Renal Denervation Catheter Market
Renal Denervation Catheter Market Size and Share Forecast Outlook 2025 to 2035
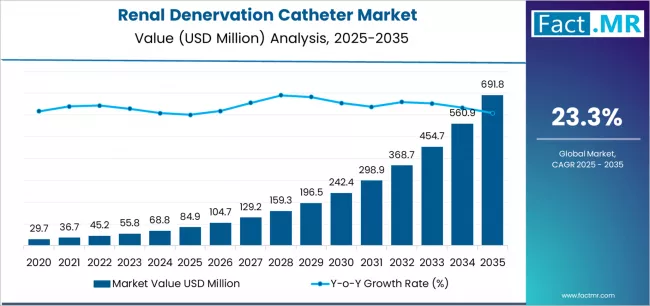
Renal denervation catheter market is projected to grow from USD 84.9 million in 2025 to USD 691.8 million by 2035, at a CAGR of 23.3%. Radiofrequency Ablation Catheters will dominate with a 62.4% market share, while hospitals will lead the end use segment with a 72.3% share.
Renal Denervation Catheter Market Forecast and Outlook 2025 to 2035
The global renal denervation catheter market is projected to reach USD 691.8 million by 2035, recording an absolute increase of USD 606.9 million over the forecast period. The market is valued at USD 84.9 million in 2025 and is set to rise at a CAGR of 23.3% during the assessment period.
The demand for renal denervation will grow 8.1 times during the same period, supported by increasing prevalence of resistant hypertension and uncontrolled blood pressure conditions worldwide, driving demand for minimally invasive catheter-based interventions and increasing investments in clinical evidence generation with randomized controlled trials across cardiovascular disease prevention and hypertension management applications globally.
Quick Stats for Renal Denervation Catheter Market
- Renal Denervation Catheter Market Value (2025): USD 84.9 million
- Renal Denervation Catheter Market Forecast Value (2035): USD 691.8 million
- Renal Denervation Catheter Market Forecast CAGR: 23.3%
- Leading Product Type in Renal Denervation Catheter Market: Radiofrequency Ablation Catheters (62.4%)
- Key Growth Regions in Renal Denervation Catheter Market: Asia Pacific, North America, and Europe
- Top Players in Renal Denervation Catheter Market: Medtronic, Recor Medical, Boston Scientific, SoniVie, Otsuka Medical Devices, Ablative Solutions, SyMap Medical, Terumo Corporation, Abbott, Johnson & Johnson MedTech
Interventional cardiologists and hypertension specialists face mounting pressure to address treatment-resistant hypertension and medication non-adherence while managing cardiovascular risk reduction and patient quality of life requirements, with modern renal denervation technologies providing documented therapeutic benefits including sustained blood pressure reduction, reduced medication burden, and improved cardiovascular outcomes compared to pharmaceutical therapy optimization alone.
Rising clinical evidence from pivotal trials and expanding regulatory approvals enabling commercial adoption create substantial opportunities for device manufacturers and cardiovascular centers. However, procedural reimbursement uncertainties and physician training requirements may pose obstacles to widespread technology adoption across diverse healthcare systems.
Radiofrequency ablation catheters dominate market activity, driven by extensive clinical validation supporting sympathetic nerve ablation and established procedural techniques across interventional cardiology practices worldwide. Cardiologists increasingly recognize the proven efficacy of radiofrequency systems, with typical product offerings providing controlled energy delivery and precise ablation targeting at competitive procedure costs through established catheterization laboratory supply networks.
Ultrasound ablation catheters demonstrat robust growth potential, supported by circumferential treatment capabilities and evidence-based sympathetic denervation integrating non-contact energy delivery in modern interventional approaches. Hospital end-use applications emerge as the dominant setting, reflecting procedural complexity requirements and cardiovascular infrastructure needs in acute care facilities. Multi-electrode radiofrequency systems represent the leading technology configuration, driven by comprehensive renal artery coverage and efficient treatment delivery enabling complete circumferential denervation.
North America maintains market leadership, supported by recent FDA approvals and established interventional cardiology expertise across cardiovascular specialty centers. Asia Pacific demonstrates the fastest growth trajectory driven by massive hypertensive patient populations and expanding device-based therapy adoption accepting innovative cardiovascular interventions, while Europe emphasizes clinical registry participation and evidence-based guideline integration for therapeutic innovation. India leads country-level growth through large hypertensive population and Medtronic training center expansion, followed by China supported by high hypertension prevalence and NMPA approvals for new devices.
The competitive landscape features moderate concentration with Medtronic Plc. maintaining a 31.4% share, while specialized players including Recor Medical, Boston Scientific, SoniVie, and Otsuka Medical Devices compete through differentiated energy modalities and comprehensive clinical evidence programs across diverse hypertension management applications.
Renal Denervation Catheter Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the renal denervation catheter market is projected to expand from USD 84.9 million to USD 198.5 million, resulting in a value increase of USD 113.6 million, which represents 18.7% of the total forecast growth for the period. This phase of development will be shaped by rising demand for resistant hypertension treatment alternatives addressing medication intolerance, product innovation in multi-electrode systems with improved ablation efficiency and real-time feedback capabilities, as well as expanding integration with cardiovascular disease prevention programs and hypertension center of excellence initiatives. Companies are establishing competitive positions through investment in pivotal clinical trial execution, physician training infrastructure development, and strategic market expansion across interventional cardiology societies, hospital catheterization laboratories, and medical education partnerships.
From 2029 to 2035, the market is forecast to grow from USD 198.5 million to USD 691.8 million, adding another USD 493.3 million, which constitutes 81.3% of the overall expansion. This period is expected to be characterized by the expansion of specialized clinical applications, including chronic kidney disease hypertension management and heart failure with preserved ejection fraction treatment tailored for specific cardiovascular patient populations, strategic collaborations between device manufacturers and pharmaceutical companies, and an enhanced focus on real-world evidence generation and long-term safety surveillance programs. The growing emphasis on personalized hypertension management protocols and rising physician preference for durable blood pressure control addressing medication adherence challenges and polypharmacy reduction will drive demand for comprehensive catheter-based renal denervation solutions across diverse hypertensive patient populations and cardiovascular risk profiles.
Renal Denervation Catheter Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 84.9 million |
| Market Forecast Value (2035) | USD 691.8 million |
| Forecast CAGR (2025-2035) | 23.3% |
Why is the Renal Denervation Catheter Market Growing?
The renal denervation catheter market grows by enabling interventional cardiologists and hypertension specialists to deliver minimally invasive sympathetic nerve ablation while addressing resistant hypertension and uncontrolled blood pressure without exclusive reliance on escalating pharmaceutical regimens.
Cardiologists aim to reduce cardiovascular risk and improve blood pressure control while managing medication side effects, patient non-adherence, and treatment-resistant hypertension affecting approximately 10-15% of hypertensive patients, with modern renal denervation procedures typically providing sustained systolic blood pressure reductions of 5-15 mmHg persisting beyond 36 months, sympathetic nervous system modulation addressing multiple cardiovascular pathways, and potential medication reduction benefits compared to pharmacological intensification alone, making catheter-based renal denervation essential for comprehensive resistant hypertension management.
The cardiovascular medicine field's need for durable non-pharmacological interventions supporting blood pressure control and cardiovascular risk reduction creates demand for specialized catheter technologies that can provide consistent denervation efficacy, enhance procedural success, and support guideline-directed medical therapy without compromising renal function or vascular integrity.
Clinical evidence validation and regulatory approval supporting renal denervation efficacy drive adoption in interventional cardiology suites, hypertension specialty clinics, and academic medical centers, where procedure outcomes have direct impact on cardiovascular morbidity and mortality reduction. The expanding global hypertension burden affecting over 1.3 billion adults worldwide with inadequate blood pressure control in approximately 50% of treated patients creates substantial unmet clinical needs for alternative therapeutic approaches.
Rising awareness about sympathetic nervous system hyperactivity and cardiovascular guideline recognition enable informed treatment decisions and adherence to evidence-based hypertension management protocols. However, procedural cost considerations and reimbursement pathway development may limit adoption rates and optimal implementation across healthcare systems serving diverse patient populations with varying insurance coverage and cardiovascular specialty access.
Segmental Analysis
The market is segmented by product, end use, and region. By product, the market is divided into radiofrequency ablation catheters (single-electrode RF systems, multi-electrode RF systems), ultrasound ablation catheters (circumferential ultrasound systems, linear ultrasound systems), and other RDN catheter technologies.
Based on end use, the market is categorized into hospitals (public hospitals, private hospitals), ambulatory surgery centers (single-specialty ASC, multi-specialty ASC), and others. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Product, Which Segment Accounts for the Dominant Market Share?
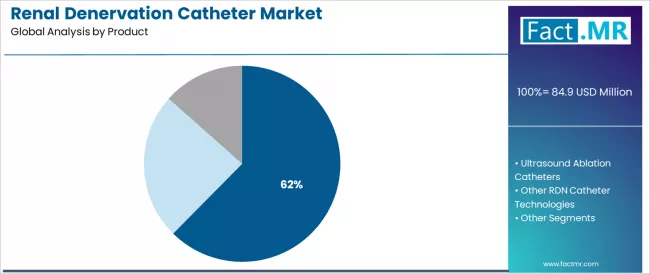
Radiofrequency ablation catheters represent the dominant force in the renal denervation catheter market, capturing 62.4% of total market share in 2025. This established product category encompasses solutions featuring proven radiofrequency energy delivery and electrode-based ablation systems, including single-electrode sequential ablation systems and multi-electrode simultaneous treatment platforms that enable controlled thermal energy application and sympathetic nerve disruption across renal artery adventitia and surrounding tissue worldwide.
The radiofrequency ablation segment's market leadership stems from its extensive clinical evidence base, with solutions capable of achieving consistent sympathetic denervation documented through multiple randomized sham-controlled trials including SPYRAL HTN-OFF MED and SPYRAL HTN-ON MED demonstrating significant blood pressure reductions while maintaining excellent safety profiles with minimal vascular complications across diverse patient populations.
Within the radiofrequency segment, multi-electrode RF systems represent 63.0% share driven by procedural efficiency advantages enabling complete circumferential treatment in single energy applications, while single-electrode RF systems account for 37.0% serving sequential point-by-point ablation approaches with precise lesion placement control.
The ultrasound ablation catheters segment maintains a substantial market share at 34.8%, serving interventional cardiologists requiring non-contact energy delivery and circumferential ablation capabilities addressing operator technique variability and electrode-tissue contact dependency including balloon-based ultrasound systems for comprehensive renal artery treatment.
Key procedural advantages driving the radiofrequency ablation catheters segment include:
- Advanced clinical evidence foundation with demonstrated blood pressure reduction efficacy across multiple pivotal randomized controlled trials validating sympathetic denervation durability
- Established procedural familiarity allowing interventional cardiologist adoption leveraging existing catheter ablation experience without extensive novel technique training complexity
- Enhanced electrode technology features enabling temperature monitoring and impedance feedback optimization while maintaining procedural safety and ablation quality consistency
- Superior regulatory clearance profile providing FDA approval status and European CE mark certification across various radiofrequency platform configurations and clinical indications
How are Ultrasound Ablation Catheters Positioned to Steer Market Growth?
Ultrasound ablation catheters offer differentiated technological functionality for physicians enabling uniform energy distribution without direct vessel wall contact while providing sufficient penetration depth to reach adventitial sympathetic nerves. The segment demonstrates strong growth potential, driven by favorable safety profile perceptions and expanding clinical evidence from Paradise Renal Denervation System trials.
Within ultrasound catheters, circumferential ultrasound systems represent 71.0% share providing comprehensive 360-degree treatment coverage, while linear ultrasound systems account for 29.0% serving targeted ablation approaches.
By End Use, Which Segment Accounts for the Largest Market Share?
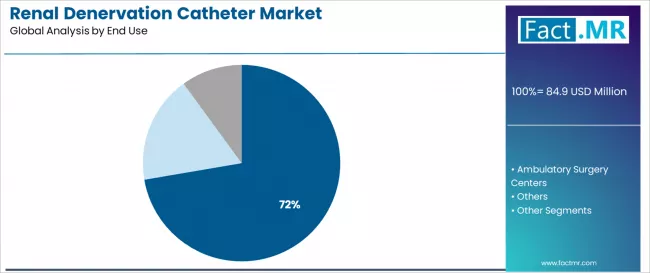
Hospitals dominate the renal denervation catheter end-use landscape with approximately 72.3% market share in 2025, reflecting the critical role of inpatient cardiovascular facilities in supporting complex interventional procedures, catheterization laboratory infrastructure requirements, and comprehensive periprocedural monitoring across acute care settings worldwide.
The hospital segment's market leadership is reinforced by procedural complexity necessitating interventional cardiology expertise, anesthesia support for conscious sedation, and immediate access to vascular complication management capabilities that characterize contemporary renal denervation practice in established cardiovascular centers.
Within this segment, private hospitals represent substantial procedure volumes, driven by elective case scheduling flexibility, dedicated hypertension programs, and premium cardiovascular service lines. This sub-segment benefits from established relationships with device manufacturers and physician preference for advanced technology adoption.
The ambulatory surgery centers segment represents an emerging category at 15.8% share, demonstrating potential expansion through specialized requirements for outpatient procedure feasibility, cost-efficiency pressures favoring ambulatory settings, and same-day discharge protocols where appropriate patient selection and procedural standardization enable catheterization laboratory alternatives. This segment benefits from healthcare system cost containment initiatives and patient preference for convenient outpatient cardiovascular interventions.
Within ambulatory settings, multi-specialty ASCs dominate over single-specialty centers through operational efficiency and broader case mix supporting cardiovascular procedure integration. The other end-use category maintains presence through specialty cardiovascular clinics, academic research institutions conducting clinical trials, and hypertension centers of excellence performing investigational procedures.
Key market dynamics supporting end-use growth include:
- Hospital procedure concentration driven by interventional cardiology infrastructure requirements and comprehensive cardiovascular care integration, requiring catheterization laboratory access and specialist availability
- Ambulatory surgery center emergence trends require appropriate patient selection criteria and standardized procedural protocols supporting safe outpatient renal denervation
- Integration of hypertension specialty programs enabling multidisciplinary team approaches and comprehensive cardiovascular risk management
- Growing emphasis on center of excellence models supporting high-volume operator experience and quality outcome optimization through procedural specialization
What are the Drivers, Restraints, and Key Trends of the Renal Denervation Catheter Market?
Mounting clinical evidence from pivotal randomized controlled trials creates expanding physician confidence and guideline recognition, with renal denervation representing validated therapeutic option for resistant hypertension in comprehensive cardiovascular disease prevention strategies, requiring widespread procedural availability.
Growing resistant hypertension prevalence and medication adherence challenges drive alternative treatment-seeking behavior and physician interest, with catheter-based denervation demonstrating substantial benefits including durable blood pressure reduction, potential medication burden reduction, and sympathetic modulation addressing multiple cardiovascular pathways by 2030. Recent regulatory approvals including FDA clearance and expanding reimbursement coverage enable commercial market development that improves patient access while meeting healthcare system cost-effectiveness expectations for interventional hypertension management.
Market restraints include limited reimbursement availability and uncertain procedural payment rates that can challenge hospital financial feasibility and patient out-of-pocket costs across healthcare systems, particularly when renal denervation procedures require USD 10,000-15,000 in device and facility costs with variable insurance coverage depending on regional reimbursement policies and medical necessity determinations.
Physician training requirements and procedural volume thresholds pose another significant obstacle, as renal denervation demands interventional cardiology expertise, femoral access proficiency, and renal artery catheterization skills, potentially affecting technology diffusion and outcome consistency. Long-term durability questions and patient selection uncertainty create additional clinical considerations, demanding comprehensive patient screening protocols and realistic expectation management for appropriate therapeutic application.
Key trends indicate accelerated multi-modality energy platform development in developed markets, particularly United States and Europe, where device manufacturers demonstrate commitment to alternative ablation technologies including ultrasound systems, pulsed electric field approaches, and chemical denervation methods.
Artificial intelligence integration and procedural guidance trends toward automated ablation parameter optimization with real-time impedance monitoring combine advanced sensing with algorithm-driven energy titration that optimizes denervation completeness and procedural efficiency. The market could face disruption if significant breakthrough medications for resistant hypertension or novel interventional approaches provide superior efficacy challenging catheter-based renal denervation positioning in hypertension treatment algorithms.
Analysis of the Renal Denervation Catheter Market by Key Countries
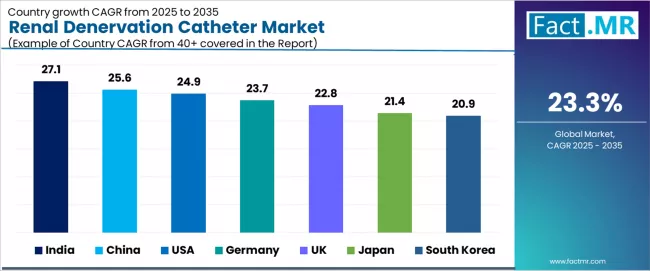
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 27.1% |
| China | 25.6% |
| USA | 24.9% |
| Germany | 23.7% |
| UK | 22.8% |
| Japan | 21.4% |
| South Korea | 20.9% |
The global renal denervation catheter market is expanding rapidly, with India leading at a 27.1% CAGR through 2035, driven by large hypertensive population exceeding 200 million adults, Medtronic training center expansion supporting physician education, and growing awareness of interventional hypertension management.
China follows at 25.6%, supported by massive hypertension prevalence affecting over 245 million people, NMPA approvals for new devices, and expanding cardiovascular intervention capabilities. USA records 24.9%, reflecting FDA approvals achieved in 2023, high resistant hypertension burden affecting approximately 12-15% of treated patients, and established interventional cardiology infrastructure.
Germany advances at 23.7%, leveraging early clinical adoption history, strong hospital cardiovascular infrastructure, and comprehensive clinical registry participation. UK posts 22.8%, focusing on rising uncontrolled hypertension prevalence and NHS innovation initiatives supporting device-based therapies, while Japan grows steadily at 21.4%, emphasizing aging population demographics, device-based therapy cultural acceptance, and cardiovascular specialty development. South Korea demonstrates 20.9% growth, anchored by expanding cardiology centers, strong technology adoption patterns, and healthcare modernization investments.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the renal denervation catheter market with a CAGR of 27.1% through 2035. The country's leadership position stems from massive hypertensive patient population estimated at over 200 million adults with inadequate blood pressure control, rapidly expanding interventional cardiology infrastructure across tier-1 and tier-2 cities, and strategic physician training initiatives including Medtronic-sponsored renal denervation centers of excellence.
Growth is concentrated in major metropolitan cardiovascular centers and emerging specialty hospitals, including Mumbai, Delhi, Bangalore, and Chennai, where interventional cardiologists are increasingly adopting catheter-based hypertension treatments for resistant hypertension management and medication intolerance cases. Distribution channels through medical device importers, hospital catheterization laboratory procurement systems, and cardiology society education programs expand technology accessibility across urban tertiary care facilities and dedicated cardiovascular institutes.
The country's growing medical tourism for cardiovascular interventions combined with domestic patient awareness provides strong momentum for innovative procedural adoption, including comprehensive skill development across cardiologists from technique training through independent procedural competency and clinical outcome tracking.
Key market factors:
- Hypertension disease burden concentrated in urban populations and expanding into rural areas with rising cardiovascular risk factor prevalence
- Training infrastructure expansion through manufacturer-sponsored education centers and interventional cardiology fellowship programs
- Comprehensive physician awareness initiatives ecosystem, including cardiology conference presentations and clinical evidence dissemination
- Medical device regulatory facilitation featuring streamlined approval pathways and government healthcare modernization supporting innovative cardiovascular technologies
Why is China Emerging as a High-Growth Market?
In major metropolitan centers including Beijing, Shanghai, Guangzhou, and Shenzhen, the adoption of renal denervation catheter technologies is accelerating across tertiary hospitals and cardiovascular specialty centers, driven by enormous hypertensive patient population exceeding 245 million adults and increasing awareness of interventional treatment options beyond pharmaceutical management. The market demonstrates strong growth momentum with a CAGR of 25.6% through 2035, linked to comprehensive healthcare system modernization and increasing focus on chronic disease management innovation.
Chinese interventional cardiologists are implementing catheter-based renal denervation procedures to address resistant hypertension cases while serving expanding patient populations seeking alternatives to lifelong medication regimens. The country's substantial cardiovascular disease burden creates ongoing demand for innovative hypertension interventions, while government emphasis on healthcare quality improvement and chronic disease control drives adoption of evidence-based cardiovascular technologies including renal denervation.
Key development areas:
- Patient population scale leading renal denervation demand with emphasis on resistant hypertension prevalence and uncontrolled blood pressure cases
- Regulatory approval acceleration through NMPA clearances for international and domestic device manufacturers
- Healthcare infrastructure investment enabling catheterization laboratory expansion and interventional cardiology capacity growth
- Clinical evidence generation initiatives alongside international trial participation supporting domestic physician confidence and guideline integration
What drives USA’s Market Resilience?
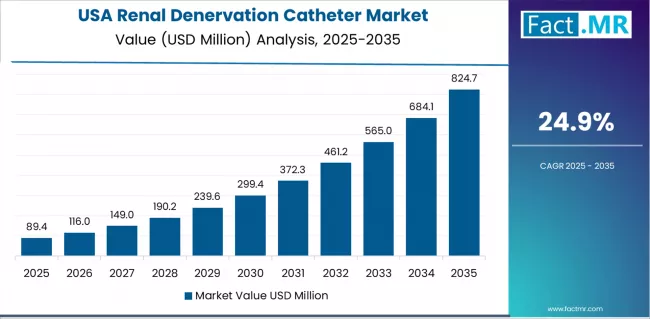
USA market expansion is driven by landmark FDA approvals achieved in 2023 for Medtronic Symplicity Spyral and Recor Paradise systems, substantial resistant hypertension patient population estimated at 9-12 million adults, and established interventional cardiology community with over 5,000 experienced operators. The country demonstrates strong growth potential with a CAGR of 24.9% through 2035, supported by pivotal clinical trial evidence from domestic study sites and increasing physician awareness through major cardiology conference presentations.
American interventional cardiologists face implementation considerations related to reimbursement coverage development and appropriate patient selection protocols, requiring demonstrated clinical value and guideline-directed therapy integration. However, established cardiovascular specialty infrastructure with over 1,500 catheterization laboratories and strong research culture investing over USD 3 billion annually create substantial baseline adoption potential for renal denervation, particularly among academic medical centers and high-volume hypertension programs where evidence-based innovation drives clinical practice evolution.
Market characteristics:
- Regulatory milestone achievement and commercial launch momentum showing robust adoption trajectory with substantial procedure volume growth potential
- Reimbursement pathway development varying between Medicare coverage determination and private payer policy establishment
- Future projections indicate continued growth with emphasis on real-world evidence generation and long-term durability documentation
- Growing emphasis on multidisciplinary hypertension teams and comprehensive cardiovascular risk reduction programs integrating device-based therapies
How does Germany Demonstrate Clinical Leadership?
The market in Germany leads in clinical research participation based on comprehensive renal denervation study enrollment history and rigorous evidence generation supporting technology validation. The country shows strong potential with a CAGR of 23.7% through 2035, driven by early adopter physician population and cardiovascular center infrastructure in major medical regions, including Bavaria, North Rhine-Westphalia, and Baden-Württemberg.
German interventional cardiologists are implementing renal denervation procedures through established clinical protocol adherence and emphasis on registry-based outcome tracking for evidence-based practice refinement, particularly in university hospitals and cardiovascular research centers demanding comprehensive clinical documentation. Distribution channels through medical device distributors and direct hospital relationships expand coverage across academic medical centers and community cardiovascular practices.
Leading market segments:
- Academic medical center leadership in major cities implementing comprehensive hypertension programs and clinical research protocols
- Clinical registry participation with German Renal Denervation Registry providing national outcome surveillance
- Strategic partnerships between device manufacturers and key opinion leader physicians advancing clinical evidence and technique optimization
- Focus on health technology assessment and cost-effectiveness analysis addressing healthcare system value demonstration requirements
What Positions UK for Healthcare System Integration?
In major cardiovascular centers including London, Manchester, Birmingham, and Edinburgh, interventional cardiologists are implementing renal denervation procedures through National Health Service commissioning pathways and clinical guideline recommendations, with documented procedure adoption showing gradual acceptance through evidence-based medicine culture. The market shows steady growth potential with a CAGR of 22.8% through 2035, linked to rising uncontrolled hypertension prevalence, NHS innovation funding supporting device-based therapy evaluation, and cardiovascular outcome improvement initiatives in major regions.
Cardiologists are adopting evidence-validated technologies with health technology appraisal guidance to enhance hypertension control while maintaining standards demanded by NHS quality frameworks and clinical commissioning group requirements. The country's established cardiovascular research infrastructure creates ongoing opportunities for renal denervation clinical studies that differentiate through rigorous methodology and comprehensive long-term follow-up documentation.
Market development factors:
- Uncontrolled hypertension burden leading treatment innovation with emphasis on resistant hypertension prevalence and cardiovascular risk reduction
- NHS innovation pathway participation providing structured evaluation frameworks for novel device technologies
- Strategic collaborations between NHS trusts and academic institutions expanding clinical trial infrastructure
- Emphasis on health economics evaluation and quality-adjusted life year analysis supporting technology adoption decisions
How Does Japan Show Demographic-Driven Demand Leadership?
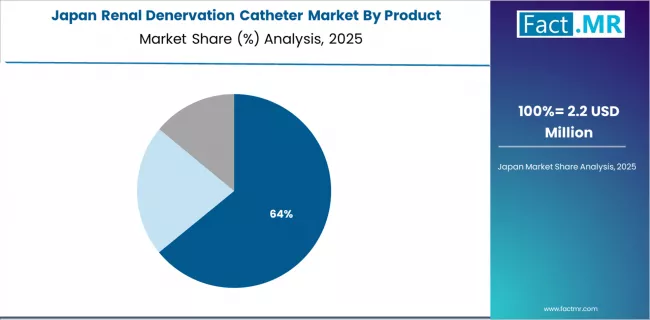
Japan's renal denervation catheter market demonstrates characteristics aligned with aging population demographics and high cardiovascular disease prevalence, with documented healthcare system interest achieving gradual technology adoption through device-based therapy acceptance across elderly hypertensive populations.
The country maintains steady growth momentum with a CAGR of 21.4% through 2035, driven by population aging with over 29% of citizens aged 65 and above and cultural preference for interventional solutions addressing medication burden reduction that aligns with Japanese healthcare delivery patterns.
Major cardiovascular centers, including Tokyo, Osaka, Kyoto, and Fukuoka facilities, showcase measured adoption of renal denervation technologies where clinical evidence requirements integrate with Japanese regulatory standards and reimbursement approval processes demanding comprehensive domestic validation.
Key market characteristics:
- Aging population demographics driving hypertension prevalence with emphasis on elderly patient management and polypharmacy reduction opportunities
- Device-based therapy acceptance supporting interventional cardiology procedure adoption and minimally invasive treatment preferences
- Regulatory pathway navigation requiring domestic clinical evidence generation and Pharmaceuticals and Medical Devices Agency approval processes
- Quality consciousness supporting reliable device performance expectations and comprehensive safety documentation requirements
What Characterizes South Korea's Market Development?
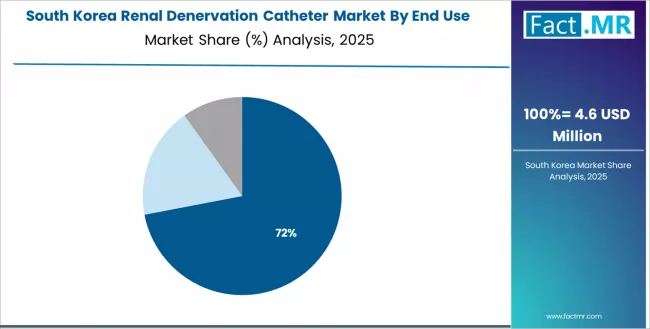
In major metropolitan centers including Seoul, Busan, Incheon, and Daegu, the adoption of renal denervation catheter solutions is expanding across university hospitals and cardiovascular specialty centers, driven by expanding cardiology infrastructure and strong technology adoption culture supporting medical innovation. The market demonstrates moderate growth potential with a CAGR of 20.9% through 2035, linked to healthcare system modernization and increasing focus on chronic disease management innovation in major urban regions.
South Korean interventional cardiologists are implementing advanced cardiovascular technologies and evaluating renal denervation for appropriate hypertensive patient populations. The country's technology-forward healthcare environment creates ongoing demand for innovative medical devices, while increasing international medical collaboration drives exposure to emerging interventional techniques including catheter-based sympathetic denervation.
Key development areas:
- Cardiology center expansion leading interventional capacity growth with emphasis on comprehensive cardiovascular service development
- Technology adoption culture supporting early evaluation of innovative medical devices and interventional techniques
- Medical device industry collaboration through domestic manufacturers and international partnerships
- Healthcare investment priorities supporting cardiovascular specialty infrastructure and advanced therapeutic modality adoption
Europe Market Split by Country
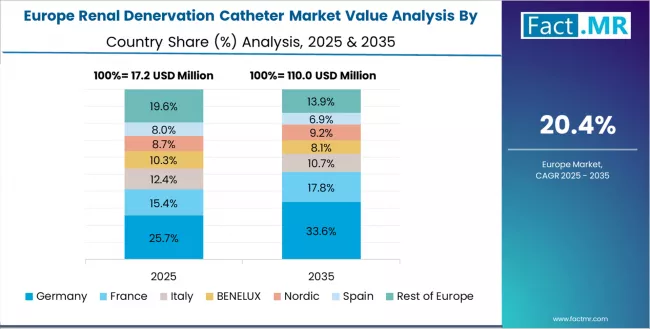
The renal denervation catheter market in Europe is projected to grow from USD 24.8 million in 2025 to USD 195.3 million by 2035, registering a CAGR of 23.2% over the forecast period. Germany is expected to maintain its leadership position with a 32.5% market share in 2025, adjusting to 32.0% by 2035, supported by its pioneering clinical research history, comprehensive cardiovascular infrastructure, and established interventional cardiology expertise serving major European markets.
UK follows with a 21.0% share in 2025, projected to reach 21.5% by 2035, driven by NHS innovation initiatives and academic medical center research participation in major cities implementing evidence-based hypertension management programs. France holds a 18.5% share in 2025, expected to maintain 19.0% by 2035 through ongoing cardiovascular center development and reimbursement pathway establishment.
Italy commands a 14.0% share, while Spain accounts for 10.0% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 4.0% to 4.0% by 2035, attributed to increasing renal denervation awareness in Nordic countries and emerging Eastern European cardiovascular centers implementing modern interventional hypertension therapies.
Competitive Landscape of the Renal Denervation Catheter Market
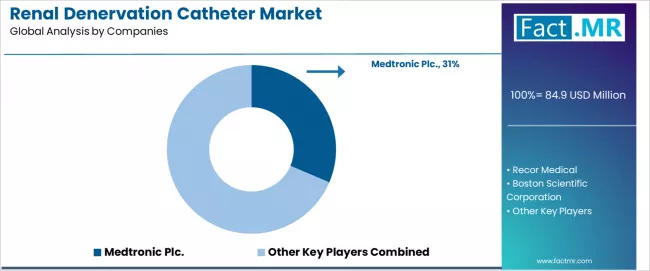
The renal denervation catheter market features approximately 10-15 meaningful players with moderate concentration, where the top three companies control roughly 55-65% of global market share through established regulatory approvals, comprehensive clinical evidence portfolios, and extensive physician training networks. Competition centers on clinical trial execution, energy modality differentiation, and procedural outcome demonstration rather than device price competition alone.
Market leaders include Medtronic Plc. with a 31.4% market share commanding significant position through Symplicity Spyral radiofrequency system. Recor Medical emphasizes Paradise ultrasound platform and Boston Scientific following recent SoniVie acquisition, which maintain competitive advantages through pivotal randomized controlled trial completion, FDA and CE mark regulatory clearances, and deep relationships with interventional cardiology key opinion leaders, creating high credibility among cardiovascular physicians seeking validated renal denervation solutions.
These companies leverage substantial clinical development investments and global commercialization capabilities to defend market positions while expanding into adjacent indications including heart failure and chronic kidney disease applications.
Challengers encompass emerging technology companies including SoniVie (now acquired by Boston Scientific) focusing on ultrasound innovation, Otsuka Medical Devices competing through alcohol-mediated chemical denervation approaches, and Ablative Solutions emphasizing perivascular administration systems. Medical device companies including Abbott, Terumo Corporation, and Johnson & Johnson MedTech evaluate strategic entry through internal development or acquisition considering substantial hypertension device market potential.
Emerging medical technology startups and alternative energy modality developers create competitive pressure through innovative ablation approaches, particularly in pulsed electric field and hybrid thermal systems where novel mechanisms and safety profiles provide differentiation opportunities against established radiofrequency and ultrasound platforms.
Market dynamics favor companies that combine robust clinical evidence with comprehensive physician education addressing complete procedural pathways from patient selection through technique execution and follow-up management. Strategic emphasis on real-world evidence generation, long-term durability demonstration, and reimbursement advocacy enables differentiation in rapidly evolving renal denervation segments across developed and emerging cardiovascular markets.
Recent Company Developments 2024 to 2025
- March 2025: Boston Scientific acquires SoniVie Ltd. to expand its renal denervation portfolio, integrating ultrasound-based sympathetic nerve ablation technology and strengthening competitive positioning in growing hypertension device market.
- January 2025: CMS initiates national coverage analysis for Medtronic's Symplicity Spyral RDN system, representing critical milestone toward Medicare reimbursement establishment and broader patient access in United States market.
- 2024: Recor Medical continues commercial expansion of Paradise Ultrasound RDN System in Europe, with increased adoption in Germany through physician training programs and clinical registry participation supporting evidence generation.
Global Renal Denervation Catheter Market - Stakeholder Contribution Framework
Renal denervation catheters represent a critical interventional technology that enables cardiologists and hypertension specialists to deliver minimally invasive sympathetic nerve ablation while addressing resistant hypertension and uncontrolled blood pressure without exclusive pharmaceutical escalation dependency, typically providing sustained systolic blood pressure reductions of 5-15 mmHg persisting beyond 36 months, sympathetic nervous system modulation addressing multiple cardiovascular pathways, and potential medication reduction benefits compared to pharmacological intensification alone while ensuring improved cardiovascular risk profiles and comprehensive patient quality of life outcomes.
With the market projected to grow from USD 84.9 million in 2025 to USD 691.8 million by 2035 at a 23.3% CAGR, these solutions offer compelling advantages for resistant hypertension applications, cardiovascular disease prevention strategies, and diverse patient populations seeking durable blood pressure control. Scaling market adoption and clinical integration requires coordinated action across cardiovascular policy, evidence generation, device manufacturers, interventional cardiologists, and reimbursement systems.
How Could Governments Spur Local Development and Adoption?
- Hypertension Management Programs: Include device-based therapies in national cardiovascular disease prevention initiatives, providing targeted support for resistant hypertension treatment access and supporting clinical research institutions through renal denervation study funding.
- Reimbursement Policy Development: Implement appropriate payment rates for renal denervation procedures, provide technology assessment pathways for innovative devices, and establish coverage criteria based on clinical evidence and appropriate patient selection guidelines.
- Regulatory Framework Optimization: Create efficient medical device approval pathways for catheter-based cardiovascular interventions, establish clear clinical evidence requirements, and develop post-market surveillance protocols ensuring patient safety.
- Healthcare Infrastructure Investment: Fund catheterization laboratory expansion in public hospitals, invest in interventional cardiology training programs, and support hypertension center of excellence development.
- Research & Innovation Support: Establish grants for cardiovascular device research, support clinical trial infrastructure and patient registry development, and create public-private partnerships advancing hypertension innovation.
How Could Industry Bodies Support Market Development?
- Clinical Guidelines Development: Define evidence-based treatment algorithms for resistant hypertension incorporating renal denervation, establish patient selection criteria and procedural technique standards, and create outcome measurement protocols supporting quality assurance.
- Physician Education Programs: Develop comprehensive training curricula for renal denervation procedure execution, patient assessment, and complication management through structured credentialing pathways.
- Registry Development: Create international renal denervation registries capturing procedural outcomes, long-term efficacy data, and safety surveillance enabling continuous quality improvement and evidence generation.
- Quality Standards Promotion: Lead device performance specifications and procedural quality metrics emphasizing ablation completeness, vascular safety, and blood pressure response documentation.
How Could Manufacturers and Technology Providers Strengthen the Ecosystem?
- Clinical Evidence Excellence: Conduct rigorous randomized controlled trials, real-world evidence studies, and long-term follow-up investigations that support regulatory submissions and guideline recommendations aligned with evidence-based medicine standards.
- Physician Training Investment: Offer comprehensive education programs including hands-on workshops, proctoring services, and simulation training that ensure successful procedure adoption and optimal patient outcomes.
- Technology Innovation: Develop next-generation catheter systems with enhanced ablation efficiency, real-time feedback mechanisms, and simplified procedural workflows that improve treatment consistency and physician confidence.
- Market Access Support: Provide health economics data, reimbursement advocacy, and coverage policy support that facilitate patient access and healthcare system adoption.
How Could Interventional Cardiologists and Healthcare Facilities Navigate the Market?
- Evidence-Based Patient Selection: Implement systematic screening protocols identifying appropriate resistant hypertension candidates, excluding secondary hypertension causes, and establishing realistic treatment expectations through comprehensive cardiovascular evaluation.
- Procedural Excellence Development: Pursue comprehensive training in renal denervation techniques, establish quality assurance protocols, and participate in registry-based outcome tracking supporting continuous performance improvement.
- Multidisciplinary Collaboration: Develop integrated hypertension management teams combining cardiologists, nephrologists, and hypertension specialists supporting comprehensive patient care and appropriate treatment selection.
- Outcomes Documentation: Implement systematic blood pressure monitoring, medication tracking, and patient-reported outcome collection enabling evidence-based practice refinement and quality reporting.
How Could Investors and Financial Enablers Unlock Value?
- Innovation Investment: Back companies developing breakthrough renal denervation technologies including novel energy modalities, procedural guidance systems, and combination device approaches enhancing clinical efficacy.
- Clinical Development Financing: Support pivotal trial execution, regulatory submission programs, and post-approval evidence generation funding companies through expensive cardiovascular device development pathways.
- Market Expansion Support: Finance international commercialization strategies, physician training infrastructure, and distribution network development enabling global market penetration for approved technologies.
- Strategic Consolidation: Support acquisitions integrating complementary technologies, expanding product portfolios, and creating comprehensive hypertension management solution platforms through medical device industry consolidation.
Key Players in the Renal Denervation Catheter Market
- Medtronic Plc.
- Recor Medical
- Boston Scientific Corporation
- SoniVie Ltd.
- Otsuka Medical Devices Co. Ltd.
- Ablative Solutions Inc.
- SyMap Medical
- Terumo Corporation
- Abbott Laboratories
- Johnson & Johnson MedTech
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 84.9 Million |
| Product | Radiofrequency Ablation Catheters (Single-electrode RF Systems, Multi-electrode RF Systems), Ultrasound Ablation Catheters (Circumferential Ultrasound Systems, Linear Ultrasound Systems), Other RDN Catheter Technologies |
| End Use | Hospitals (Public Hospitals, Private Hospitals), Ambulatory Surgery Centers (Single-specialty ASC, Multi-specialty ASC), Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | India, China, USA, Germany, UK, Japan, South Korea, and 40+ countries |
| Key Companies Profiled | Medtronic, Recor Medical, Boston Scientific, SoniVie, Otsuka Medical Devices, Ablative Solutions, SyMap Medical, Terumo Corporation, Abbott, Johnson & Johnson MedTech |
| Additional Attributes | Dollar sales by product and end-use categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with medical device manufacturers and interventional cardiology companies, device specifications and clinical performance requirements, integration with hypertension management programs and cardiovascular disease prevention initiatives, innovations in energy modality development and procedural guidance systems, and development of specialized applications with resistant hypertension treatment and sympathetic nervous system modulation capabilities. |
Renal Denervation Catheter Market by Segments
-
Product :
- Radiofrequency Ablation Catheters
- Single-electrode RF Systems
- Multi-electrode RF Systems
- Ultrasound Ablation Catheters
- Circumferential Ultrasound Systems
- Linear Ultrasound Systems
- Other RDN Catheter Technologies
- Radiofrequency Ablation Catheters
-
End Use :
- Hospitals
- Public Hospitals
- Private Hospitals
- Ambulatory Surgery Centers
- Single-specialty ASC
- Multi-specialty ASC
- Others
- Hospitals
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product , 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product , 2025 to 2035
- Radiofrequency Ablation Catheters
- Ultrasound Ablation Catheters
- Other RDN Catheter Technologies
- Y to o to Y Growth Trend Analysis By Product , 2020 to 2024
- Absolute $ Opportunity Analysis By Product , 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Hospitals
- Ambulatory Surgery Centers
- Others
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product
- By End Use
- Competition Analysis
- Competition Deep Dive
- Medtronic Plc.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Recor Medical
- Boston Scientific Corporation
- SoniVie Ltd.
- Otsuka Medical Devices Co. Ltd.
- Ablative Solutions Inc.
- SyMap Medical
- Terumo Corporation
- Abbott Laboratories
- Johnson & Johnson MedTech
- Medtronic Plc.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Product
- Figure 6: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by End Use
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 22: North America Market Attractiveness Analysis by Product
- Figure 23: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by End Use
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 29: Latin America Market Attractiveness Analysis by Product
- Figure 30: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 32: Latin America Market Attractiveness Analysis by End Use
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 36: Western Europe Market Attractiveness Analysis by Product
- Figure 37: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 39: Western Europe Market Attractiveness Analysis by End Use
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Product
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 50: East Asia Market Attractiveness Analysis by Product
- Figure 51: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 53: East Asia Market Attractiveness Analysis by End Use
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Product
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Product
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the renal denervation catheter market in 2025?
The global renal denervation catheter market is estimated to be valued at USD 84.9 million in 2025.
What will be the size of renal denervation catheter market in 2035?
The market size for the renal denervation catheter market is projected to reach USD 691.8 million by 2035.
How much will be the renal denervation catheter market growth between 2025 and 2035?
The renal denervation catheter market is expected to grow at a 23.3% CAGR between 2025 and 2035.
What are the key product types in the renal denervation catheter market?
The key product types in renal denervation catheter market are radiofrequency ablation catheters , ultrasound ablation catheters and other rdn catheter technologies.
Which end use segment to contribute significant share in the renal denervation catheter market in 2025?
In terms of end use, hospitals segment to command 72.3% share in the renal denervation catheter market in 2025.