COVID-19 Clinical Trials Market
COVID-19 Clinical Trials Market Size and Share Forecast Outlook 2025 to 2035
Covid-19 clinical trials market is projected to grow from USD 6.2 billion in 2025 to USD 13.8 billion by 2035, at a CAGR of 8.4%. Phase II will dominate with a 30.3% market share, while vaccines will lead the product segment with a 75.0% share.
COVID-19 Clinical Trials Market Forecast and Outlook 2025 to 2035
The global COVID-19 clinical trials market is projected to reach USD 13.83 billion by 2035, registering an absolute increase of USD 7.68 billion between 2025 and 2035. The market is valued at USD 6.15 billion in 2025 and is expected to expand at a CAGR of 8.4% during the forecast period.
Growth is supported by continuing vaccine innovation programs, expanded antiviral and immunomodulatory drug pipelines, and research investments targeting variant-specific and broad-spectrum formulations. The market’s trajectory is further reinforced by ongoing investigations into long-term immunity, post-acute COVID syndrome, and combination therapeutic strategies that require specialized clinical research and adaptive trial management frameworks.
Quick Stats for COVID-19 Clinical Trials Market
- COVID-19 Clinical Trials Market Value (2025): USD 6.15 billion
- COVID-19 Clinical Trials Market Forecast Value (2035): USD 13.83 billion
- COVID-19 Clinical Trials Market Forecast CAGR: 8.4%
- Leading Phase in COVID-19 Clinical Trials Market: Phase II
- Key Growth Regions in COVID-19 Clinical Trials Market: North America, Europe, and Asia Pacific
- Top Players in COVID-19 Clinical Trials Market: Pfizer Inc., Moderna Inc., Johnson & Johnson, GlaxoSmithKline plc, Gilead Sciences Inc., AbbVie Inc., BioNTech SE, Novavax Inc., Takeda Pharmaceutical Co.

Pharmaceutical and biotechnology companies continue to allocate significant research and development (R&D) budgets toward pandemic preparedness initiatives. The establishment of specialized clinical networks and adaptive trial platforms has improved the efficiency of vaccine and therapeutic testing.
These frameworks allow faster protocol modification in response to emerging viral variants and enable the inclusion of real-world data for outcome validation. The development of long-acting monoclonal antibodies, mRNA-based booster candidates, and small-molecule antivirals remains central to global COVID-19 therapeutic research.
Evolving scientific understanding of SARS-CoV-2 mutations and immune evasion mechanisms has created sustained demand for next-generation vaccine designs. Several studies have demonstrated improved protection from adjuvanted formulations and enhanced outcomes from early antiviral intervention.
This evidence supports continued investment in both preventive and therapeutic clinical trials. Public health efforts are also shifting toward the development of universal coronavirus vaccines capable of providing cross-variant protection, expanding research activity beyond variant-specific immunization approaches.
Contract research organizations (CROs) are increasingly integrating decentralized and hybrid trial models into COVID-19 studies. These approaches facilitate participant recruitment, reduce operational costs, and improve patient engagement.
The use of digital data collection systems and remote monitoring tools allows for higher-quality evidence generation while maintaining regulatory compliance. Real-world evidence platforms and cloud-based data management systems have become essential components of modern COVID-19 clinical trial operations, supporting large-scale, multi-site studies across diverse geographies.
The market faces emerging challenges as the acute phase of the pandemic recedes. Declining infection rates and reduced urgency for emergency vaccine development may slow trial enrollment and funding momentum. Regulatory complexities related to streamlined approvals for variant-updated vaccines also influence sponsor strategies. Competition for funding within broader infectious disease research categories may divert resources from COVID-19–specific investigations.
Sustaining long-term investment in COVID-19 clinical research depends on establishing stable financing channels and maintaining clear regulatory guidance for adaptive vaccine and therapeutic trials. Academic institutions and smaller biotechnology firms in developing regions continue to face structural barriers, including limited research infrastructure and constrained access to global trial networks.
Despite these challenges, ongoing research on long-term immunity, combination immunization platforms, and persistent post-infection conditions ensures that the COVID-19 clinical trials market remains a significant component of global biomedical innovation throughout the forecast period.
COVID-19 Clinical Trials Market Year-over-Year Forecast (2025 to 2035)
Between 2025 and 2030, the COVID-19 clinical trials market is projected to expand from USD 6.15 billion to USD 9.21 billion, resulting in a value increase of USD 3.06 billion, which represents 39.8% of the total forecast growth for the decade.
This phase of development will be shaped by continued demand for variant-adapted vaccine trials and booster optimization studies, therapeutic innovation in broad-spectrum antivirals and immunomodulatory agents, as well as expanding integration with real-world evidence platforms and decentralized trial methodologies.
Companies are establishing competitive positions through investment in adaptive trial platform development, variant surveillance capabilities, and strategic market expansion across next-generation vaccine programs, therapeutic pipeline advancement, and long COVID treatment investigations.
From 2030 to 2035, the market is forecast to grow from USD 9.21 billion to USD 13.83 billion, adding another USD 4.62 billion, which constitutes 60.2% of the overall ten-year expansion.
This period is expected to be characterized by the expansion of universal coronavirus vaccine research programs, including pan-sarbecovirus candidates and broadly neutralizing antibody platforms tailored for comprehensive coronavirus protection, strategic collaborations between pharmaceutical companies and government pandemic preparedness initiatives, and an enhanced focus on combination immunization strategies and mucosal vaccine delivery systems.
The growing emphasis on pandemic preparedness infrastructure and rapid response platform technologies will drive demand for innovative clinical trial approaches across diverse coronavirus research applications.
COVID-19 Clinical Trials Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 6.15 billion |
| Market Forecast Value (2035) | USD 13.83 billion |
| Forecast CAGR (2025-2035) | 8.4% |
Why is the COVID-19 Clinical Trials Market Growing?
The COVID-19 clinical trials market grows by enabling pharmaceutical companies, biotechnology firms, and academic research institutions to access specialized clinical research infrastructure that supports accelerated drug and vaccine development while meeting stringent regulatory requirements for emergency use authorization and full licensure pathways.
Pharmaceutical sponsors face sustained pressure to develop next-generation COVID-19 interventions with improved efficacy against emerging variants, with modern vaccine platforms and therapeutic candidates typically achieving 70-90% protective efficacy or clinical benefit rates in well-designed trials, making specialized COVID-19 research capabilities essential for competitive product development in pandemic response and preparedness categories.
The clinical research industry's need for adaptive trial designs and rapid enrollment capabilities creates demand for flexible research platforms that can provide real-time data analysis, maintain protocol compliance across diverse populations, and ensure regulatory acceptance without compromising scientific rigor or patient safety standards.
Government initiatives promoting pandemic preparedness and rapid response infrastructure drive continued investment in COVID-19 clinical research, vaccine platform optimization, and therapeutic pipeline development, where specialized clinical trials have a direct impact on public health preparedness and outbreak response capabilities.
The pharmaceutical industry's growing focus on universal coronavirus vaccines and broad-spectrum antivirals further expands market opportunities, with next-generation platforms demonstrating potential for protection against multiple coronavirus species and future pandemic threats following successful proof-of-concept clinical validation.
Recruitment challenges as pandemic urgency diminishes and the technical complexity of variant-adapted vaccine trials requiring immunobridging studies may limit trial feasibility among smaller sponsors and developing regions with limited infrastructure for sophisticated immunological assessments and correlates of protection research.
Segmental Analysis
The market is segmented by phase, product, and region. By phase, the market is divided into Phase II, Phase III, and others. Based on product, the market is categorized into vaccines and therapeutics. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
Why Does Phase II Account for a Dominant Market Share?
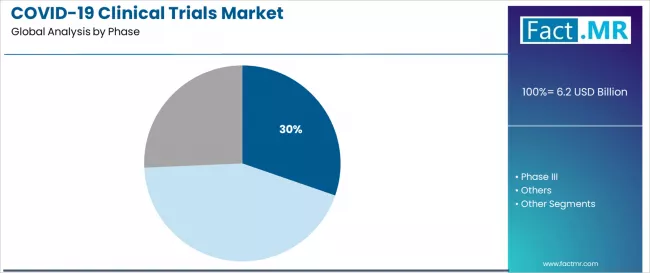
Phase II clinical trials capture 30.3% of the total market share in 2025. This established clinical phase category encompasses dose-ranging studies, immunogenicity evaluations, and proof-of-concept trials for novel vaccine platforms and therapeutic candidates that enable preliminary efficacy assessment and safety characterization across targeted patient populations.
Phase II clinical trials’ leadership stems from its critical role in COVID-19 product development, with trials capable of generating essential immunological data while maintaining manageable trial sizes and accelerated timelines across all development programs.
Phase III clinical trials are expected to be the fastest-growing category, driven by large-scale efficacy trials for variant-adapted vaccines, pivotal registration studies for novel therapeutics, and comprehensive safety databases required for regulatory approvals.
This segment benefits from regulatory requirements for substantial evidence generation that meets specific licensure standards, real-world effectiveness protocols, and long-term safety monitoring expectations in competitive pharmaceutical markets.
Key advantages driving the Phase II segment include:
- Flexible trial design capabilities with adaptive protocols that reduce development complexity and enable rapid decision-making for program advancement
- Immunogenicity assessment focus allowing comprehensive immune response characterization across different vaccine platforms without requiring large safety databases
- Proven regulatory acceptance, delivering critical efficacy signals while maintaining cost competitiveness against larger Phase III programs
- Accelerated timeline opportunities enabling streamlined development pathways and expedited variant response capabilities
How do Vaccines Dominate Product Distribution in the Market?

Vaccines dominate the product segment with approximately 75.0% market share in 2025, reflecting the critical role of immunization strategies in supporting global COVID-19 prevention and pandemic control operations worldwide.
The vaccines segment's market leadership is reinforced by continued need for variant-adapted formulations, booster optimization programs, and rising requirements for next-generation vaccine platforms including mucosal vaccines, universal coronavirus vaccines, and combination immunization approaches across developed and emerging markets.
The therapeutics segment represents the fastest-growing product category, capturing growing market share through broad-spectrum antiviral development, immunomodulatory agent trials, and combination therapy evaluations.
This segment benefits from expanding treatment options beyond initial monoclonal antibody platforms that meet specific clinical requirements, oral antiviral availability standards, and outpatient treatment protocols in competitive pharmaceutical markets.
Key market dynamics supporting product growth include:
- Vaccine expansion driven by variant emergence and waning immunity concerns, requiring continuous vaccine platform optimization in global markets
- Therapeutic modernization trends require high-quality, broad-spectrum antivirals for variant-independent efficacy and accessibility compliance
- Integration of combination approaches enabling synergistic protection and comprehensive outbreak response strategies
- Growing emphasis on product diversity driving demand for comprehensive, multi-platform pandemic response solutions
What are the Drivers, Restraints, and Key Trends of the COVID-19 Clinical Trials Market?
Continued emergence of SARS-CoV-2 variants with immune escape capabilities creates ongoing demand for variant-adapted vaccines and therapeutics, with global variant surveillance systems identifying new lineages every 3-6 months worldwide, requiring rapid clinical evaluation infrastructure.
Government and international health organization initiatives promoting pandemic preparedness and rapid response platforms drive sustained investment in COVID-19 research infrastructure, with many countries implementing standing research networks targeting outbreak response by 2030.
Technological advancements in mRNA vaccine platforms, viral vector optimization, and antiviral drug discovery enable more effective and rapidly deployable interventions that reduce development timelines while improving efficacy profiles and manufacturing scalability capabilities.
Market restraints include declining public health urgency as populations achieve hybrid immunity through vaccination and natural infection that can deter continued research investment, particularly in regions where COVID-19 transitions to endemic circulation patterns with reduced clinical severity.
Regulatory uncertainty regarding variant-adapted vaccine approval pathways poses another significant challenge, as streamlined authorization processes demand extensive immune-bridging data and correlates of protection validation, potentially causing development delays and increased trial costs.
Clinical trial recruitment challenges among populations with prior vaccination or infection history create additional obstacles for placebo-controlled efficacy trials, demanding innovative trial designs including active comparator studies and immunogenicity-focused endpoints. Key trends indicate continued research activity in developed markets, particularly USA and European Union, where robust pandemic preparedness funding and established clinical research infrastructure drive comprehensive COVID-19 trial programs.
Technology integration trends toward decentralized trial models with remote patient monitoring, digital consent platforms, and telemedicine-enabled study visits enable participant-centric research approaches that optimize recruitment and retention while minimizing site visit burdens. The industry could face disruption if significant advances in universal coronavirus vaccines providing decades-long protection or complete viral elimination through global vaccination programs substantially reduce ongoing clinical research requirements.
Analysis of the COVID-19 Clinical Trials Market by Key Country

| Country | CAGR (2025-2035) |
|---|---|
| India | 9.0% |
| China | 8.9% |
| USA | 8.7% |
| Brazil | 8.4% |
| Japan | 8.4% |
| Germany | 8.3% |
| UK | 8.2% |
The COVID-19 clinical trials market is expanding steadily, with India leading at a 9.0% CAGR through 2035, driven by government fast-track and DCGI waivers, cost-effective trial ecosystem attracting global sponsors and expanding clinical research infrastructure. China follows at 8.9%, supported by rapid CRO expansion, public funding and strong government support for vaccine development, growing domestic pharmaceutical innovation capacity.
Brazil records 8.4%, reflecting participation in WHO Solidarity II trials, large patient populations enabling rapid recruitment and established vaccine trial expertise. USA posts 8.7%, anchored by NIH and BARDA-backed ACTIV partnership, CRO leadership and robust pandemic preparedness funding infrastructure. Japan grows at 8.4%, with local pharma engagement including Takeda and Daiichi Sankyo, regional trial coordination for Asia Pacific populations.
Germany advances at 8.3%, emphasizing accelerated vaccine R&D via ACCORD and AGILE platforms, strong regulatory collaboration and EU clinical research leadership, while UK grows steadily at 8.2%, focusing on major host of multinational trials, recovery trial platform expertise and MHRA adaptive licensing frameworks.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the COVID-19 clinical trials market with a CAGR of 9.0% through 2035. The country's leadership position stems from government fast-track and DCGI waivers, cost-effective trial ecosystem attracting global pharmaceutical sponsors, and expanding clinical research infrastructure enabling large-scale vaccine and therapeutic trials.
Growth is concentrated in major clinical research hubs, including Mumbai, Pune, Hyderabad, and Bangalore, where contract research organizations and expanding hospital networks are implementing COVID-19 trial programs for multinational pharmaceutical companies and domestic vaccine manufacturers. Clinical research channels through established CRO networks, hospital trial sites, and government research institutions expand deployment across vaccine efficacy studies and therapeutic intervention trials. The country's growing clinical trial sector provides regulatory support for accelerated approvals, including emergency use pathways for COVID-19 interventions.
Key market factors:
- Clinical research demand concentrated in metropolitan centers and tier-2 cities with comprehensive trial infrastructure programs
- CRO ecosystem growth through specialized COVID-19 research capabilities and regulatory expertise initiatives
- Comprehensive research infrastructure, including established clinical trial sites with proven patient recruitment capabilities
- Technology integration featuring electronic data capture systems, remote monitoring platforms, and digital consent technologies
Why is China Emerging as a High-Growth Market?
In Beijing, Shanghai, Guangzhou, and Wuhan, the adoption of COVID-19 clinical research is accelerating across vaccine development programs and therapeutic trial categories, driven by government funding initiatives and domestic pharmaceutical innovation priorities. The market demonstrates strong growth momentum with a CAGR of 8.9% through 2035, linked to rapid CRO expansion, public funding and strong government support for vaccine development, growing domestic pharmaceutical innovation capacity, and increasing integration with international collaborative research networks.
Research institutions in China are implementing comprehensive COVID-19 trial programs to advance domestic vaccine platforms while meeting government pandemic preparedness objectives and supporting global health security initiatives. The country's national pandemic response strategy creates persistent demand for clinical research infrastructure, while increasing emphasis on scientific leadership drives adoption of advanced trial methodologies and regulatory science approaches.
Key development areas:
- Pharmaceutical companies and research institutions leading COVID-19 trial implementation with comprehensive government support programs
- Clinical research infrastructure providing integrated trial capabilities with rapidly expanding site networks
- Technology partnerships between Chinese CROs and international pharmaceutical sponsors are expanding research capacity
- Integration of real-world data platforms and comprehensive population health surveillance systems
What Drives Strong Research Activity in Brazil?
The COVID-19 clinical trials market’s expansion in Brazil is driven by diverse clinical trial demand, including vaccine efficacy studies in São Paulo and Rio de Janeiro, and therapeutic trials across multiple research centers. The country demonstrates promising growth potential with a CAGR of 8.4% through 2035, supported by participation in WHO Solidarity II trials, large patient populations enabling rapid recruitment, and established vaccine trial expertise from previous epidemic responses.
Research institutions in Brazil face implementation challenges related to regulatory complexity and funding sustainability, requiring continued international collaboration and support from global pharmaceutical sponsors. However, large treatment-naïve populations and experienced clinical research infrastructure create compelling opportunities for COVID-19 trial execution, particularly in major urban centers where research capacity has a direct impact on global clinical development programs.
Market characteristics:
- Vaccine and therapeutic trial segments showing sustained activity with participation in major international research collaborations
- Regional research concentration focused on southeastern states with established clinical trial infrastructure
- Future projections indicate continued role in global COVID-19 research through Phase III efficacy trials and real-world effectiveness studies
- Growing emphasis on local research capacity development and technology transfer in clinical trial operations
How Does the USA Demonstrate Research Leadership?
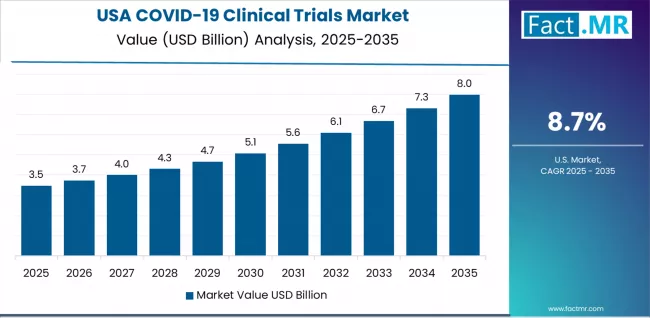
COVID-19 clinical trials in the USA lead based on integration with NIH-supported research networks and BARDA-funded development programs for advanced pandemic response capabilities. The country shows strong potential with a CAGR of 8.7% through 2035, driven by NIH and BARDA-backed ACTIV partnership, CRO leadership and robust pandemic preparedness funding infrastructure, and the expansion of specialized COVID-19 research platforms in major pharmaceutical and academic centers, including Boston, San Francisco, Research Triangle Park, and Houston.
American pharmaceutical companies and research institutions are adopting advanced trial methodologies including master protocol designs and platform trials for pandemic response optimization, particularly in regions with world-class clinical research expertise and specialized capabilities demanding comprehensive regulatory compliance. Research deployment channels through established pharmaceutical industry networks and academic medical center partnerships expand coverage across vaccine development programs and therapeutic investigation initiatives.
Leading market segments:
- Pharmaceutical companies and academic medical centers in major research hubs implementing comprehensive COVID-19 development programs
- Government-industry partnerships with ACTIV, BARDA, and Operation Warp Speed successors, achieving rapid vaccine and therapeutic advancement
- Strategic collaborations between American pharmaceutical companies and international research networks are expanding global trial reach
- Focus on next-generation vaccine platforms and universal coronavirus vaccine development requirements
What Emphasizes Regional Coordination in Japan?
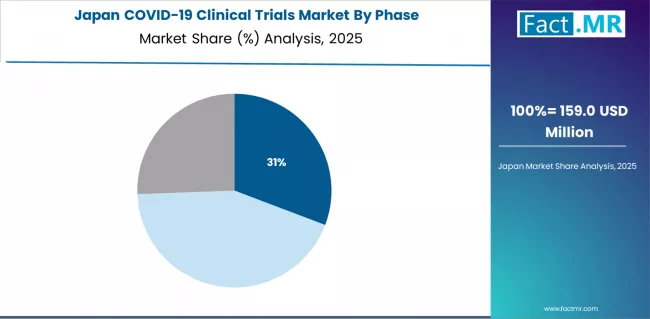
In major pharmaceutical centers including Tokyo, Osaka, Kyoto, and Fukuoka, research institutions are implementing comprehensive COVID-19 trial programs to evaluate vaccine efficacy in Asian populations and advance domestic therapeutic development, with documented participation in multinational vaccine trials through regional coordinating centers.
The market shows strong growth potential with a CAGR of 8.4% through 2035, linked to local pharma engagement including Takeda and Daiichi Sankyo, regional trial coordination for Asia Pacific populations, and emerging collaborative research frameworks in major pharmaceutical regions.
Research organization in Japan are conducting COVID-19 clinical trials to validate vaccine performance while maintaining rigorous quality standards demanded by PMDA regulations and pharmaceutical industry requirements. The country's established pharmaceutical infrastructure creates sustained demand for clinical research participation and regional trial coordination solutions that integrate with global development programs.
Market development factors:
- Pharmaceutical companies and academic institutions leading COVID-19 research participation across Japan
- Regulatory collaboration programs providing PMDA framework support for accelerated development pathways
- Strategic partnerships between Japanese pharmaceutical companies and international vaccine developers are expanding clinical capabilities
- Emphasis on population-specific immunogenicity data and regional efficacy validation across Asian demographics
How Does Germany Show Research Excellence?
Germany's COVID-19 clinical trials market demonstrates sophisticated research infrastructure focused on accelerated vaccine development and adaptive trial platforms, with documented leadership in European regulatory science through BfArM and Paul-Ehrlich-Institut collaboration, achieving streamlined approval pathways for pandemic response trials.
The country maintains steady growth momentum with a CAGR of 8.3% through 2035, driven by accelerated vaccine R&D via ACCORD and AGILE platforms, strong regulatory collaboration and EU clinical research leadership, and pharmaceutical industry emphasis on innovation that aligns with EMA regulatory frameworks applied to COVID-19 interventions.
Major pharmaceutical research regions, including Frankfurt, Munich, Berlin, and Hamburg, showcase advanced deployment of COVID-19 clinical research where trial platforms integrate seamlessly with existing pharmaceutical development infrastructure and comprehensive regulatory compliance systems.
Key market characteristics:
- Pharmaceutical companies and research institutions driving COVID-19 trial implementation with emphasis on regulatory science and adaptive designs
- Research platform partnerships enabling efficient multi-arm trial execution with comprehensive regulatory acceptance
- Technology collaboration between German research institutions and international pharmaceutical sponsors is expanding research capabilities
- Emphasis on variant surveillance integration and evidence-based regulatory decision-making methodologies
Why Does the UK Emphasize Platform Trials?
The UK's COVID-19 clinical trials market demonstrates sophisticated platform trial expertise, characterized by pioneering RECOVERY trial implementation and adaptive randomization methodologies across pharmaceutical sponsors, academic medical centers, and NHS research networks. The country shows steady growth momentum with a CAGR of 8.2% through 2035, driven by major host of multinational trials, RECOVERY trial platform expertise and MHRA adaptive licensing frameworks.
The UK's emphasis on pragmatic trial designs and rapid evidence generation creates requirements for flexible research platforms that support comprehensive pandemic response and regulatory innovation initiatives in clinical research operations.
The market benefits from strong partnerships between NHS research infrastructure and international pharmaceutical companies, creating comprehensive trial ecosystems that prioritize rapid enrollment and regulatory acceptance programs.
Research centers in major urban regions showcase advanced platform trial implementations where COVID-19 studies achieve unprecedented recruitment rates through integrated healthcare system participation.
Key market characteristics:
- NHS research networks and academic institutions driving platform trial requirements with emphasis on pragmatic designs and rapid evidence generation
- Regulatory innovation partnerships enabling adaptive licensing pathways with comprehensive post-authorization monitoring programs
- Technology collaboration between UK research institutions and global pharmaceutical sponsors is expanding clinical methodology capabilities
- Emphasis on real-world evidence generation and continuous protocol optimization methodologies
Europe Market Split by Country

The COVID-19 clinical trials market in Europe is projected to grow from USD 1.89 billion in 2025 to USD 4.20 billion by 2035, registering a CAGR of 8.3% over the forecast period. Germany is expected to maintain its leadership position with a 36.0% market share in 2025, supported by its extensive pharmaceutical research infrastructure, advanced regulatory science capabilities, and comprehensive EU clinical trial coordination networks serving European COVID-19 research programs.
The UK follows with a 32.8% share in 2025, driven by comprehensive RECOVERY platform trial implementation in NHS research networks advancing evidence-based COVID-19 treatment adoption. France holds a 15.3% share through ongoing vaccine development programs and therapeutic investigation networks.
Italy commands a 9.5% share, while Spain accounts for 4.2% in 2025. The rest of Europe region maintains a 2.2% collective share, attributed to emerging COVID-19 research participation in Nordic countries and Eastern European research institutions implementing collaborative trial programs.
Competitive Landscape of the COVID-19 Clinical Trials Market

The COVID-19 clinical trials market features approximately 15-20 meaningful pharmaceutical sponsors with moderate concentration, where the Pfizer-BioNTech alliance controls roughly 11-12% of global clinical trial investment share through established mRNA vaccine programs and comprehensive booster development initiatives. Competition centers on platform technology innovation, variant adaptation speed, and regulatory pathway expertise rather than trial volume alone.
Market leaders include Pfizer Inc. (with BioNTech SE), Moderna Inc., and Johnson & Johnson, which maintain competitive advantages through proven vaccine platform portfolios, extensive clinical trial networks, and deep expertise in regulatory science and pandemic response development, creating high switching costs for research collaborators and government procurement programs.
These companies leverage established relationships with regulatory agencies including FDA, EMA, and WHO and ongoing public health partnerships to defend market positions while expanding into next-generation universal coronavirus vaccine research and combination immunization strategies.
Challengers encompass GlaxoSmithKline plc and Gilead Sciences Inc., which compete through specialized adjuvant technologies and antiviral therapeutic platforms with strong clinical validation in COVID-19 treatment settings.
Pharmaceutical specialists including AbbVie Inc., Novavax Inc., and Takeda Pharmaceutical Co. focus on specific product categories or regional trial leadership, offering differentiated capabilities in combination antiviral therapies, protein-based vaccine platforms, and Asia Pacific clinical development coordination.
Emerging biotechnology companies and academic research consortia create competitive pressure through innovative vaccine designs and novel therapeutic mechanisms, particularly in next-generation approaches including mucosal vaccines, broadly neutralizing antibodies, and universal coronavirus vaccine candidates, where scientific innovation provides advantages in intellectual property and regulatory interest.
Market dynamics favor companies that combine advanced platform technologies with comprehensive clinical development expertise that addresses the complete product lifecycle from preclinical proof-of-concept through global Phase III efficacy trials and post-authorization effectiveness monitoring.
Global COVID-19 Clinical Trials Market - Stakeholder Contribution Framework
COVID-19 clinical trials represent critical research infrastructure that enables pharmaceutical companies, biotechnology firms, and public health agencies to develop and validate pandemic interventions without reliance on observational data alone, typically providing scientifically rigorous evidence from randomized controlled trials that meets regulatory standards for authorization and licensure decisions.
With the market projected to grow from USD 6.15 billion in 2025 to USD 13.83 billion by 2035 at an 8.4% CAGR, these specialized research programs offer compelling advantages - regulatory-grade evidence generation, accelerated development timelines, and adaptive design capabilities - making them essential for variant-adapted vaccine development (growing segment), novel therapeutic validation (expanding activity), and diverse pandemic preparedness applications seeking evidence-based intervention strategies.
Scaling research capacity and maintaining trial quality requires coordinated action across regulatory agencies, public health organizations, pharmaceutical sponsors, contract research organizations, and academic research institutions.
How Could Governments Support Pandemic Research Infrastructure?
- Sustained Funding Programs: Maintain dedicated pandemic preparedness research budgets beyond emergency appropriations, providing sustained funding for COVID-19 clinical trial networks and supporting academic medical centers through infrastructure grants and operational support for standing research capacity.
- Regulatory Science Enhancement: Develop clear regulatory pathways for variant-adapted vaccines requiring minimal bridging data, establish framework guidance for universal coronavirus vaccine evaluation, and create international harmonization protocols that facilitate coordinated clinical development across multiple jurisdictions.
- Research Network Maintenance: Support standing clinical trial networks including ACTIV, RECOVERY, and WHO Solidarity platforms that enable rapid trial activation during outbreak situations, provide infrastructure funding for site maintenance, and establish rapid ethics review processes that minimize trial startup delays.
- International Collaboration Facilitation: Fund collaborative research initiatives linking high-income and low-middle income country research sites, support technology transfer for local clinical trial capacity building, and establish data sharing frameworks that enable global evidence synthesis and meta-analysis approaches.
How Could Public Health Organizations Support Research Development?
- Trial Design Guidance: Develop standardized endpoint definitions for COVID-19 clinical trials across vaccine and therapeutic categories, establish correlates of protection frameworks for immunobridging studies, and create master protocol templates that sponsors can adapt for specific investigational products.
- Evidence Synthesis & Communication: Lead scientific messaging that demonstrates continued need for COVID-19 research, emphasizing variant surveillance data, waning immunity evidence, and therapeutic gap analyses that justify ongoing clinical investigation investments.
- Priority Setting Frameworks: Conduct systematic research prioritization exercises identifying critical knowledge gaps in COVID-19 prevention and treatment, comprehensive target product profiles for needed interventions, and resource allocation guidance that optimizes public health impact per research dollar invested.
- Surveillance Integration: Connect variant surveillance systems with clinical trial networks enabling rapid protocol amendments for emerging variants, real-time immunogenicity monitoring against circulating strains, and proactive identification of immune escape requiring new vaccine formulations.
How Could Pharmaceutical Sponsors and CROs Strengthen Research Capabilities?
- Platform Technology Investment: Develop next-generation vaccine platforms with rapid strain-change capabilities, improved thermostability enabling cold-chain-free distribution, and enhanced immunogenicity requiring fewer booster doses while maintaining durable protection.
- Decentralized Trial Infrastructure: Expand remote monitoring capabilities that integrate telemedicine visits, home specimen collection, digital symptom reporting, and electronic consent platforms, enabling participant-centric trial designs that maximize recruitment and retention across diverse populations.
- Data Standards & Interoperability: Implement common data standards enabling cross-trial analysis and meta-analysis approaches, develop interoperable electronic data capture systems facilitating rapid data sharing, and create standardized specimen repositories supporting immunological correlates research across multiple trial programs.
- Capacity Building Partnerships: Establish collaborative research training programs in low-middle income countries, transfer Good Clinical Practice expertise and quality management systems, and develop sustainable local research infrastructure that persists beyond current pandemic supporting future outbreak response readiness.
Key Players in the COVID-19 Clinical Trials Market
- Pfizer Inc.
- Moderna Inc.
- Johnson & Johnson
- GlaxoSmithKline plc
- Gilead Sciences Inc.
- AbbVie Inc.
- BioNTech SE
- Novavax Inc.
- Takeda Pharmaceutical Co.
- Sanofi S.A.
- AstraZeneca plc
- Merck & Co. Inc.
- Regeneron Pharmaceuticals Inc.
- Eli Lilly and Company
- Vir Biotechnology Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 6.15 Billion |
| Phase | Phase II, Phase III, Others |
| Product | Vaccines, Therapeutics |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Country Covered | USA, Germany, UK, China, Japan, India, Brazil, and 40+ countries |
| Key Companies Profiled | Pfizer Inc., Moderna Inc., Johnson & Johnson, GlaxoSmithKline plc, Gilead Sciences Inc., AbbVie Inc., BioNTech SE, Novavax Inc., Takeda Pharmaceutical Co. |
| Additional Attributes | Dollar investment by phase and product categories, regional trial activity trends across North America, Europe, and Asia Pacific, competitive landscape with pharmaceutical sponsors and biotechnology companies, regulatory pathway requirements and trial design specifications, integration with pandemic preparedness platforms and adaptive trial networks. |
COVID-19 Clinical Trials Market by Segments
-
Phase :
- Phase II
- Phase III
- Others
-
Product :
- Vaccines
- Therapeutics
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Latin America
- Brazil
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Union
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Phase
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Phase, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Phase, 2025 to 2035
- Phase II
- Phase III
- Others
- Y to o to Y Growth Trend Analysis By Phase, 2020 to 2024
- Absolute $ Opportunity Analysis By Phase, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2025 to 2035
- Vaccines
- Therapeutics
- Y to o to Y Growth Trend Analysis By Product, 2020 to 2024
- Absolute $ Opportunity Analysis By Product, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Phase
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Phase
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Phase
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Phase
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Phase
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Phase
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Phase
- By Product
- By Country
- Market Attractiveness Analysis
- By Country
- By Phase
- By Product
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Phase
- By Product
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Phase
- By Product
- Competition Analysis
- Competition Deep Dive
- Pfizer Inc.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Moderna Inc.
- Johnson & Johnson
- GlaxoSmithKline plc
- Gilead Sciences Inc.
- AbbVie Inc.
- BioNTech SE
- Novavax Inc.
- Takeda Pharmaceutical Co.
- Sanofi S.A.
- AstraZeneca plc
- Merck & Co. Inc.
- Regeneron Pharmaceuticals Inc.
- Eli Lilly and Company
- Vir Biotechnology Inc.
- Pfizer Inc.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Phase, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Phase, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Phase, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Phase, 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Phase, 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Phase, 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Phase, 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Phase, 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by Product, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Phase, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Phase, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Phase
- Figure 6: Global Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Product
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Phase, 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Phase, 2025 to 2035
- Figure 22: North America Market Attractiveness Analysis by Phase
- Figure 23: North America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Product
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Phase, 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Phase, 2025 to 2035
- Figure 29: Latin America Market Attractiveness Analysis by Phase
- Figure 30: Latin America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 32: Latin America Market Attractiveness Analysis by Product
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Phase, 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Phase, 2025 to 2035
- Figure 36: Western Europe Market Attractiveness Analysis by Phase
- Figure 37: Western Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 39: Western Europe Market Attractiveness Analysis by Product
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Phase, 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Phase, 2025 to 2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Phase
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by Product
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Phase, 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Phase, 2025 to 2035
- Figure 50: East Asia Market Attractiveness Analysis by Phase
- Figure 51: East Asia Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 53: East Asia Market Attractiveness Analysis by Product
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Phase, 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Phase, 2025 to 2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Phase
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by Product
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Phase, 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Phase, 2025 to 2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Phase
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by Product
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the covid-19 clinical trials market in 2025?
The global covid-19 clinical trials market is estimated to be valued at USD 6.2 billion in 2025.
What will be the size of covid-19 clinical trials market in 2035?
The market size for the covid-19 clinical trials market is projected to reach USD 13.8 billion by 2035.
How much will be the covid-19 clinical trials market growth between 2025 and 2035?
The covid-19 clinical trials market is expected to grow at a 8.4% CAGR between 2025 and 2035.
What are the key product types in the covid-19 clinical trials market?
The key product types in covid-19 clinical trials market are phase ii, phase iii and others.
Which product segment to contribute significant share in the covid-19 clinical trials market in 2025?
In terms of product, vaccines segment to command 75.0% share in the covid-19 clinical trials market in 2025.