Cannabis Pharmaceuticals Market
Cannabis Pharmaceuticals Market Size and Share Forecast Outlook 2025 to 2035
Cannabis pharmaceuticals market is projected to grow from USD 1.2 billion in 2025 to USD 90.4 billion by 2035, at a CAGR of 54.1%. Epidiolex will dominate with a 65.2% market share, while seizures will lead the application segment with a 42.5% share.
Cannabis Pharmaceuticals Market Forecast and Outlook 2025 to 2035
The global cannabis pharmaceuticals market is projected to reach USD 90.4 billion by 2035, recording an absolute increase of USD 89.2 billion over the forecast period. The market is valued at USD 1.2 billion in 2025 and is set to rise at a CAGR of 54.1% during the assessment period.
Quick Stats for Cannabis Pharmaceuticals Market
- Cannabis Pharmaceuticals Market Value (2025): USD 1.2 billion
- Cannabis Pharmaceuticals Market Forecast Value (2035): USD 90.4 billion
- Cannabis Pharmaceuticals Market Forecast CAGR: 54.1%
- Leading Brand Type in Cannabis Pharmaceuticals Market: Epidiolex
- Key Growth Regions in Cannabis Pharmaceuticals Market: North America, Europe, and Asia Pacific
- Top Players in Cannabis Pharmaceuticals Market: Jazz Pharmaceuticals plc, GW Pharmaceuticals, Canopy Growth Corporation, Tilray Brands, Inc., Aurora Cannabis Inc., Cronos Group Inc., Corbus Pharmaceuticals Holdings, Inc., AbbVie Inc., Emerald Health Pharmaceuticals Inc., INSYS Therapeutics, Inc.
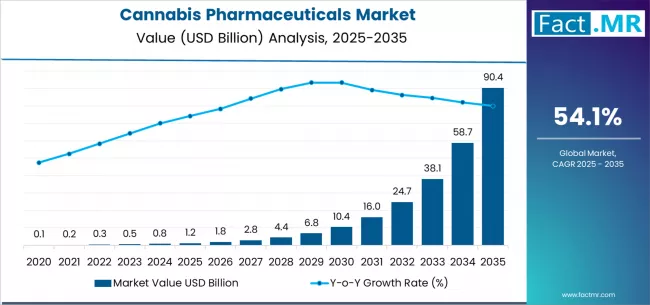
The overall market size is expected to grow by approximately 75.3 times during the same period, supported by increasing regulatory approvals for cannabis-based medications and expanding clinical evidence driving demand for pharmaceutical-grade cannabinoid formulations and increasing investments in cannabinoid drug development and therapeutic application research globally.
The pharmaceutical and neurology sectors face mounting pressure to deliver effective treatment alternatives while meeting evolving patient requirements for refractory epilepsy management and chronic pain control, with modern cannabis pharmaceutical products providing documented anticonvulsant efficacy and symptom relief capabilities compared to traditional treatment alternatives.
Rising acceptance of medical cannabis and expanding prescription authorization across developed economies create substantial opportunities for pharmaceutical manufacturers and specialty healthcare providers. However, regulatory complexity and inconsistent reimbursement policies may pose obstacles to market accessibility expansion.
The Epidiolex segment dominates market activity with approximately 65.2% share in 2025, driven by the extensive clinical evidence base supporting FDA-approved cannabidiol treatment with proven seizure reduction properties across drug-resistant epilepsy applications worldwide.
Neurologists and epileptologists increasingly recognize the transformative benefits of Epidiolex therapy, with typical treatment protocols providing effective seizure frequency reduction and improved quality of life at specialized neurology centers through established pharmaceutical distribution networks. Sativex demonstrates meaningful presence, supported by European regulatory approvals and multiple sclerosis spasticity management applications driving continued prescription in MS patient populations.
Seizures emerge as an important therapeutic category with a 42.5% share, reflecting neurologist emphasis on achieving seizure control in treatment-resistant epilepsy syndromes including Dravet syndrome and Lennox-Gastaut syndrome. Hospital pharmacies represent an important distribution channel segment, driven by specialty medication management requirements and controlled substance dispensing protocols for cannabis pharmaceutical administration across hospital-based neurology and pain management departments.
Regional dynamics show North America maintaining market leadership with strong presence in 2025, supported by FDA regulatory framework establishment and comprehensive medical cannabis acceptance across USA and Canada.
Europe demonstrates accelerating adoption trends driven by expanding national medical cannabis programs and pharmaceutical reimbursement integration, while Asia Pacific emphasizes emerging regulatory reforms and clinical research expansion.
The USA leads country-level growth at 54.2% CAGR through regulatory support evolution and growing epilepsy treatment acceptance, followed by Israel at 55.0% supported by advanced clinical pipeline development and pharmaceutical export capabilities.
The competitive landscape features moderate concentration with Jazz Pharmaceuticals plc holding a 19.8% market share, while established players including GW Pharmaceuticals, Canopy Growth Corporation, and Tilray Brands, Inc. compete through comprehensive product portfolios and pharmaceutical development capabilities across diverse therapeutic applications.
Cannabis Pharmaceuticals Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the cannabis pharmaceuticals market is projected to expand from USD 1.2 billion to USD 7.0 billion, resulting in a value increase of USD 5.8 billion, which represents 6.5% of the total forecast growth for the period. This phase of development will be shaped by accelerating regulatory approvals for cannabis-based medications in epilepsy and pain management indications, expanded clinical trial programs validating therapeutic efficacy across multiple neurological conditions, as well as increasing integration with specialty pharmacy networks and neurology treatment protocols. Companies are establishing competitive positions through investment in pharmaceutical-grade cultivation facilities, cannabinoid extraction technologies, and strategic market expansion across epilepsy treatment, multiple sclerosis management, and chronic pain applications.
From 2029 to 2035, the market is forecast to grow from USD 7.0 billion to USD 90.4 billion, adding another USD 83.4 billion, which constitutes 93.5% of the overall expansion. This period is expected to be characterized by the expansion of novel cannabinoid pharmaceutical applications, including mental health disorders and neurodegenerative disease treatments tailored for specific patient populations, strategic collaborations between cannabis pharmaceutical companies and major pharmaceutical corporations, and an enhanced focus on synthetic cannabinoid development and precision medicine formulations. The growing emphasis on opioid alternative therapies in pain management and rising demand for evidence-based cannabinoid medications will drive comprehensive cannabis pharmaceutical solutions across diverse therapeutic applications.
Cannabis Pharmaceuticals Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 1.2 billion |
| Market Forecast Value (2035) | USD 90.4 billion |
| Forecast CAGR (2025-2035) | 54.1% |
Why is the Cannabis Pharmaceuticals Market Growing?
The cannabis pharmaceuticals market grows by enabling neurologists and pain management specialists to access FDA-approved cannabinoid medications while providing evidence-based treatment alternatives without compromising pharmaceutical quality standards. Physicians and healthcare systems face mounting pressure to address treatment-resistant epilepsy and chronic pain conditions while managing patient safety concerns across diverse neurological disorders and symptom management needs, with modern cannabis pharmaceutical products typically providing superior standardized dosing and clinical validation compared to unregulated medical cannabis alternatives, making pharmaceutical-grade adoption essential for evidence-based medicine positioning. The neurology and pain management sectors' need for effective anticonvulsant therapies and non-opioid analgesic capabilities creates demand for comprehensive cannabis pharmaceutical solutions that can provide superior symptom control, maintain consistent therapeutic effects, and ensure regulatory compliance without compromising patient safety or prescribing physician liability standards.
Growing clinical evidence from randomized controlled trials drives adoption in epilepsy centers, neurology departments, and pain management clinics, where treatment performance has a direct impact on seizure control outcomes and quality-of-life improvements. The opioid crisis has created lasting changes in pain management prescribing patterns and alternative therapy evaluation, supporting sustained demand for effective cannabis-based analgesic products across all healthcare settings. Expanding insurance coverage for FDA-approved cannabinoid medications in developed markets enables greater patient access to expensive pharmaceutical-grade treatments with proven therapeutic benefit and safety profiles. However, regulatory restrictions and stigma concerns may limit prescriber adoption among conservative medical communities in regions with limited cannabis pharmaceutical education and clinical experience.
Segmental Analysis
The market is segmented by brand type, application, distribution channel, and region. By brand type, the market is divided into Epidiolex, Sativex, and others. Based on application, the market is categorized into pain management, nausea, seizures, multiple sclerosis (MS), and others. By distribution channel, the market includes hospital pharmacies, retail pharmacies, and online pharmacies. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Brand Type, Which Segment Accounts for the Dominant Market Share?
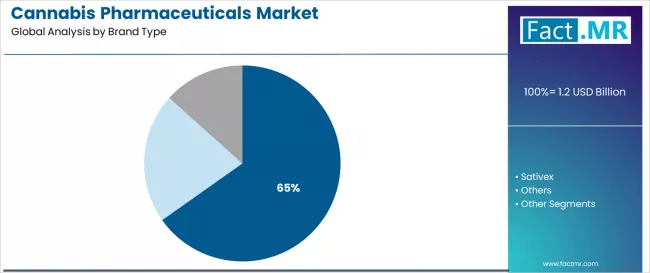
The Epidiolex segment represents the dominant force in the cannabis pharmaceuticals market, capturing approximately 65.2% of total market share in 2025. This established pharmaceutical product encompasses solutions featuring FDA-approved cannabidiol formulation and rigorous clinical validation, including advanced seizure reduction capabilities and comprehensive safety data that enable superior acceptance among neurologists and regulatory confidence across all drug-resistant epilepsy treatment applications worldwide. The Epidiolex segment's market leadership stems from its pioneering regulatory approval status, with formulations capable of addressing severe childhood epilepsy syndromes while maintaining pharmaceutical-grade consistency and extensive clinical evidence supporting epilepsy specialist utilization across diverse patient populations.
The Sativex segment maintains meaningful market presence, serving multiple sclerosis patients and healthcare providers who require oromucal spray formulations with balanced THC:CBD ratios for spasticity management and neuropathic pain control in MS populations. These solutions offer targeted symptom relief for patients experiencing muscle stiffness while providing sufficient efficacy to meet European regulatory standards and clinical guidelines. The Sativex segment demonstrates established market position in European markets, driven by regulatory approvals and reimbursement coverage supporting MS specialist prescribing.
Within the Epidiolex segment, Dravet syndrome and Lennox-Gastaut syndrome indications command dominant share, driven by orphan drug designation and demonstrated efficacy in reducing convulsive seizure frequency in pediatric epilepsy populations. This application benefits from extensive clinical trial evidence and neurologist familiarity with prescribing protocols across specialized epilepsy treatment centers.
Key advantages driving the Epidiolex segment include:
- FDA regulatory approval status with pharmaceutical-grade manufacturing standards that enhance prescriber confidence and ensure consistent therapeutic delivery
- Established clinical protocols enabling evidence-based epilepsy treatment across different seizure syndrome categories without regulatory uncertainty
- Enhanced safety profile features enabling pediatric use while maintaining favorable adverse event characteristics and drug interaction management
- Superior market positioning providing first-mover advantage for pharmaceutical-grade cannabinoid medications across epilepsy treatment segments and neurology specialist acceptance
By Application, Which Segment Accounts for the Largest Market Share?
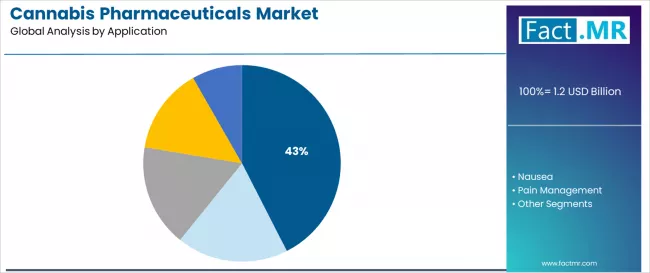
Seizures dominate the cannabis pharmaceuticals application landscape with approximately 42.5% market share in 2025, reflecting the critical role of cannabidiol therapy in supporting treatment-resistant epilepsy management and pediatric seizure disorder control across specialized neurology centers worldwide. The seizures segment's market leadership is reinforced by FDA approval for specific epilepsy syndromes, extensive clinical trial evidence, and orphan drug status combined with neurologist confidence in pharmaceutical-grade cannabinoid anticonvulsant therapy.
Within this segment, drug-resistant epilepsy applications including Dravet syndrome and Lennox-Gastaut syndrome represent dominant share, driven by limited alternative treatment options and demonstrated Epidiolex efficacy in reducing seizure burden. This sub-segment benefits from pediatric epilepsy specialist adoption and comprehensive caregiver support programs facilitating treatment access.
The pain management segment represents an important application category, demonstrating growth potential through specialized requirements for chronic pain treatment, neuropathic pain management, and opioid-sparing analgesic approaches in pain medicine applications. This segment benefits from growing physician interest in non-opioid pain management alternatives that address prescription opioid concerns.
The multiple sclerosis segment maintains meaningful presence through Sativex utilization for MS spasticity management and symptom control, while nausea applications serve chemotherapy-induced nausea and vomiting management in oncology supportive care settings.
Key market dynamics supporting application growth include:
- Seizure disorder focus driven by regulatory approval pathways and orphan drug incentives, requiring specialized neurology expertise
- Pain management evolution requiring non-opioid alternatives and evidence-based cannabinoid analgesic validation
- Integration of cannabis pharmaceuticals across neurological applications enabling comprehensive therapeutic benefits beyond single indication focus
- Growing emphasis on quality-of-life outcomes driving adoption in symptom management and palliative care applications
What are the Drivers, Restraints, and Key Trends of the Cannabis Pharmaceuticals Market?
The market is driven by three concrete demand factors tied to regulatory advancement and therapeutic validation. First, accelerating FDA and international regulatory approvals for cannabis-based pharmaceutical products create expanding treatment options for previously underserved patient populations, with pharmaceutical-grade cannabinoid medications representing critical therapeutic advances for refractory epilepsy worldwide, requiring comprehensive clinical development programs. Second, growing clinical evidence from rigorous randomized controlled trials and real-world studies drives physician confidence in cannabinoid therapy efficacy, with major medical associations increasingly recognizing legitimate therapeutic applications for pharmaceutical cannabis products by 2030. Third, opioid crisis response initiatives and alternative pain management mandates enable broader acceptance of cannabis pharmaceuticals as non-addictive treatment options that improve patient safety while reducing prescription opioid dependence and overdose risks.
Market restraints include complex regulatory frameworks with inconsistent international cannabis pharmaceutical policies that can challenge manufacturers in achieving global market access capabilities, particularly in jurisdictions where cannabis scheduling conflicts with pharmaceutical approval pathways and prescribing authorization proves limited. High development costs and extensive clinical trial requirements for cannabis pharmaceutical approval pose another significant challenge, as regulatory validation depends on comprehensive safety and efficacy data generation with substantial investment timelines, potentially affecting smaller companies and limiting pipeline development. Reimbursement uncertainty and limited insurance coverage for cannabis pharmaceuticals create additional complexity for patient access, demanding manufacturer patient assistance programs and prior authorization navigation to overcome affordability barriers.
Key trends indicate accelerated synthetic cannabinoid development in pharmaceutical pipelines, particularly North America and Europe, where companies demonstrate investment in novel cannabinoid molecules with improved pharmacokinetic profiles and targeted receptor selectivity. Pharmaceutical industry partnership trends toward major pharmaceutical companies acquiring cannabis pharmaceutical specialists and licensing cannabinoid compounds enable validation approaches that leverage established development expertise while accessing novel therapeutic mechanisms. However, the market thesis could face disruption if adverse safety signals emerge from long-term cannabinoid exposure studies or if alternative non-cannabinoid therapies demonstrate superior efficacy in core epilepsy and pain management applications.
Analysis of the Cannabis Pharmaceuticals Market by Key Countries
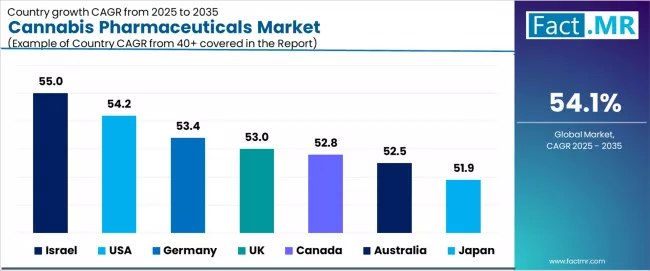
| Country | CAGR (2025-2035) |
|---|---|
| Israel | 55.0% |
| USA | 54.2% |
| Germany | 53.4% |
| UK | 53.0% |
| Canada | 52.8% |
| Australia | 52.5% |
| Japan | 51.9% |
The global cannabis pharmaceuticals market is expanding explosively, with Israel leading at a 55.0% CAGR through 2035, driven by advanced clinical pipeline development, government research support, and pharmaceutical export opportunities supporting cannabinoid drug innovation. USA follows at 54.2%, supported by FDA regulatory framework maturation, growing epilepsy treatment acceptance, and expanding state-level medical cannabis integration.
Germany records 53.4%, reflecting rising medical cannabis prescriptions, insurance reimbursement inclusion, and pharmaceutical industry engagement. UK grows at 53.0%, anchored by expanding NHS clinical trials and regulatory pathway development.
Canada advances at 52.8%, leveraging federal legalization framework and pharmaceutical R&D capabilities. Australia posts 52.5%, focusing on Therapeutic Goods Administration reforms and medical cannabis access expansion, while Japan grows steadily at 51.9%, emphasizing legal progress and CBD pharmaceutical adoption.
How is Israel Leading Global Market Innovation?
Israel demonstrates the strongest growth potential in the cannabis pharmaceuticals market with a CAGR of 55.0% through 2035. The country's leadership position stems from pioneering cannabis research infrastructure, government support for cannabinoid pharmaceutical development, and extensive clinical trial capabilities driving therapeutic innovation.
Growth is concentrated in pharmaceutical research institutions and biotechnology clusters, including Tel Aviv, Jerusalem, and Haifa, where research organizations are advancing novel cannabinoid compounds and conducting comprehensive clinical studies for regulatory approval pathways.
Pharmaceutical development through academic-industry partnerships and government-funded research programs expands innovation capacity across previously unexplored therapeutic applications. The country's established medical cannabis framework and export-oriented pharmaceutical strategy provide strong momentum for cannabis pharmaceutical commercialization, including extensive international partnership development across regulatory jurisdictions.
Key market factors:
- Research excellence concentrated in academic medical centers with established cannabinoid pharmacology expertise
- Government innovation support through research grants and pharmaceutical development incentives
- Comprehensive clinical trial infrastructure, including patient recruitment capabilities and regulatory experience
- Export market development featuring partnerships with international pharmaceutical companies seeking cannabinoid therapeutics
Why is USA Emerging as a High-Growth Market?
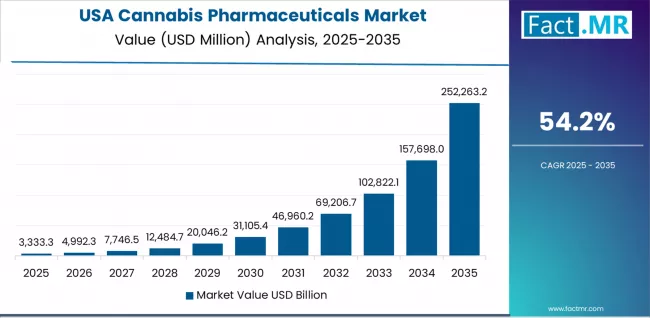
In major medical centers across regions including Massachusetts, California, New York, and Colorado, the adoption of FDA-approved cannabis pharmaceuticals is accelerating among neurologists and pain specialists, driven by Epidiolex approval precedent and expanding clinical acceptance of cannabinoid therapeutics.
The market demonstrates exceptional growth momentum with a CAGR of 54.2% through 2035, linked to federal regulatory pathway establishment and increasing physician education on pharmaceutical-grade cannabinoid prescribing. American healthcare providers are implementing evidence-based cannabis pharmaceutical protocols and establishing specialty pharmacy distribution systems to provide controlled access while meeting regulatory compliance expectations for Schedule V controlled substance management.
The country's robust pharmaceutical development infrastructure creates ongoing opportunities for pipeline advancement, while increasing state-level medical cannabis programs drive broader therapeutic acceptance and patient demand for standardized pharmaceutical products.
Key development areas:
- Epilepsy centers and neurology departments leading Epidiolex adoption with emphasis on pediatric seizure disorder management
- Pharmaceutical pipeline expansion through biotech companies developing novel cannabinoid therapeutics
- Specialty pharmacy network integration enabling controlled distribution and patient monitoring programs
- Growing clinical research infrastructure supporting investigational cannabinoid drug trials across multiple therapeutic areas
What Drives the Cannabis Pharmaceuticals Market Growth in Germany?
Germany’s market expansion is driven by comprehensive medical cannabis prescription framework, including physician authorization pathways and health insurance reimbursement coverage supporting pharmaceutical-grade cannabinoid access.
The country demonstrates strong growth potential with a CAGR of 53.4% through 2035, supported by established pharmaceutical industry engagement and expanding clinical guideline integration from medical specialty societies.
Physicians face evolving therapeutic landscapes related to cannabis pharmaceutical prescribing education and patient selection criteria, requiring manufacturers to provide comprehensive medical information and clinical support resources.
Expanding insurance coverage and specialized pharmacy distribution create substantial treatment access infrastructure for cannabis pharmaceutical utilization, particularly in pain management applications where conventional therapy limitations drive alternative medication consideration.
Market characteristics:
- Medical cannabis prescription growth across pain clinics and specialized treatment centers
- Insurance reimbursement frameworks providing coverage for approved cannabis pharmaceutical indications
- Future projections indicate continued pharmaceutical development with emphasis on standardized therapeutic products
- Growing emphasis on clinical evidence generation and real-world outcomes supporting reimbursement justification
How does the UK Demonstrate Clinical Development in the Cannabis Pharmaceuticals Domain?
The UK market demonstrates advancing cannabis pharmaceutical adoption based on expanding NHS clinical trial programs and regulatory framework evolution for medicinal cannabis products. The country shows strong potential with a CAGR of 53.0% through 2035, driven by specialist prescribing pathways and academic medical center leadership in major healthcare regions, including London, Manchester, Birmingham, and Edinburgh teaching hospitals.
Clinicians are participating in cannabinoid pharmaceutical research and implementing specialized prescribing protocols for eligible patient populations, particularly in epilepsy and palliative care demanding comprehensive evidence-based treatment credentials. NHS commissioning through specialist clinics and tertiary care centers expands coverage across patient populations requiring ongoing cannabis pharmaceutical therapy under specialist supervision.
Leading market segments:
- Academic medical centers and NHS trusts implementing cannabis pharmaceutical prescribing programs
- Clinical trial networks with pharmaceutical sponsors achieving high-quality evidence generation rates
- Strategic patient access initiatives expanding availability through specialist prescriber networks
- Focus on pediatric epilepsy and refractory symptom management addressing unmet medical needs
What Positions Canada for Market Expansion?
In provinces including Ontario, Quebec, British Columbia, and Alberta, healthcare providers are implementing cannabis pharmaceutical prescribing within comprehensive federal legalization framework featuring pharmaceutical-grade product standards and medical access pathways.
The market shows solid growth potential with a CAGR of 52.8% through 2035, linked to advanced research and development infrastructure, government pharmaceutical innovation support, and established medical cannabis patient populations.
Healthcare providers in Canada are optimizing cannabis pharmaceutical utilization in neurological disorders and chronic pain applications to achieve therapeutic outcomes while maintaining regulatory compliance standards demanded by federal cannabis regulations and provincial healthcare requirements.
The country's pharmaceutical research capabilities create ongoing opportunities for cannabinoid drug development that differentiates through clinical innovation and regulatory expertise.
Market development factors:
- Federal legalization framework providing regulatory clarity for pharmaceutical cannabis development
- Research funding supporting cannabinoid therapeutic development through academic and industry partnerships
- Established patient populations with medical cannabis experience facilitating pharmaceutical product transition
- Emphasis on pharmaceutical-grade standardization and quality control addressing medical product requirements
How does Australia show Expansion Prospects amid Regulatory Progress?
Australia's cannabis pharmaceuticals market demonstrates expanding therapeutic access focused on Therapeutic Goods Administration regulatory reforms and special access scheme utilization, with documented patient access growth showing substantial progress in cannabinoid medicine availability across specialized medical applications.
The country maintains strong growth momentum with a CAGR of 52.5% through 2035, driven by regulatory pathway development emphasizing pharmaceutical cannabis product approval and medical practitioner prescribing authorization that align with therapeutic goods standards applied to scheduled medicines.
Major metropolitan regions, including Sydney, Melbourne, Brisbane, and Perth, showcase advancing cannabis pharmaceutical prescribing where specialist physicians integrate cannabinoid therapies with conventional treatment protocols and comprehensive patient monitoring systems.
Key market characteristics:
- Specialist physicians and cannabis clinics driving pharmaceutical cannabinoid prescribing with emphasis on regulatory compliance
- Therapeutic Goods Administration pathway enabling pharmaceutical product registration and patient access
- Clinical evidence generation supporting regulatory submissions and medical community acceptance
- Emphasis on pharmaceutical-grade quality and standardized dosing addressing medical safety requirements
What Characterizes the Market’s Evolution in Japan?
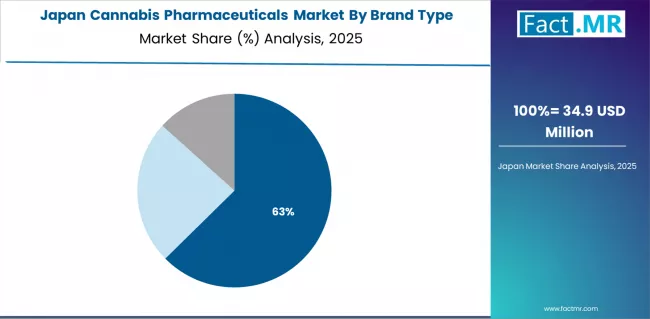
Japan's cannabis pharmaceuticals market demonstrates emerging therapeutic acceptance focused on CBD-based pharmaceutical development and regulatory framework evolution, with documented legal progress showing substantial advancement in cannabinoid medicine consideration across pharmaceutical regulatory pathways.
The country maintains solid growth momentum with a CAGR of 51.9% through 2035, driven by pharmaceutical industry interest emphasizing controlled cannabinoid compound development and medical application validation that align with Japanese pharmaceutical standards applied to novel therapeutic substances.
Major pharmaceutical hubs, including Tokyo, Osaka, Nagoya, and Fukuoka, showcase evolving cannabis pharmaceutical research where biotechnology companies integrate cannabinoid drug candidates with rigorous development protocols and comprehensive regulatory navigation strategies.
Key market characteristics:
- Pharmaceutical companies and research institutions driving cannabinoid therapeutic development with emphasis on regulatory pathway navigation
- Legal framework evolution enabling CBD pharmaceutical consideration while maintaining controlled substance regulation
- Clinical research collaboration supporting international pharmaceutical partnership and development expertise transfer
- Emphasis on rigorous safety validation and pharmaceutical quality standards addressing Japanese regulatory requirements
Europe Market Split by Country
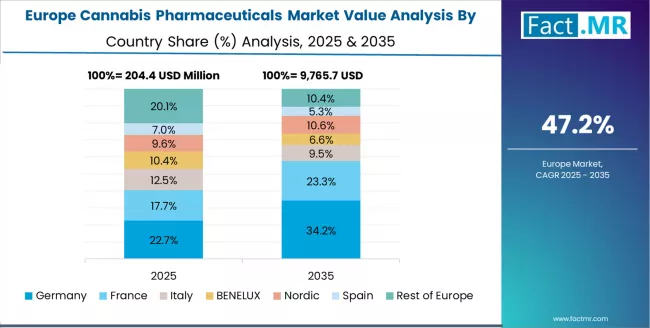
The cannabis pharmaceuticals market in Europe is projected to grow from USD 285.0 million in 2025 to USD 21.5 billion by 2035, registering a CAGR of 53.2% over the forecast period. Germany is expected to maintain its leadership position with a 32.5% market share in 2025, adjusting slightly to 32.2% by 2035, supported by its comprehensive medical cannabis prescription framework, established pharmaceutical industry engagement, and progressive insurance reimbursement policies serving major European patient populations.
The UK follows with a 24.0% share in 2025, projected to reach 24.3% by 2035, driven by comprehensive NHS clinical trial programs in major medical centers implementing cannabis pharmaceutical research protocols. France holds a 18.5% share in 2025, expected to maintain 18.6% by 2035 through ongoing medical cannabis experimentation programs and pharmaceutical regulatory pathway development.
Italy commands a 13.0% share, while Spain accounts for 9.0% in 2025. The rest of Europe is anticipated to gain momentum, expanding its collective share from 3.0% to 3.4% by 2035, attributed to increasing cannabis pharmaceutical interest in Nordic countries and emerging Eastern European medical cannabis frameworks implementing pharmaceutical product regulations.
Competitive Landscape of the Cannabis Pharmaceuticals Market
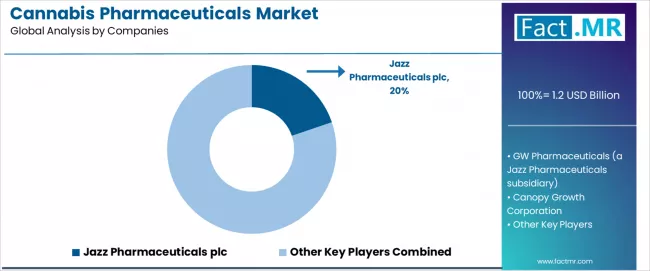
The cannabis pharmaceuticals market features approximately 10-15 meaningful players with moderate concentration, where the top three companies control roughly 45-50% of global market share through established pharmaceutical product franchises and comprehensive development pipelines.
Competition centers on regulatory approval achievement, clinical evidence generation, and pharmaceutical-grade manufacturing capabilities rather than differentiation through cultivation alone.
Market leaders include Jazz Pharmaceuticals plc, GW Pharmaceuticals (a Jazz Pharmaceuticals subsidiary), and Canopy Growth Corporation, which maintain competitive advantages through FDA-approved product portfolios, extensive clinical development expertise, and deep pharmaceutical industry experience, creating strong relationships among neurologists and specialty pharmacies.
These companies leverage established regulatory pathways and ongoing pipeline development initiatives to defend market positions while expanding into adjacent therapeutic indications and international market opportunities.
Challengers encompass Tilray Brands, Inc. and Aurora Cannabis Inc., which compete through diversified pharmaceutical development programs and vertical integration strategies in key cannabis pharmaceutical markets.
Specialty pharmaceutical and biotechnology companies, including Cronos Group Inc., Corbus Pharmaceuticals Holdings, Inc., and emerging pipeline developers, focus on novel cannabinoid molecules or specific therapeutic applications, offering differentiated capabilities in synthetic cannabinoid development, rare disease programs, and specialized formulation technologies.
Established pharmaceutical companies including AbbVie Inc. and other major pharmaceutical corporations create competitive presence through cannabinoid pharmaceutical acquisitions, in-licensing agreements, and internal development programs, particularly in markets with proven therapeutic validation.
Market dynamics favor companies that combine pharmaceutical development expertise with comprehensive clinical evidence generation that addresses regulatory approval requirements from early-stage research through post-marketing surveillance and real-world evidence programs.
Strategic emphasis on synthetic cannabinoid innovation, precision medicine applications, and pharmaceutical partnership development enables differentiation in increasingly competitive therapeutic landscapes across epilepsy, pain management, and emerging neurological indications.
Global Cannabis Pharmaceuticals Market - Stakeholder Contribution Framework
Cannabis pharmaceutical solutions represent a transformative therapeutic category that enables neurologists, pain specialists, and healthcare systems to access FDA-approved cannabinoid medications and evidence-based treatment alternatives without relying on unregulated medical cannabis products, typically providing pharmaceutical-grade standardization and rigorous clinical validation compared to botanical cannabis alternatives while ensuring consistent therapeutic effects and regulatory compliance outcomes.
With the market projected to grow from USD 1.2 billion in 2025 to USD 90.4 billion by 2035 at a 54.1% CAGR, these solutions offer compelling advantages - regulatory approval status, clinical efficacy validation, and pharmaceutical quality assurance - making them essential for Epidiolex applications (65.2% brand share), seizure disorder management (42.5% application share), and diverse patient populations requiring evidence-based cannabinoid therapeutics. Scaling therapeutic access and clinical adoption requires coordinated action across pharmaceutical policy, healthcare infrastructure, drug manufacturers, specialty pharmacies, and medical education initiatives.
How Could Governments Spur Local Development and Adoption?
- Pharmaceutical Policy Development: Include cannabis pharmaceuticals in essential medicines lists, provide regulatory clarity for cannabinoid drug approval pathways, and support clinical research through government funding and academic partnership programs.
- Reimbursement Policy & Access Support: Implement comprehensive insurance coverage for FDA-approved cannabis pharmaceuticals in validated indications, establish patient assistance programs reducing financial barriers for expensive cannabinoid medications, and create streamlined prior authorization processes that facilitate appropriate prescribing.
- Regulatory Framework Harmonization: Develop consistent cannabis pharmaceutical regulations balancing controlled substance oversight with therapeutic access needs, establish clear prescriber authorization pathways and dispensing protocols, and create international regulatory cooperation enabling multi-national drug development and approval processes.
- Medical Education & Training: Fund continuing medical education programs for physicians on evidence-based cannabis pharmaceutical prescribing, invest in specialty training for neurologists and pain specialists managing cannabinoid therapy patients, and support pharmacist education on cannabis pharmaceutical dispensing and patient counseling.
- Research Infrastructure Support: Establish government-funded cannabis pharmaceutical research centers, provide clinical trial infrastructure supporting cannabinoid drug development, and create data registries tracking real-world cannabis pharmaceutical outcomes and safety monitoring.
How Could Industry Bodies Support Market Development?
- Clinical Guidelines & Standards: Define evidence-based prescribing guidelines for cannabis pharmaceuticals across validated therapeutic indications, establish quality standards for pharmaceutical-grade cannabinoid products, and create best practice protocols that physicians can reference.
- Medical Education & Awareness: Lead professional education demonstrating cannabis pharmaceutical therapeutic benefits, emphasizing regulatory approval status and clinical evidence differentiation from unregulated medical cannabis products.
- Prescriber Support Programs: Develop continuing education certification for cannabis pharmaceutical prescribing, provide clinical decision support tools and patient selection criteria, and establish peer networks facilitating knowledge sharing among experienced prescribers.
- Quality Assurance Standards: Run certification programs for pharmaceutical manufacturing facilities, analytical testing laboratories, and dispensing pharmacies ensuring pharmaceutical-grade cannabinoid product quality throughout supply chain operations.
How Could Pharmaceutical Manufacturers and Biotechnology Companies Strengthen the Ecosystem?
- Clinical Development Programs: Conduct rigorous phase III trials demonstrating cannabinoid pharmaceutical efficacy and safety, develop biomarker strategies enabling patient selection optimization, and generate long-term safety data addressing regulatory requirements and prescriber confidence building.
- Pharmaceutical Innovation: Provide novel cannabinoid molecules with improved therapeutic profiles, develop enhanced delivery systems optimizing bioavailability and patient convenience, and create combination products addressing unmet medical needs through synergistic mechanisms.
- Patient Support Programs: Offer comprehensive patient assistance including financial support for uninsured patients, nurse educator services supporting therapy initiation and monitoring, and adherence programs optimizing treatment outcomes through patient engagement.
- Medical Affairs & Education: Build comprehensive healthcare provider education capabilities, provide clinical research support for investigator-initiated studies, and develop real-world evidence programs documenting therapeutic value in diverse patient populations.
How Could Healthcare Systems and Specialty Pharmacies Navigate the Market?
- Specialty Pharmacy Development: Establish cannabis pharmaceutical dispensing programs within hospital and retail pharmacy settings offering comprehensive patient counseling, medication therapy management, and regulatory compliance protocols, with particular focus on controlled substance handling and adverse event monitoring.
- Prescriber Education Initiatives: Implement continuing education programs for physicians covering cannabis pharmaceutical evidence, appropriate patient selection, and dosing protocols, while strengthening coordination across neurology departments, pain clinics, and palliative care services.
- Patient Access Programs: Develop navigation services combining specialty pharmacy support with financial assistance coordination, prior authorization management, and insurance appeal support that optimize therapy access and initiation success.
- Outcomes Monitoring: Participate in registry programs and real-world evidence initiatives documenting cannabis pharmaceutical effectiveness, safety profiles, and quality-of-life improvements that inform clinical practice optimization and reimbursement policy development.
How Could Investors and Financial Enablers Unlock Value?
- Pipeline Development Financing: Provide growth capital for companies developing novel cannabinoid pharmaceuticals, particularly programs addressing high unmet need therapeutic areas including neurodegenerative diseases, psychiatric disorders, and inflammatory conditions.
- Manufacturing Infrastructure Investment: Back pharmaceutical-grade cannabis cultivation and extraction facilities, support GMP manufacturing capacity development, and finance analytical testing laboratories ensuring product quality and regulatory compliance.
- Regulatory Milestone Funding: Finance clinical trial programs through FDA approval milestones, support regulatory strategy development and submission preparation, and provide commercialization capital enabling product launch and market access initiatives.
- Strategic Consolidation Opportunities: Support pharmaceutical industry acquisitions of promising cannabis biotechnology companies, finance licensing agreements enabling major pharmaceutical companies to access cannabinoid therapeutics, and back platform companies developing multiple cannabinoid drug candidates across diverse indications.
Key Players in the Cannabis Pharmaceuticals Market
- Jazz Pharmaceuticals plc
- GW Pharmaceuticals (a Jazz Pharmaceuticals subsidiary)
- Canopy Growth Corporation
- Tilray Brands, Inc.
- Aurora Cannabis Inc.
- Cronos Group Inc.
- Corbus Pharmaceuticals Holdings, Inc.
- AbbVie Inc.
- Emerald Health Pharmaceuticals Inc.
- INSYS Therapeutics, Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 1.2 Billion |
| Brand Type | Epidiolex, Sativex, Others |
| Application | Pain Management, Nausea, Seizures, Multiple Sclerosis (MS), Others |
| Distribution Channel | Hospital Pharmacies, Retail Pharmacies, Online Pharmacies |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | USA, Canada, Germany, UK, Israel, Australia, Japan, and 40+ countries |
| Key Companies Profiled | Jazz Pharmaceuticals plc, GW Pharmaceuticals, Canopy Growth Corporation, Tilray Brands, Inc., Aurora Cannabis Inc., Cronos Group Inc., Corbus Pharmaceuticals Holdings, Inc., AbbVie Inc., Emerald Health Pharmaceuticals Inc., INSYS Therapeutics, Inc. |
| Additional Attributes | Dollar sales by brand type and application categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with pharmaceutical manufacturers and cannabis biotechnology companies, product specifications and regulatory approval requirements, integration with specialty pharmacy networks and neurology treatment protocols, innovations in cannabinoid drug development and pharmaceutical formulation technologies, and development of specialized applications with seizure control efficacy and pharmaceutical-grade quality standards. |
Cannabis Pharmaceuticals Market by Segments
-
Brand Type :
- Epidiolex
- Sativex
- Others
-
Application :
- Pain Management
- Nausea
- Seizures
- Multiple Sclerosis (MS)
- Others
-
Distribution Channel :
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Union
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Brand Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Brand Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Brand Type, 2025 to 2035
- Epidiolex
- Sativex
- Others
- Y to o to Y Growth Trend Analysis By Brand Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Brand Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- Seizures
- Nausea
- Pain Management
- Multiple Sclerosis (MS)
- Others
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Brand Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Brand Type
- By Application
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Brand Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Brand Type
- By Application
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Brand Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Brand Type
- By Application
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Brand Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Brand Type
- By Application
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Brand Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Brand Type
- By Application
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Brand Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Brand Type
- By Application
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Brand Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Brand Type
- By Application
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Brand Type
- By Application
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Brand Type
- By Application
- Competition Analysis
- Competition Deep Dive
- Jazz Pharmaceuticals plc
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- GW Pharmaceuticals (a Jazz Pharmaceuticals subsidiary)
- Canopy Growth Corporation
- Tilray Brands, Inc.
- Aurora Cannabis Inc.
- Cronos Group Inc.
- Corbus Pharmaceuticals Holdings, Inc.
- AbbVie Inc.
- Emerald Health Pharmaceuticals Inc.
- INSYS Therapeutics, Inc.
- Jazz Pharmaceuticals plc
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Brand Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Brand Type, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Brand Type, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Brand Type, 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Brand Type, 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Brand Type, 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Brand Type, 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Brand Type, 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by Application, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Brand Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Brand Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Brand Type
- Figure 6: Global Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Application
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Brand Type, 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Brand Type, 2025 to 2035
- Figure 22: North America Market Attractiveness Analysis by Brand Type
- Figure 23: North America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Application
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Brand Type, 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Brand Type, 2025 to 2035
- Figure 29: Latin America Market Attractiveness Analysis by Brand Type
- Figure 30: Latin America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 32: Latin America Market Attractiveness Analysis by Application
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Brand Type, 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Brand Type, 2025 to 2035
- Figure 36: Western Europe Market Attractiveness Analysis by Brand Type
- Figure 37: Western Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 39: Western Europe Market Attractiveness Analysis by Application
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Brand Type, 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Brand Type, 2025 to 2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Brand Type
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by Application
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Brand Type, 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Brand Type, 2025 to 2035
- Figure 50: East Asia Market Attractiveness Analysis by Brand Type
- Figure 51: East Asia Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 53: East Asia Market Attractiveness Analysis by Application
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Brand Type, 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Brand Type, 2025 to 2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Brand Type
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Brand Type, 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Brand Type, 2025 to 2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Brand Type
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the cannabis pharmaceuticals market in 2025?
The global cannabis pharmaceuticals market is estimated to be valued at USD 1.2 billion in 2025.
What will be the size of cannabis pharmaceuticals market in 2035?
The market size for the cannabis pharmaceuticals market is projected to reach USD 90.4 billion by 2035.
How much will be the cannabis pharmaceuticals market growth between 2025 and 2035?
The cannabis pharmaceuticals market is expected to grow at a 54.1% CAGR between 2025 and 2035.
What are the key product types in the cannabis pharmaceuticals market?
The key product types in cannabis pharmaceuticals market are epidiolex, sativex and others.
Which application segment to contribute significant share in the cannabis pharmaceuticals market in 2025?
In terms of application, seizures segment to command 42.5% share in the cannabis pharmaceuticals market in 2025.