe is projected to grow from USD 12.8 million in 2025 to USD 68.4 million by 2035, registering a CAGR of 18.2% over the forecast period. Germany is expected to maintain its leadership position with a 28.4% market share in 2025, rising to 29.6% by 2035, supported by its advanced healthcare infrastructure, comprehensive biosimilar adoption policies, and established pharmaceutical market supporting biosimilar prescribing across hospital and ambulatory care settings.
The UK follows with a 22.8% share in 2025, projected to reach 23.4% by 2035, driven by NHS biosimilar promotion initiatives, systematic adoption targets, and comprehensive clinical evidence generation supporting prescriber confidence. France holds a 18.6% share in 2025, expected to increase to 18.9% by 2035, supported by healthcare system cost management and biosimilar utilization policies. Italy commands a 14.2% share in 2025, projected to reach 13.8% by 2035, while Spain accounts for 10.8% in 2025, expected to reach 10.2% by 2035.
The rest of Europe region, including Nordic countries with progressive biosimilar adoption, Eastern European emerging markets with developing biosimilar access, and smaller Western European healthcare systems, is anticipated to hold 5.2% in 2025, declining slightly to 4.1% by 2035, attributed to market consolidation toward larger core markets with established healthcare infrastructure and comprehensive biosimilar integration capabilities.
Competitive Landscape of the Biosimilar Market
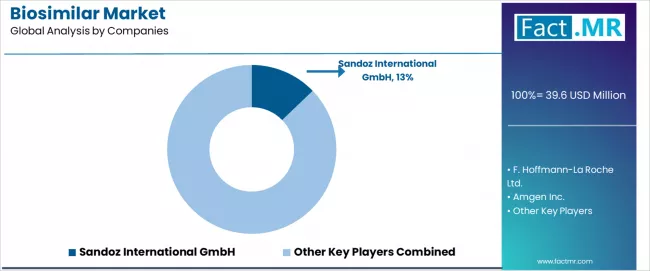
The biosimilar market is characterized by intense competition among established pharmaceutical companies, specialized biosimilar developers, and integrated biopharmaceutical organizations focused on delivering clinically equivalent, regulatory compliant, and commercially viable biosimilar alternatives to originator biologics.
Companies are investing in biosimilar pipeline expansion, manufacturing capacity development, strategic market access partnerships, and comprehensive stakeholder education programs to deliver effective, accessible, and cost-competitive biosimilar solutions meeting rigorous regulatory standards and healthcare stakeholder requirements.
Interchangeability designation achievement, real-world evidence generation, and specialty distribution network development are central to strengthening competitive position and market penetration success.
Sandoz leads the market with a 12.8% market share, offering comprehensive biosimilar portfolio with focus on established market presence and diversified therapeutic category coverage for hospital and specialty pharmacy applications. Amgen provides biosimilar products leveraging biologic manufacturing expertise and established pharmaceutical distribution infrastructure across global healthcare markets.
Roche focuses on biosimilar development through strategic business units while maintaining originator biologic franchises. Dr. Reddy's delivers biosimilar portfolio emphasizing emerging market leadership and cost-competitive manufacturing capabilities.
Teva operates biosimilar business leveraging specialty pharmaceutical expertise and global distribution networks. Pfizer provides biosimilar products through integrated pharmaceutical operations and established market access capabilities.
Samsung Bioepis specializes in biosimilar development with focus on monoclonal antibody portfolio and strategic partnership commercialization models. Biocon delivers biosimilar products emphasizing cost-competitive manufacturing and emerging market penetration.
Viatris and Celltrion Healthcare focus on comprehensive biosimilar portfolios and vertical integration strategies spanning development, manufacturing, and commercialization, emphasizing clinical evidence generation and market access optimization through strategic healthcare system partnerships.
Key Players in the Biosimilar Market
- Amgen Inc.
- F. Hoffmann-La Roche Ltd.
- Sandoz International GmbH
- Dr. Reddy’s Laboratories Ltd.
- Teva Pharmaceutical Industries Ltd.
- Pfizer Inc.
- Samsung Bioepis Co., Ltd.
- Biocon Limited
- Viatris Inc.
- Celltrion Healthcare Co., Ltd.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 39.6 Million |
| Drug Class | Monoclonal Antibodies, Growth Factors & Hematopoietic Agents, Insulin & Analogues, Osteoporosis/Bone Agents, Others |
| Indication | Autoimmune Disorders, Oncology, Diabetes Mellitus, Ophthalmic Disorders, Hematologic/Rare Disorders, Others |
| End Use | Hospitals, Specialty Pharmacies, Online & Retail |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | usa, Germany, UK, Japan, India, China, Brazil and 40+ countries |
| Key Companies Profiled | Amgen, Roche, Sandoz, Dr. Reddy's, Teva, Pfizer, Samsung Bioepis, Biocon, Viatris, Celltrion Healthcare |
| Additional Attributes | Dollar sales by drug class, indication, end use, regional demand trends, competitive landscape, healthcare provider preferences for specific biosimilar products, integration with specialty pharmacy networks, innovations in biosimilar development technologies, interchangeability designation achievement, and market access optimization capabilities |
Biosimilar Market by Segments
-
Drug Class :
- Monoclonal Antibodies
- Growth Factors & Hematopoietic Agents
- Insulin & Analogues
- Osteoporosis/Bone Agents
- Others
-
Indication :
- Autoimmune Disorders
- Oncology
- Diabetes Mellitus
- Ophthalmic Disorders
- Hematologic/Rare Disorders
- Others
-
End Use :
- Hospitals
- Specialty Pharmacies
- Online & Retail
-
Region :
-
North America
- USA
- Canada
- Mexico
-
Europe
- Germany
- France
- UK
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
-
Asia Pacific
- China
- India
- Japan
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
-
Latin America
- Brazil
- Argentina
- Chile
- Rest of Latin America
-
Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Countries
- Rest of Middle East & Africa
-
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Drug Class
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Drug Class, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Drug Class, 2025 to 2035
- Monoclonal Antibodies
- Growth Factors & Hematopoietic Agents
- Insulin & Analogues
- Osteoporosis/Bone Agents
- Others
- Y to o to Y Growth Trend Analysis By Drug Class, 2020 to 2024
- Absolute $ Opportunity Analysis By Drug Class, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Indication
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Indication, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Indication, 2025 to 2035
- Autoimmune Disorders
- Oncology
- Diabetes Mellitus
- Ophthalmic Disorders
- Hematologic/Rare Disorders
- Others
- Y to o to Y Growth Trend Analysis By Indication, 2020 to 2024
- Absolute $ Opportunity Analysis By Indication, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Hospitals
- Specialty Pharmacies
- Online & Retail
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Drug Class
- By Indication
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Indication
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Drug Class
- By Indication
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Indication
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Drug Class
- By Indication
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Indication
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Drug Class
- By Indication
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Indication
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Drug Class
- By Indication
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Indication
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Drug Class
- By Indication
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Indication
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Drug Class
- By Indication
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Indication
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Indication
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Drug Class
- By Indication
- By End Use
- Competition Analysis
- Competition Deep Dive
- Sandoz International GmbH
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- F. Hoffmann-La Roche Ltd.
- Amgen Inc.
- Dr. Reddy’s Laboratories Ltd.
- Teva Pharmaceutical Industries Ltd.
- Pfizer Inc.
- Samsung Bioepis Co., Ltd.
- Biocon Limited
- Viatris Inc.
- Celltrion Healthcare Co., Ltd.
- Sandoz International GmbH
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Drug Class
- Figure 6: Global Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Indication, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Indication
- Figure 9: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by End Use
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Drug Class
- Figure 26: North America Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Indication, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Indication
- Figure 29: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by End Use
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Drug Class
- Figure 36: Latin America Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Indication, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Indication
- Figure 39: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by End Use
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Drug Class
- Figure 46: Western Europe Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Indication, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Indication
- Figure 49: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by End Use
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Drug Class
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Indication, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Indication
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Drug Class
- Figure 66: East Asia Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Indication, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Indication
- Figure 69: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by End Use
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Drug Class
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Indication, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Indication
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Drug Class, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Drug Class
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Indication, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Indication
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the biosimilar market in 2025?
The global biosimilar market is estimated to be valued at USD 39.6 million in 2025.
What will be the size of biosimilar market in 2035?
The market size for the biosimilar market is projected to reach USD 220.5 million by 2035.
How much will be the biosimilar market growth between 2025 and 2035?
The biosimilar market is expected to grow at a 18.7% CAGR between 2025 and 2035.
What are the key product types in the biosimilar market?
The key product types in biosimilar market are monoclonal antibodies, growth factors & hematopoietic agents, insulin & analogues, osteoporosis/bone agents and others.
Which indication segment to contribute significant share in the biosimilar market in 2025?
In terms of indication, autoimmune disorders segment to command 40.0% share in the biosimilar market in 2025.