Pegfilgrastim Biosimilar Market
Pegfilgrastim Biosimilar Market Size and Share Forecast Outlook 2025 to 2035
Pegfilgrastim biosimilar market is projected to grow from USD 4.5 billion in 2025 to USD 9.3 billion by 2035, at a CAGR of 7.5%. Subcutaneous prefilled will dominate with a 82.0% market share, while hospital/oncology clinics will lead the setting segment with a 64.0% share.
Pegfilgrastim Biosimilar Market Forecast and Outlook 2025 to 2035
The global pegfilgrastim biosimilar market is valued at USD 4.5 billion in 2025 and is slated to reach USD 9.3 billion by 2035, recording an absolute increase of USD 4.8 billion over the forecast period. This translates into a total growth of 106.7%, with the market forecast to expand at a compound annual growth rate (CAGR) of 7.5% between 2025 and 2035.
The overall market size is expected to grow by nearly 2.1X during the same period, supported by increasing cancer treatment demands, growing biosimilar adoption, and rising healthcare cost containment initiatives across diverse oncology treatment and specialty pharmacy applications.
Quick Stats for Pegfilgrastim Biosimilar Market
- Pegfilgrastim Biosimilar Market Value (2025): USD 4.5 billion
- Pegfilgrastim Biosimilar Market Forecast Value (2035): USD 9.3 billion
- Pegfilgrastim Biosimilar Market Forecast CAGR: 7.5%
- Leading Route in Pegfilgrastim Biosimilar Market: Subcutaneous prefilled (82.0%)
- Key Growth Regions in Pegfilgrastim Biosimilar Market: North America, Europe, and Asia Pacific
- Key Players in Pegfilgrastim Biosimilar Market: Viatris/Biocon, Sandoz, Coherus, Fresenius Kabi, Pfizer, Teva
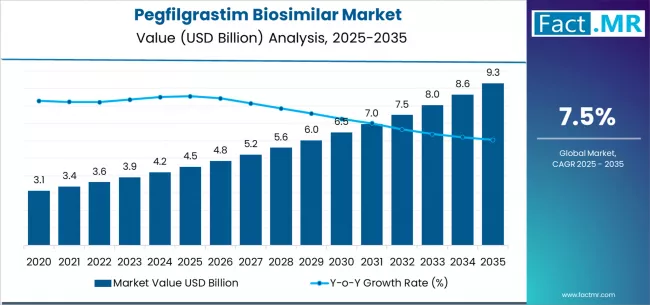
Between 2025 and 2030, the pegfilgrastim biosimilar market is projected to expand from USD 4.5 billion to USD 6.4 billion, resulting in a value increase of USD 1.9 billion, which represents 39.6% of the total forecast growth for the decade.
This phase of development will be shaped by increasing oncology treatment expansion, rising biosimilar acceptance, and growing adoption of cost-effective treatment solutions in healthcare systems. Producers are expanding their manufacturing capabilities to address the growing demand for accessible cancer supportive care and enhanced treatment affordability.
Pegfilgrastim Biosimilar Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value in (2025E) | USD 4.5 billion |
| Forecast Value in (2035F) | USD 9.3 billion |
| Forecast CAGR (2025 to 2035) | 7.5% |
From 2030 to 2035, the market is forecast to grow from USD 6.4 billion to USD 9.3 billion, adding another USD 2.9 billion, which constitutes 60.4% of the overall ten-year expansion. This period is expected to be characterized by the expansion of advanced delivery systems, the integration of innovative manufacturing technologies, and the development of enhanced biosimilar formulations for major healthcare providers.
The growing adoption of value-based healthcare programs and efficient treatment protocols will drive demand for pegfilgrastim biosimilars with enhanced efficacy standards and improved cost-effectiveness profiles.
Between 2020 and 2025, the pegfilgrastim biosimilar market experienced robust recovery growth, driven by increasing oncology treatment demand and growing recognition of biosimilars as essential components for cancer supportive care and chemotherapy-induced neutropenia management.
The market developed as healthcare providers and oncology centers recognized the potential for these products to enhance treatment accessibility while maintaining clinical efficacy and improving healthcare economics.
Technological advancement in biosimilar manufacturing and delivery systems began emphasizing the critical importance of maintaining therapeutic equivalence and operational efficiency in oncology care operations.
Why is the Pegfilgrastim Biosimilar Market Growing?
Market expansion is being supported by the increasing global cancer incidence and the corresponding need for cost-effective supportive care treatments that can maintain therapeutic efficacy while supporting diverse oncology treatment applications across various healthcare environments.
Modern healthcare providers and oncology specialists are increasingly focused on implementing treatment solutions that can reduce healthcare costs, maintain clinical outcomes, and provide consistent performance in cancer care operations.
Pegfilgrastim biosimilars' proven ability to deliver therapeutic equivalence, cost-effectiveness, and versatile treatment applications make them essential components for contemporary oncology care operations and healthcare cost management solutions.
The growing emphasis on healthcare cost containment and treatment accessibility is driving demand for pegfilgrastim biosimilars that can support affordable care delivery, reduce treatment burden, and enable efficient healthcare operations across varying oncology care configurations.
Healthcare provider preference for treatments that combine clinical efficacy with cost-effectiveness and treatment convenience is creating opportunities for innovative biosimilar implementations.
The rising influence of value-based healthcare and treatment accessibility initiatives is also contributing to increased adoption of pegfilgrastim biosimilars that can provide advanced therapeutic benefits without compromising clinical outcomes or healthcare sustainability.
Opportunity Pathways - Pegfilgrastim Biosimilar Market
The pegfilgrastim biosimilar market is poised for robust growth and transformation. As healthcare providers and oncology centers across both developed and emerging markets seek supportive care treatments that are effective, affordable, accessible, and clinically equivalent, pegfilgrastim biosimilar systems are gaining prominence not just as alternative therapies but as strategic components for cost optimization, treatment accessibility, clinical equivalence, and healthcare sustainability.
Rising cancer treatment demand and healthcare cost pressures in North America, Europe, and Asia Pacific amplify demand, while producers are picking up on innovations in manufacturing technologies and delivery system methods.
Pathways like advanced delivery systems, specialized formulations, and enhanced manufacturing promise strong margin uplift, especially in developed markets. Geographic expansion and healthcare system integration will capture volume, particularly where oncology care is growing or biosimilar infrastructure requires development. Quality pressures around clinical equivalence, manufacturing efficiency, cost optimization, and regulatory compliance give structural support.
- Pathway A - Advanced Delivery Systems. Healthcare providers increasingly require treatments that offer convenient administration and patient compliance. Producers who specialize in innovative delivery or enhance convenience capabilities can command a premium. Expected revenue pool: USD 930.0-1116.0 million
- Pathway B - Specialized Manufacturing & Quality. Enhanced manufacturing systems -- improved biosimilar production, advanced purification, quality optimization -- improve product consistency and regulatory compliance. Opportunity: USD 744.0-900.0 million
- Pathway C - Healthcare System Integration. Direct partnerships with healthcare systems, customized protocols, and clinical support optimize treatment delivery and enhance provider relationships. Systems with stronger integration capabilities will allow premium positioning. Revenue lift: USD 581.0-744.0 million
- Pathway D - Specialty Pharmacy Development. Extending reach into specialty pharmacy channels, patient support programs, and specialized distribution markets. Healthcare systems will look for biosimilar suppliers who provide comprehensive, accessible solutions. Pool: USD 525.0-688.0 million
- Pathway E - Emerging Market Expansion. Strong growth in developing healthcare markets, oncology care expansion, and biosimilar adoption. Local partnerships and manufacturing facilities help penetration. Expected upside: USD 465.0-581.0 million
- Pathway F - Clinical Support Services. Medical affairs services, clinical education, and outcomes research help healthcare providers ensure treatment effectiveness and patient outcomes. Service approaches benefit from long-term relationships. USD 325.0-418.0 million
- Pathway G - Cost Optimization Solutions. Healthcare economics, value demonstration, and cost-effectiveness programs create competitive advantages and healthcare system benefits. Pool: USD 279.0-372.0 million
Segmental Analysis
The market is segmented by route, setting, and product. By route, the market is divided into subcutaneous prefilled and vials/others. By setting, it covers hospital/oncology clinics, specialty pharmacies, and others. By product, it is segmented into on-body injector and standard PFS. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
By Route, the Subcutaneous Prefilled Segment Accounts for 82.0% Market Share
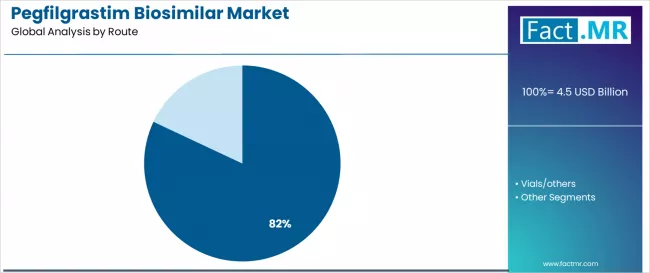
The subcutaneous prefilled route is projected to account for 82.0% of the pegfilgrastim biosimilar market in 2025, reaffirming its position as the leading administration category. Healthcare providers and oncology specialists increasingly utilize subcutaneous prefilled systems for their administration convenience, established clinical protocols, and proven effectiveness across oncology treatment, supportive care, and patient management applications. Subcutaneous prefilled route's established administration procedures and consistent delivery output directly address the healthcare requirements for reliable treatment delivery and clinical efficiency in diverse oncology environments.
This route segment forms the foundation of current market operations, as it represents the delivery method with the greatest clinical acceptance and established healthcare infrastructure across multiple applications and treatment scenarios. Healthcare investments in enhanced administration methods and patient convenience continue to strengthen adoption among oncology providers. With healthcare providers prioritizing treatment convenience and patient compliance, subcutaneous prefilled operations align with both clinical objectives and healthcare efficiency requirements, making them the central component of comprehensive pegfilgrastim biosimilar strategies.
By Setting, the Hospital/Oncology Clinics Segment Accounts for 64.0% Market Share
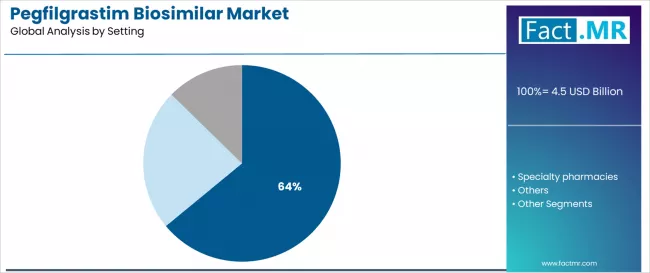
Hospital/oncology clinics settings are projected to represent 64.0% of pegfilgrastim biosimilar utilization in 2025, underscoring their critical role as the primary care requirement for oncology treatment and cancer supportive care. Hospital/oncology clinics prefer pegfilgrastim biosimilars for their clinical integration, established treatment protocols, and ability to provide comprehensive care while supporting patient outcomes and healthcare efficiency requirements. Positioned as essential treatment setting for modern oncology care, hospital/oncology clinics offer both clinical advantages and care coordination benefits.
The segment is supported by continuous innovation in oncology care and the growing availability of specialized treatment methods that enable comprehensive care with enhanced clinical capabilities. Additionally, healthcare providers are investing in care optimization to support patient outcomes and treatment efficiency delivery. As oncology care becomes more prevalent and treatment integration requirements increase, hospital/oncology clinics will continue to dominate the care setting market while supporting advanced clinical positioning and comprehensive care strategies.
By Product, the On-Body Injector Segment Accounts for 51.0% Market Share
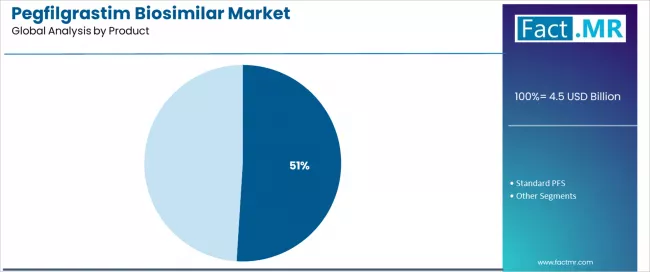
On-body injector products are projected to represent 51.0% of pegfilgrastim biosimilar delivery in 2025, underscoring their critical role as the primary product requirement for patient convenience and treatment compliance. Healthcare providers prefer on-body injectors for their administration convenience, patient independence, and ability to provide consistent delivery while supporting treatment compliance and care efficiency requirements. Positioned as essential delivery system for modern oncology supportive care, on-body injectors offer both convenience advantages and patient benefits.
The segment is supported by continuous innovation in delivery technology and the growing availability of specialized device methods that enable enhanced patient experience with improved compliance capabilities. Additionally, manufacturers are investing in device optimization to support patient convenience and healthcare efficiency delivery. As patient-centric care becomes more prevalent and convenience requirements increase, on-body injectors will continue to dominate the product market while supporting advanced patient experience and treatment compliance strategies.
What are the Drivers, Restraints, and Key Trends of the Pegfilgrastim Biosimilar Market?
The pegfilgrastim biosimilar market is advancing steadily due to increasing cancer treatment demand and growing adoption of cost-effective therapeutic alternatives that provide clinical equivalence and healthcare cost benefits across diverse oncology care applications. However, the market faces challenges, including complex regulatory requirements, manufacturing complexity costs, and varying adoption rates across different healthcare environments. Innovation in biosimilar manufacturing and delivery technologies continues to influence product development and market expansion patterns.
Rising Cancer Incidence and Healthcare Cost Pressures
The growing expansion of cancer treatment needs and healthcare cost containment initiatives is enabling producers to develop pegfilgrastim biosimilars that provide superior clinical equivalence, enhanced cost-effectiveness, and reliable therapeutic performance in oncology care environments. Advanced manufacturing systems provide improved biosimilar quality while allowing more effective cost reduction and consistent therapeutic delivery across various applications and treatment requirements. Producers are increasingly recognizing the competitive advantages of cost-effective manufacturing capabilities for market positioning and healthcare system targeting.
Manufacturing Innovation and Regulatory Excellence Drive Biosimilar Development
Modern pegfilgrastim biosimilar producers are incorporating advanced manufacturing methods and quality control systems to enhance therapeutic equivalence, improve manufacturing efficiency, and ensure consistent performance delivery to healthcare providers and oncology specialists. These technologies improve clinical standards while enabling new applications, including specialized delivery systems and patient-centric formulations. Advanced manufacturing integration also allows producers to support premium product positioning and clinical optimization beyond traditional biosimilar supply.
Analysis of the Pegfilgrastim Biosimilar Market by Key Countries

| Country | CAGR (2025-2035) |
|---|---|
| USA | 8.1% |
| Mexico | 7.7% |
| Germany | 7.3% |
| France | 7.2% |
| UK | 7.0% |
| South Korea | 6.9% |
| Japan | 6.8% |
The pegfilgrastim biosimilar market is experiencing robust growth globally, with the USA leading at an 8.1% CAGR through 2035, driven by extensive oncology care expansion, advanced healthcare infrastructure investments, and significant adoption of biosimilar treatment technologies.
Mexico follows at 7.7%, supported by growing healthcare development, rapid oncology modernization, and growing adoption of cost-effective treatment systems. Germany shows growth at 7.3%, emphasizing quality standards and healthcare capabilities.
France and UK record strong growth, focusing on healthcare system development and treatment optimization. South Korea demonstrates 6.9% growth, supported by healthcare trends and biosimilar adoption. Japan demonstrates 6.8% growth, supported by advanced healthcare but constrained by regulatory complexity.
The report covers an in-depth analysis of 40+ countries; seven top-performing countries are highlighted below.
USA Demonstrates Healthcare Innovation
The USA market emphasizes advanced delivery features, including precision administration control and integration with comprehensive oncology platforms that manage treatment optimization, cost containment, and patient care applications through unified biosimilar systems.
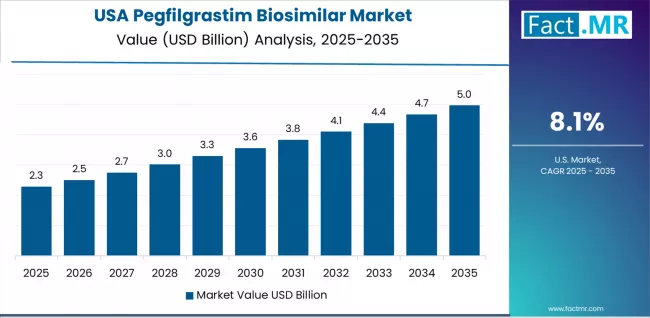
The country demonstrates strong growth at 8.1% CAGR, driven by oncology care expansion, healthcare cost pressures, and emerging value-based care applications that support biosimilar system integration. American healthcare providers prioritize clinical effectiveness with pegfilgrastim biosimilars delivering consistent therapeutic performance through advanced manufacturing capabilities and specialized treatment adaptation.
Technology deployment channels include major health systems, specialized oncology suppliers, and hospital procurement programs that support professional applications for complex cancer treatment and supportive care applications. Healthcare platform integration capabilities with established oncology systems expand market appeal across diverse treatment requirements seeking clinical benefits and cost efficiency. The resilient healthcare sector and expanding oncology capacity additions create sustained demand, while innovative applications in patient-centric care open new growth avenues.
Performance Metrics:
- Oncology care facilities in California, Texas, and New York leading pegfilgrastim biosimilar adoption for cancer treatment applications
- Specialty pharmacy channels maintaining 74% market share for complex oncology integration applications
- Hospital programs supporting 41% of biosimilar acquisitions across oncology and healthcare facilities
- Healthcare platform compatibility with major oncology systems driving procurement selection criteria
Mexico Maintains Healthcare Growth
Mexico's expanding healthcare market demonstrates sophisticated pegfilgrastim biosimilar adoption with documented clinical effectiveness in oncology care applications and healthcare facilities through integration with existing healthcare systems and treatment infrastructure.
The country leverages growing healthcare development and oncology care integration to maintain strong growth at 7.7% CAGR. Healthcare centers, including Mexico City, Guadalajara, and Monterrey, showcase premium applications where biosimilar systems integrate with comprehensive healthcare platforms and treatment management systems to optimize clinical outcomes and cost effectiveness.
Mexican healthcare companies prioritize treatment accessibility and clinical quality in pegfilgrastim biosimilar development, creating demand for premium biosimilar systems with advanced features, including cost optimization integration and healthcare delivery systems.
The market benefits from established healthcare infrastructure and a willingness to invest in oncology technologies that provide long-term treatment benefits and compliance with international healthcare and clinical standards. Premium oncology applications, healthcare delivery systems, and treatment programs drive diversified demand across multiple end-use segments.
Market Intelligence Brief:
- Healthcare market focuses on treatment accessibility and clinical quality compliance, driving premium segment growth
- Healthcare partnerships providing 43% faster development cycles
- Technology collaboration between Mexican healthcare companies and international biosimilar manufacturers
- Clinical training programs expanding pegfilgrastim biosimilar system integration in healthcare and oncology scenarios
Germany Emphasizes Clinical Excellence
Germany demonstrates steady market development with a 7.3% CAGR, distinguished by healthcare providers' preference for high-quality pegfilgrastim biosimilars that integrate seamlessly with existing oncology systems and provide reliable long-term therapeutic performance in specialized cancer care applications.
The market prioritizes advanced features, including precision clinical control, manufacturing quality, and integration with comprehensive healthcare platforms that reflect German healthcare expectations for therapeutic sophistication and clinical excellence.
Growth drivers encompass oncology care optimization applications, expanding healthcare requirements, and advanced treatment system integration. German healthcare providers emphasize quality control systems and comprehensive clinical documentation that align with domestic healthcare standards. The convergence of advanced biosimilar technology, oncology care presence, and traditional healthcare heritage creates diversified demand across multiple application segments.
Market Characteristics:
- Premium focus on subcutaneous prefilled systems with advanced manufacturing capabilities and precision clinical features
- Integration requirements with existing oncology and healthcare platforms
- Emphasis on therapeutic reliability and long-term clinical benefits in oncology applications
South Korea Emphasizes Healthcare Innovation
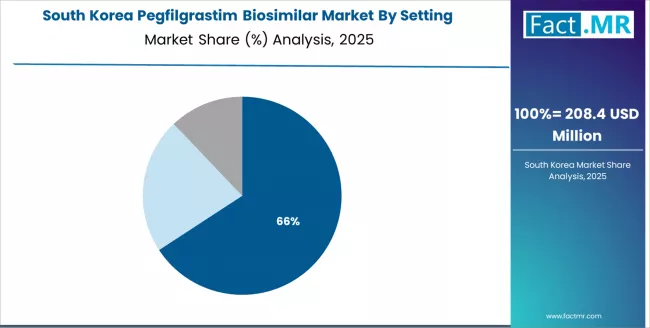
South Korea maintains robust development at 6.9% CAGR through healthcare expansion, oncology growth, and specialty treatment applications. South Korean healthcare facilities and oncology centers are implementing advanced pegfilgrastim biosimilar systems to enhance treatment capabilities and support clinical operations that align with healthcare regulations and quality standards.
Market expansion benefits from government healthcare programs that mandate biosimilar performance capabilities in oncology specifications, creating sustained demand where therapeutic flexibility and clinical compliance represent critical requirements.
Strategic Market Indicators:
- Healthcare and oncology facilities leading adoption with treatment modernization programs requiring advanced pegfilgrastim biosimilar systems
- Government healthcare programs providing regulatory support for advanced biosimilar system acquisition
- Clinical compliance requirements driving demand for certified systems with international therapeutic compatibility
- Specialized oncology and healthcare segments adopting comprehensive pegfilgrastim biosimilar solutions for treatment optimization
France Shows Healthcare Development
France demonstrates steady expansion at 7.2% CAGR through diversified demand from oncology programs, healthcare activities, and specialty treatment projects. Major healthcare hubs in regions throughout the country drive pegfilgrastim biosimilar adoption for oncology and specialty care production.
Healthcare heritage and oncology programs create sustained market demand, while specialty and advanced applications provide additional growth opportunities. Healthcare provider preference for clinical treatments and healthcare sophistication supports consistent market development.
Market Characteristics:
- Strong healthcare heritage and established oncology culture supporting premium positioning
- Advanced specialty care and oncology sectors driving demand for high-quality biosimilar treatments
- Clinical positioning capabilities and healthcare market presence supporting diversified revenue streams
United Kingdom Demonstrates Healthcare Innovation
The UK market maintains strong growth at 7.0% CAGR, driven by healthcare modernization, oncology development, and specialty treatment applications. British healthcare facilities and oncology centers are implementing advanced pegfilgrastim biosimilar systems to enhance treatment capabilities and support clinical operations that align with healthcare regulations and quality standards.
Market expansion benefits from healthcare programs that mandate treatment performance capabilities in oncology specifications, creating sustained demand where therapeutic flexibility and clinical compliance represent critical requirements.
Strategic Market Indicators:
- Healthcare and oncology facilities leading adoption with treatment modernization programs requiring advanced pegfilgrastim biosimilar systems
- Healthcare programs providing regulatory support for advanced biosimilar system acquisition
- Clinical compliance requirements driving demand for certified systems with therapeutic operational compatibility
- Specialized oncology and healthcare segments adopting comprehensive pegfilgrastim biosimilar solutions for treatment optimization
Japan Shows Clinical-Focused Development

Japan maintains steady expansion at 6.8% CAGR, distinguished by healthcare providers' preference for high-quality pegfilgrastim biosimilars that integrate seamlessly with existing treatment systems and provide reliable long-term therapeutic performance in specialized oncology applications.
The market prioritizes advanced features, including precision clinical control, manufacturing quality, and integration with comprehensive healthcare platforms that reflect Japanese healthcare expectations for therapeutic sophistication and clinical excellence.
High-specification oncology and clinical applications drive demand, supported by advanced biosimilar research and development initiatives. Japanese healthcare providers emphasize treatment purity, consistent performance characteristics, and comprehensive clinical documentation that aligns with stringent healthcare standards. The focus on clinical applications and therapeutic excellence supports stable growth despite regulatory complexity.
Market Characteristics:
- Clinical focus on on-body injector systems with advanced manufacturing capabilities and precision therapeutic features
- Integration requirements with existing healthcare and oncology platforms
- Emphasis on therapeutic reliability and long-term clinical benefits in oncology
Europe Market Split by Countries
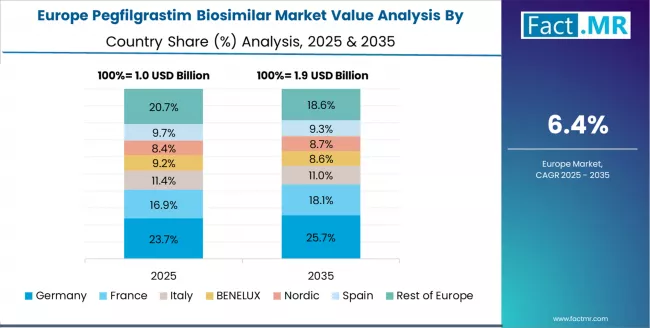
The pegfilgrastim biosimilar market in Europe is projected to grow from USD 1.35 billion in 2025 to USD 2.79 billion by 2035, registering a CAGR of 7.5% over the forecast period. Germany is expected to maintain its leadership position with a 28.9% market share in 2025, increasing to 29.4% by 2035, supported by its advanced healthcare infrastructure, comprehensive oncology capabilities, and major treatment facilities serving European and international markets.
France follows with a 24.8% share in 2025, projected to reach 25.2% by 2035, driven by established healthcare heritage, oncology programs, and clinical sophistication, supported by growing biosimilar adoption trends. United Kingdom holds a 22.1% share in 2025, expected to reach 22.6% by 2035, supported by healthcare modernization and oncology development.
The Netherlands commands a 11.7% share in 2025, projected to reach 11.4% by 2035, while Spain accounts for 8.9% in 2025, expected to reach 8.7% by 2035. The rest of Europe region is anticipated to maintain momentum, with its collective share from 3.6% to 2.7% by 2035, attributed to increasing healthcare development across Eastern European countries and growing oncology care across various European markets implementing treatment modernization programs.
Competitive Landscape of the Pegfilgrastim Biosimilar Market

The pegfilgrastim biosimilar market is characterized by competition among established biopharmaceutical companies, specialized biosimilar manufacturers, and integrated healthcare companies. Companies are investing in advanced manufacturing research, regulatory development, clinical optimization, and comprehensive product portfolios to deliver effective, equivalent, and cost-effective pegfilgrastim biosimilar solutions.
Innovation in manufacturing methods, delivery technologies, and clinical support capabilities is central to strengthening market position and competitive advantage. Viatris/Biocon leads the biosimilar market with an 18.0% market share, offering specialized biosimilar production with a focus on clinical equivalence and cost-effective manufacturing design for oncology care operations. Sandoz provides comprehensive biosimilar portfolios with an emphasis on regulatory excellence, manufacturing innovation, and global healthcare services.
Coherus delivers diverse biosimilar offerings with a focus on specialized delivery systems and healthcare market capabilities. Fresenius Kabi specializes in hospital-focused products with emphasis on healthcare integration and clinical optimization.
Pfizer focuses on integrated biopharmaceutical solutions with global operations and advanced manufacturing capabilities. Teva offers biosimilar products with emphasis on specialty pharmacy leadership and cost optimization. Celltrion provides biosimilar solutions with Asian market positioning and manufacturing efficiency.
The competitive landscape is evolving as companies invest in advanced manufacturing technologies, specialized delivery systems, and healthcare provider relationships. Market leaders are differentiating through regulatory expertise, proprietary manufacturing methods, and comprehensive clinical programs.
Innovation in manufacturing efficiency, therapeutic equivalence enhancement, and healthcare support platforms drives competitive positioning, while strategic partnerships with healthcare systems and oncology centers support market expansion and clinical development initiatives.
Key Players in the Pegfilgrastim Biosimilar Market
- Viatris Inc./Biocon Biologics Ltd.
- Sandoz International GmbH
- Coherus BioSciences Inc.
- Fresenius Kabi AG
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd.
- Celltrion Inc.
- Samsung Bioepis Co. Ltd.
- Mundipharma International Ltd.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 4.5 Billion |
| Route | Subcutaneous prefilled, Vials/others |
| Setting | Hospital/oncology clinics, Specialty pharmacies, Others |
| Product | On-body injector, Standard PFS |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | United States, Canada, Germany, United Kingdom, France, Japan, South Korea, Mexico, China and 40+ countries |
| Key Companies Profiled | Viatris/Biocon, Sandoz, Coherus, Fresenius Kabi, Pfizer, Teva, and Celltrion |
| Additional Attributes | Sales by route and setting category, regional demand trends, competitive landscape, technological advancements in biosimilar systems, manufacturing development, clinical innovation, and therapeutic optimization |
Pegfilgrastim Biosimilar Market by Segments
-
Route:
- Subcutaneous prefilled
- Vials/others
-
Setting:
- Hospital/oncology clinics
- Specialty pharmacies
- Others
-
Product:
- On-body injector
- Standard PFS
-
Region:
-
North America
- United States
- Canada
- Mexico
-
Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
-
Asia Pacific
- China
- Japan
- India
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
-
Latin America
- Brazil
- Chile
- Rest of Latin America
-
Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Union
- Rest of Middle East & Africa applications
-
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Route
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Route , 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Route , 2025 to 2035
- Subcutaneous prefilled
- Vials/others
- Y to o to Y Growth Trend Analysis By Route , 2020 to 2024
- Absolute $ Opportunity Analysis By Route , 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Setting
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Setting, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Setting, 2025 to 2035
- Hospital/oncology clinics
- Specialty pharmacies
- Others
- Y to o to Y Growth Trend Analysis By Setting, 2020 to 2024
- Absolute $ Opportunity Analysis By Setting, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2025 to 2035
- On-body injector
- Standard PFS
- Y to o to Y Growth Trend Analysis By Product, 2020 to 2024
- Absolute $ Opportunity Analysis By Product, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Route
- By Setting
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Route
- By Setting
- By Product
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Route
- By Setting
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Route
- By Setting
- By Product
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Route
- By Setting
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Route
- By Setting
- By Product
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Route
- By Setting
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Route
- By Setting
- By Product
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Route
- By Setting
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Route
- By Setting
- By Product
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Route
- By Setting
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Route
- By Setting
- By Product
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Route
- By Setting
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Route
- By Setting
- By Product
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Route
- By Setting
- By Product
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Route
- By Setting
- By Product
- Competition Analysis
- Competition Deep Dive
- Viatris Inc./Biocon Biologics Ltd.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Sandoz International GmbH
- Coherus BioSciences Inc.
- Fresenius Kabi AG
- Pfizer Inc.
- Teva Pharmaceutical Industries Ltd.
- Celltrion Inc.
- Samsung Bioepis Co. Ltd.
- Mundipharma International Ltd.
- Viatris Inc./Biocon Biologics Ltd.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Route , 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Setting, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Route , 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Setting, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Route , 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Setting, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Route , 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Setting, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Route , 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Setting, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Route , 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Setting, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Route , 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Setting, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Route , 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Setting, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by Product, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Route , 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Route , 2025-2035
- Figure 5: Global Market Attractiveness Analysis by Route
- Figure 6: Global Market Value Share and BPS Analysis by Setting, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Setting, 2025-2035
- Figure 8: Global Market Attractiveness Analysis by Setting
- Figure 9: Global Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Product, 2025-2035
- Figure 11: Global Market Attractiveness Analysis by Product
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025-2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025-2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025-2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025-2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025-2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Route , 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Route , 2025-2035
- Figure 25: North America Market Attractiveness Analysis by Route
- Figure 26: North America Market Value Share and BPS Analysis by Setting, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Setting, 2025-2035
- Figure 28: North America Market Attractiveness Analysis by Setting
- Figure 29: North America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Product, 2025-2035
- Figure 31: North America Market Attractiveness Analysis by Product
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Route , 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Route , 2025-2035
- Figure 35: Latin America Market Attractiveness Analysis by Route
- Figure 36: Latin America Market Value Share and BPS Analysis by Setting, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Setting, 2025-2035
- Figure 38: Latin America Market Attractiveness Analysis by Setting
- Figure 39: Latin America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Product, 2025-2035
- Figure 41: Latin America Market Attractiveness Analysis by Product
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Route , 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Route , 2025-2035
- Figure 45: Western Europe Market Attractiveness Analysis by Route
- Figure 46: Western Europe Market Value Share and BPS Analysis by Setting, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Setting, 2025-2035
- Figure 48: Western Europe Market Attractiveness Analysis by Setting
- Figure 49: Western Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by Product, 2025-2035
- Figure 51: Western Europe Market Attractiveness Analysis by Product
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Route , 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Route , 2025-2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Route
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Setting, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Setting, 2025-2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Setting
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by Product, 2025-2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by Product
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Route , 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Route , 2025-2035
- Figure 65: East Asia Market Attractiveness Analysis by Route
- Figure 66: East Asia Market Value Share and BPS Analysis by Setting, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Setting, 2025-2035
- Figure 68: East Asia Market Attractiveness Analysis by Setting
- Figure 69: East Asia Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by Product, 2025-2035
- Figure 71: East Asia Market Attractiveness Analysis by Product
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Route , 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Route , 2025-2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Route
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Setting, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Setting, 2025-2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Setting
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by Product, 2025-2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by Product
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Route , 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Route , 2025-2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Route
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Setting, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Setting, 2025-2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Setting
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by Product, 2025-2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by Product
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the pegfilgrastim biosimilar market in 2025?
The global pegfilgrastim biosimilar market is estimated to be valued at USD 4.5 billion in 2025.
What will be the size of pegfilgrastim biosimilar market in 2035?
The market size for the pegfilgrastim biosimilar market is projected to reach USD 9.3 billion by 2035.
How much will be the pegfilgrastim biosimilar market growth between 2025 and 2035?
The pegfilgrastim biosimilar market is expected to grow at a 7.5% CAGR between 2025 and 2035.
What are the key product types in the pegfilgrastim biosimilar market?
The key product types in pegfilgrastim biosimilar market are subcutaneous prefilled and vials/others.
Which setting segment to contribute significant share in the pegfilgrastim biosimilar market in 2025?
In terms of setting, hospital/oncology clinics segment to command 64.0% share in the pegfilgrastim biosimilar market in 2025.