Fusion Protein Biosimilars Market
Fusion Protein Biosimilars Market By Product (Cytokines Recombinant protein, Immunoglobin (Ig) fusion protein), By application (Cancer, HIV-AIDS), By end-users (Hospitals, Research institutes) & By region: Global Industry Analysis 2025 to 2035
Analysis of Fusion Protein Biosimilars market covering 30+ countries including analysis of US, Canada, UK, Germany, France, Nordics, GCC countries, Japan, Korea and many more
Fusion Protein Biosimilars Market Outlook 2025 to 2035
The Fusion Protein Biosimilars Market is projected to grow from USD 42.7 billion in 2025 to USD 64.4 billion by 2035, with a CAGR of 4.2%. Increased biologics demand and biosimilar approvals are fostering growth. Cancer-focused biosimilars lead usage, particularly for rare or orphan indications. The U.S. and Germany remain critical markets due to pricing reforms and biosimilar support programs.
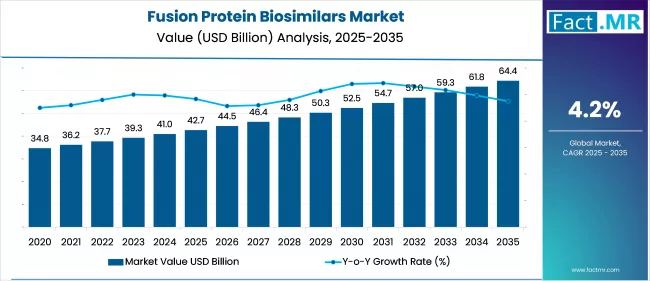
| Metric | Value |
|---|---|
| Fusion Protein Biosimilars Market Estimated Value in (2025 E) | USD 42.7 billion |
| Fusion Protein Biosimilars Market Forecast Value in (2035 F) | USD 64.4 billion |
| Forecast CAGR (2025 to 2035) | 4.2% |
Biologic drugs are commonly used to treat various diseases but their high cost has raised the emerging scope for biosimilars in the market. A fusion protein biosimilars has come forth as a new modality, as an alternate of biologic products. The fusion protein biosimilars combines the customized pharmacological properties of biological ligands, together with multiple functions of the fragmented crystallizable domain of immunoglobulins.
The fusion protein biosimilars are used in several clinical studies based on applications like cancer, HIV-AIDS, chronic inflammatory diseases, and many other chronic diseases. Moreover, the first therapeutic Fc fusion protein was established for the AIDS treatment.
Thus the wide applications of fusion protein biosimilars has increased the demand for fusion protein biosimilars in the global market. Currently, 11 fusion proteins have been approved by the Food and Drug Administration (FDA) and there are several new Fc fusion proteins being in the pre-clinical and clinical development stage. Thus, the rising prevalence of such chronic diseases expects to surge the demand for biosimilars in the market in the coming years.
| Countries | CAGR |
|---|---|
| U.S. | 4.6% |
| Germany | 4.2% |
| Japan | 3.9% |
| South Korea | 3.6% |
What are the key Trends Impacting Growth of the Fusion Protein Biosimilars Market?
Government initiatives to pioneer fusion protein biosimilars is another trending factor that expects to explore lucrative opportunities in the fusion protein biosimilars market in coming years.
The U.S. Food and Drug Administration established an abbreviated licensure pathway Biologics Price Competition and Innovation Act (BPCIA) for biological products that are demonstrated to be biosimilar or interchangeable. This pathway intended to allow more options for chronic disease treatment that expects to increase access to lifesaving medications, and potentially lower health care costs through competition.
Along which shift towards improvement of regulatory environment across the global markets are evolving rapidly, with widespread global industrial biologic development and manufacturing experience, go along with the rising standards of clinical care.
What are the Key Factors that Expected to Drive the Fusion Protein Biosimilars Market growth?
The wide applications of fusion protein biosimilar in various chronic diseases like cardiovascular disorder, cancer, HIV-AIDS, and chronic inflammatory disorders is the key factor that expects to drive the demand in the fusion protein biosimilars market during the forecast period.
Approval of fusion protein biosimilar is expected to benefit manufacturers and explore lucrative opportunities for organizations specializing in generics and biosimilars to grow their businesses rapidly. For instance, in November 2019, Celgene received FDA approval for activin receptor-IgG1 Fc fusion protein, rDNA [luspatercept-aamt - Reblozyl] used to treat anemia in adults with beta thalassemia. Additionally, the launch of new fusion protein biosimilars in the market also benefits the patients by increasing treatment options and lowering the costs of complex, life-saving treatments.
Thus, the surge in demand for cost-effective fusion protein biosimilars expects to explore lucrative opportunities for the manufacturers to invest more in the fusion protein biosimilars development which in turn drives the fusion protein biosimilars market growth.
Favorable insurance by Medicare or Medicaid, the Centers for Medicare & Medicaid Services (CMS) or any other plan providers, along with FDA approval pathway for fusion protein biosimilar medications for cost reductions projects to high patient access which in turn surge the demand for the biosimilars that expects to fuel the fusion protein biosimilars market during the forecast period.
What are the Restraining Factors that Expected to Hamper the Growth of the Fusion Protein Biosimilars Market?
As the fusion proteins are highly complex structure the analytical characterization of fusion protein is far more difficult than that of monoclonal antibodies that has surged the demand for conventional generic platform. Development of alternatives expects to impede the fusion protein biosimilars market during the forecast period.
As biosimilars have to undergo extensive clinical trials, to compare them with the original biologic drug to ensure their similarity and interchangeable property that needs time to study. Thus, stringent regulatory approvals and time-consuming factor for clinical studies expects to hamper the fusion protein biosimilars market growth.
Key Segments of the Fusion Protein Biosimilars Market Covered in the Report
-
Based on the product, the fusion protein biosimilars market has been segmented as
- Cytokines Recombinant protein
- Immunoglobin (Ig) fusion protein
- Parathyroid Hormone (PTPH) fusion protein
- Others
-
Based on application, the fusion protein biosimilars market has been segmented as
- Cancer
- HIV-AIDS
- Respiratory disease
- Cardiovascular disorder
- Ophthalmology
- Others
-
Based on end-users, the fusion protein biosimilars market has been segmented as
- Hospitals
- Research institutes
-
Based on the region, the fusion protein biosimilars market has been segmented as
- North America
- Latin America
- Europe
- South Asia
- East Asia
- Oceania
- Middle East & Africa
Why Cancer Holds the Highest Share the Fusion Protein Biosimilars Market?
Cancer expects to hold the higher share in the fusion protein biosimilars market owing to the rising cancer cases among the growing population in the world. Cancer is a growing global concern stated by the World Health Organization (WHO). Biosimilars are in high demand for cancer therapies. According to the Globocan 2020 data, in 2020, the incidence of new cancer cases was estimated to be around 19,292,789, with nearly about 9,958,133 deaths due to cancers.
Competitive Landscape
The prominent key players operating are
- Pfizer Inc.
- Eli Lilly and Company
- Sandoz International GmbH
- F. Hoffmann-La Roche Ltd.
- AbbVie Inc.
- Dr. Reddy’s Laboratories Ltd.
- Samsung Bioepis
- Teva Pharmaceutical Industries Ltd.
- Mylan N.V., Novartis AG
- Fresenius Kabi AG
- STADA Arzneimittel AG
- Celltrion Healthcare Co.Ltd.
- Thermo Fisher Scientific Inc.
- Daiichi Sankyo Co Ltd.
- Aurobindo Pharma
- Absolute Antibody
- Bioverativ Therapeutics Inc.
- and others are actively involved in the fusion protein biosimilars market.
What Strategies are the Key Players Adopting for Increasing their Market Share in the Fusion Protein Biosimilars Market?
Increasing competition among pharmaceutical and biotechnological companies to penetrate the fusion protein biosimilars market has led the manufacturers to actively invest in research areas.
Manufacturers have increased focus on the development and launch of effective biosimilars that would meet the similarity in the composition, efficacy, and results that are similar to biological products. New product launches and collaborations help companies to gain maximum revenue share and expand regional presence and existing product portfolio.
For instance, In January 2020, Coherus BioSciences, Inc. entered into a licensing agreement with Innovent Biologics, (Suzhou) Co., Ltd., a leading Chinese biopharmaceutical company, in order to commercialize biosimilar candidate to Avastin (bevacizumab) in North American countries like U.S and Canada.
Additionally, in September 2018, SOTIO had acquired Cytune Pharma. This agreement was signed to allow the company the usage of Cytune’s SO-C101, a human fusion protein of IL-15 along with their clinical programs in lung, prostate, and ovarian cancer which is based on their autologous dendritic cell therapy platform DCVAC.
What are the Key Opportunities in Fusion Protein Biosimilars Market?
Biosimilars are expected to be a cost-effective alternative to high-priced branded biologics, resulting in a positive impact that can emerge opportunities for the stakeholders in the fusion protein biosimilars market. The regulatory approvals of an accelerated timeline for biosimilars signal a change in the industry and the potential for severe drops in profits for pharmaceutical companies.
Increased research efforts and pipeline drugs expect to lay lucrative opportunities for the fusion protein biosimilars market growth in the coming years. For instance, approximately 37 therapeutic fusion products are in clinical development and thirteen products have been approved by the FDA and the EMA. They follow the commercial successes of mAbs with five blockbuster products (aflibercept, etanercept, dulaglutide, abatacept and efmoroctocog α.
Why Europe has witnessed to be dominant Sales of Fusion Protein Biosimilars Market?
Europe expects to dominate the fusion protein biosimilars market due to the presence of a well-defined regulatory framework for biosimilars and the presence of giant pharmaceutical companies such as Novartis, Johnson & Johnson, Pfizer Inc., Merck & Co., Sanofi, AstraZeneca, GlaxoSmithKline and many others.
Furthermore, favorable regulatory scenario, well-developed healthcare infrastructure and a growing number of product launches have fueled the European fusion protein biosimilars market growth. Several biosimilars are introduced in the European fusion protein biosimilars market owing to applicability in various indications and higher cost arbitrage.
Along, the rising prevalence of chronic disease in the region has raised the demand for clinical studies which in turn has surge demand for biosimilars in the European fusion protein biosimilars market. For instance, as per Joint Research Centre (JRC) of the European Commission, the cancer burden was estimated to have risen to 2.7 million new cases (including all types of cancers, except non-melanoma skin cancer) while around 1.3 million deaths were found in 2020.
What is the Repercussion of the Covid-19 Pandemic on the Fusion Protein Biosimilars Market Growth?
The COVID-19 impact projected a significant impact on the fusion protein biosimilars market due to the lockdown and break on transportation across the world, shortage in supply chain and raw materials, has negatively impacted biosimilar production. During amid COVID-19 pandemic, have shifted their priorities towards COVID-19 medication and vaccine development, this shift has contributed a remarkable impact on the fusion protein biosimilars market.
Most of the pipeline products are exhibiting a slow pace of research and development activities as most of the clinical trials has been focusing to combat the COVID-19 crisis and reduce the exposure of coronavirus infection worldwide. For instance, as per JCO Global Oncology 2020, in the COVID-19 pandemic, nearly about 88% of the cancer care centres faced difficult challenges. The challenges associated in delivering usual cancer attention and caution due to lack of personal protective equipment, strict precautionary measures, and staff shortage.
Additionally, this impact was observed in low-income and middle income countries. Therefore, COVID-19 has projected a short term negative impact on cancer therapies, which indirectly is expected to project a short-term negative impact on the fusion protein biosimilars market.
The report covers exhaustive analysis on:
- Fusion protein biosimilars market Segments
- Fusion protein biosimilars market Dynamics
- Fusion protein biosimilars market Size
- Supply & Demand
- Current Trends/Issues/Challenges
- Competition & Companies involved
- Technology
- Value Chain
Regional Analysis Includes
- North America (U.S., Canada)
- Latin America ( Brazil, Mexico, Argentina, Rest of LATAM)
- Europe (Germany, Italy, UK, Spain, France, Russia, BENELUX, Rest of Europe)
- East Asia (China, Japan, S. Korea,)
- South Asia (India, Indonesia, Malaysia, Thailand, Rest of South Asia)
- Oceania (Australia, New Zealand)
- The Middle East and Africa (GCC, Turkey, South Africa, Rest Of MEA)
Report Highlights
- Detailed overview of fusion protein biosimilars market
- Changing market dynamics in the industry
- In-depth fusion protein biosimilars market segmentation
- Historical, current and projected market size in terms of volume and value
- Recent industry trends and developments
- Competitive landscape
- Strategies of key players and products offered
- Potential and niche segments, geographical regions exhibiting promising growth
- A neutral perspective on market performance
- Must-have information for market players to sustain and enhance their market footprint.
NOTE - All statements of fact, opinion, or analysis expressed in reports are those of the respective analysts. They do not necessarily reflect formal positions or views of the company.
Table of Content
- Executive Summary
- Global Market Outlook
- Demand-side Trends
- Supply-side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Inclusion and Exclusion
- Value Added Insights
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Supply Side Participants and their Roles
- Producers
- Mid-Level Participants (Traders/ Agents/ Brokers)
- Wholesalers and Distributors
- Value Added and Value Created at Node in the Supply Chain
- List of Raw Material Suppliers
- List of Existing and Potential Buyers
- Supply Side Participants and their Roles
- Pricing Analysis of Market
- Pricing Analysis By Region
- Pricing Analysis By Country
- Pricing Analysis By End Use
- Pricing Analysis By Distributor
- Pricing Analysis By Application
- Value Chain Analysis
- Profit Margin Analysis
- Wholesalers and Distributors
- Retailers
- Product Portfolio Analysis
- Regulatory Landscape
- By Key Regions
- By Key Countries
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Investment Feasibility Matrix
- PESTLE Analysis
- Political Factors
- Government stability and change
- Taxation policy and incentives
- Trade tariffs and import/export regulations
- Political risk and corruption levels
- Economic Factors
- GDP growth and economic cycles
- Inflation and interest rates
- Exchange rate fluctuations
- Unemployment and consumer spending power
- Social Factors
- Demographic trends (age, population growth)
- Cultural attitudes and lifestyle changes
- Education levels and workforce skills
- Health consciousness and social values
- Technological Factors
- Rate of technological innovation
- R&D activity and automation trends
- Digital infrastructure and connectivity
- Intellectual property protection
- Legal Factors
- Employment and labor laws
- Health & safety regulations
- Consumer protection laws
- Data privacy and antitrust legislation
- Environmental Factors
- Environmental regulations and compliance
- Climate change impacts and carbon footprint
- Resource availability and sustainability practices
- Waste management and recycling initiatives
- Political Factors
- Market Background and Dynamics
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- SWOT Analysis of Global Market
- Strengths
- Weaknesses
- Opportunities
- Threats
- Market Dynamics
- Global Market Analysis 2020-2024 and Forecast, 2025-2035
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Projections, 2025-2035
- Y-o-Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Revenue Forecast Scenario, 2020-2035
- Conservative Scenario
- Likely Scenario
- Optimistic Scenario
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis By Application, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By Application, 2025-2035
- Y-o-Y Growth Trend Analysis By Application, 2020-2024
- Absolute $ Opportunity Analysis By Application, 2025-2035
- Market Attractiveness Analysis By Application, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis By End Use, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By End Use, 2025-2035
- Y-o-Y Growth Trend Analysis By End Use, 2020-2024
- Absolute $ Opportunity Analysis By End Use, 2025-2035
- Market Attractiveness Analysis By End Use, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Region
- Introduction
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis By Region, 2020-2024
- Current Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By Region, 2025-2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific & Pacific
- MEA
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020-2024 and Forecast 2025-2035
- Introduction
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By Market Taxonomy, 2025-2035
- By Country
- USA
- Canada
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- USA Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Canada Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Mexico Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- USA Market
- Latin America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Brazil
- Argentina
- Chile
- Rest of Latin America
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Brazil Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Argentina Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Chile Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of Latin America Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Brazil Market
- Western Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Germany Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- UK Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Italy Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Spain Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- France Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Nordic Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- BENELUX Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of Western Europe Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Germany Market
- Eastern Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Russia Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Poland Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Hungary Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Balkan & Baltic Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of Eastern Europe Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Russia Market
- East Asia Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- China
- Japan
- South Korea
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- China Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Japan Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- South Korea Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- China Market
- South Asia and Pacific Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- India Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- ASEAN Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Australia & New Zealand Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of South Asia and Pacific Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- India Market
- MEA Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Kingdom of Saudi Arabia Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Other GCC Countries Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Turkiye Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- South Africa Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- South Africa Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of MEA Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of MEA Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Kingdom of Saudi Arabia Market
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Region
- By Application
- By End Use
- Competition Analysis
- Competition Deep Dive
- Global Key Players
- Key Players by Region
- Key Players by Country
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (US$ Mn) Forecast by Region, 2020 to 2035
- Table 2: Global Market Volume (Units) Forecast by Region, 2020 to 2035
- Table 3: Global Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 4: Global Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 5: Global Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 6: Global Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 7: North America Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 8: North America Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 9: North America Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 10: North America Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 11: North America Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 12: North America Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 13: Latin America Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 14: Latin America Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 15: Latin America Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 16: Latin America Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 17: Latin America Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 18: Latin America Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 19: Europe Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 20: Europe Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 21: Europe Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 22: Europe Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 23: Europe Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 24: Europe Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 25: East Asia Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 26: East Asia Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 27: East Asia Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 28: East Asia Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 29: East Asia Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 30: East Asia Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 31: South Asia Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 32: South Asia Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 33: South Asia Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 34: South Asia Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 35: South Asia Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 36: South Asia Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 37: Oceania Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 38: Oceania Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 39: Oceania Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 40: Oceania Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 41: Oceania Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 42: Oceania Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 43: MEA Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 44: MEA Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 45: MEA Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 46: MEA Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 47: MEA Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 48: MEA Market Volume (Units) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 2: Global Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 3: Global Market Value (US$ Mn) by Region, 2020 to 2035
- Figure 4: Global Market Value (US$ Mn) Analysis by Region, 2020 to 2035
- Figure 5: Global Market Volume (Units) Analysis by Region, 2020 to 2035
- Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2020 to 2035
- Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2020 to 2035
- Figure 8: Global Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 9: Global Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 10: Global Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 11: Global Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 12: Global Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 13: Global Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 14: Global Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 15: Global Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 16: Global Market Attractiveness by Application, 2020 to 2035
- Figure 17: Global Market Attractiveness by End Use, 2020 to 2035
- Figure 18: Global Market Attractiveness by Region, 2020 to 2035
- Figure 19: North America Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 20: North America Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 21: North America Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 22: North America Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 23: North America Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 24: North America Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 25: North America Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 26: North America Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 27: North America Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 28: North America Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 29: North America Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 30: North America Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 31: North America Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 32: North America Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 33: North America Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 34: North America Market Attractiveness by Application, 2020 to 2035
- Figure 35: North America Market Attractiveness by End Use, 2020 to 2035
- Figure 36: North America Market Attractiveness by Country, 2020 to 2035
- Figure 37: Latin America Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 38: Latin America Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 39: Latin America Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 40: Latin America Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 41: Latin America Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 42: Latin America Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 43: Latin America Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 44: Latin America Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 45: Latin America Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 46: Latin America Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 48: Latin America Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 49: Latin America Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 50: Latin America Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 51: Latin America Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 52: Latin America Market Attractiveness by Application, 2020 to 2035
- Figure 53: Latin America Market Attractiveness by End Use, 2020 to 2035
- Figure 54: Latin America Market Attractiveness by Country, 2020 to 2035
- Figure 55: Europe Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 56: Europe Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 57: Europe Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 58: Europe Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 59: Europe Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 60: Europe Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 61: Europe Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 62: Europe Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 63: Europe Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 64: Europe Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 65: Europe Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 66: Europe Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 67: Europe Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 68: Europe Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 69: Europe Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 70: Europe Market Attractiveness by Application, 2020 to 2035
- Figure 71: Europe Market Attractiveness by End Use, 2020 to 2035
- Figure 72: Europe Market Attractiveness by Country, 2020 to 2035
- Figure 73: East Asia Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 74: East Asia Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 75: East Asia Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 76: East Asia Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 77: East Asia Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 78: East Asia Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 79: East Asia Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 80: East Asia Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 81: East Asia Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 82: East Asia Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 83: East Asia Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 84: East Asia Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 85: East Asia Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 86: East Asia Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 87: East Asia Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 88: East Asia Market Attractiveness by Application, 2020 to 2035
- Figure 89: East Asia Market Attractiveness by End Use, 2020 to 2035
- Figure 90: East Asia Market Attractiveness by Country, 2020 to 2035
- Figure 91: South Asia Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 92: South Asia Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 93: South Asia Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 94: South Asia Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 95: South Asia Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 96: South Asia Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 97: South Asia Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 98: South Asia Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 99: South Asia Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 100: South Asia Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 101: South Asia Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 102: South Asia Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 103: South Asia Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 104: South Asia Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 105: South Asia Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 106: South Asia Market Attractiveness by Application, 2020 to 2035
- Figure 107: South Asia Market Attractiveness by End Use, 2020 to 2035
- Figure 108: South Asia Market Attractiveness by Country, 2020 to 2035
- Figure 109: Oceania Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 110: Oceania Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 111: Oceania Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 112: Oceania Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 113: Oceania Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 114: Oceania Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 115: Oceania Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 116: Oceania Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 117: Oceania Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 118: Oceania Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 119: Oceania Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 120: Oceania Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 121: Oceania Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 122: Oceania Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 123: Oceania Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 124: Oceania Market Attractiveness by Application, 2020 to 2035
- Figure 125: Oceania Market Attractiveness by End Use, 2020 to 2035
- Figure 126: Oceania Market Attractiveness by Country, 2020 to 2035
- Figure 127: MEA Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 128: MEA Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 129: MEA Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 130: MEA Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 131: MEA Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 132: MEA Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 133: MEA Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 134: MEA Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 135: MEA Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 136: MEA Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 137: MEA Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 138: MEA Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 139: MEA Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 140: MEA Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 141: MEA Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 142: MEA Market Attractiveness by Application, 2020 to 2035
- Figure 143: MEA Market Attractiveness by End Use, 2020 to 2035
- Figure 144: MEA Market Attractiveness by Country, 2020 to 2035
- FAQs -
How much is Fusion Protein Biosimilars Market Worth?
The fusion protein biosimilars market worth is currently valued at nearly US$ 27.5 Billion.
Why Fusion Protein Biosimilars Market has Higher Growth Potential?
The development of cost-effective products is driving the fusion protein biosimilars market.
Where are the Most Opportunities for Fusion Protein Biosimilars Industry Players?
Favorable medical insurance policies provide the most opportunities for the fusion protein biosimilars industry players.