Retinal Vein Occlusion Treatment Market
Retinal Vein Occlusion Treatment Market Size and Share Forecast Outlook 2025 to 2035
Retinal vein occlusion treatment market is projected to grow from USD 2.5 billion in 2025 to USD 5.1 billion by 2035, at a CAGR of 7.2%. Central Retinal Vein Occlusion (CRVO) will dominate with a 66.9% market share, while anti-vegf will lead the treatment type segment with a 65.8% share.
Retinal Vein Occlusion Treatment Market Forecast and Outlook 2025 to 2035
The global retinal vein occlusion treatment market is set to grow from USD 2.54 billion in 2025 to USD 5.07 billion by 2035, adding USD 2.53 billion in new revenue and advancing at a CAGR of 7.2%. Growth is driven by rising diabetic retinopathy prevalence, escalating aging population demographics, and expanding ophthalmic care infrastructure in emerging economies seeking comprehensive retinal disease management solutions.
Central retinal vein occlusion holds a 66.9% share in 2025, favored in clinical treatment environments for its severe visual impairment characteristics, established anti-VEGF response patterns, and comprehensive therapeutic protocol requirements addressing diverse patient severity profiles.
Quick Stats for Retinal Vein Occlusion Treatment Market
- Retinal Vein Occlusion Treatment Market Value (2025): USD 2.54 billion
- Retinal Vein Occlusion Treatment Market Forecast Value (2035): USD 5.07 billion
- Retinal Vein Occlusion Treatment Market Forecast CAGR: 7.2%
- Leading Disease Type in Retinal Vein Occlusion Treatment Market: Central Retinal Vein Occlusion (CRVO)
- Key Growth Regions in Retinal Vein Occlusion Treatment Market: North America, Asia Pacific, and Europe
- Top Players in Retinal Vein Occlusion Treatment Market: F. Hoffmann-La Roche Ltd., Regeneron Pharmaceuticals Inc., AbbVie Inc., Chugai Pharmaceutical Co. Ltd., Pfizer Inc.

Anti-VEGF therapies represent 65.8% and remain essential in treatment algorithms where vascular endothelial growth factor inhibition and macular edema reduction match clinical effectiveness requirements and evidence-based guideline recommendations. Bevacizumab and ranibizumab at 48% within anti-VEGF modalities are critical among first-line therapeutic options as treatment experience accumulates and cost-effectiveness considerations gain traction in diverse healthcare markets.
Retail pharmacies remain the dominant distribution channel at 42.8%, reflecting the need for accessibility convenience, prescription fulfillment efficiency, and patient-centered medication dispensing across diverse geographic regions. Hospitals and clinics at 39.5% maintain substantial market presence as intravitreal injection procedures concentrate in specialized ophthalmic facilities and retinal specialist practices in metropolitan healthcare centers.
From 2025-2030, the market will witness the market climbing from USD 2.54 billion to approximately USD 3.58 billion, adding USD 1.04 billion in value, which constitutes 41% of the total forecast growth period. This phase will be characterized by the continued dominance of established anti-VEGF agents in developed markets, combined with accelerating biosimilar anti-VEGF penetration in emerging economies where cost-effective therapeutic alternatives and patent expiration consequences create favorable adoption conditions.
Manufacturers will concentrate on extended-duration formulation development, intravitreal injection burden reduction for improved patient compliance, and retinal specialist network expansion in underserved territories where retinal vein occlusion awareness remains limited among general ophthalmology practitioners. Enhanced port delivery system implementation and sustained-release implant integration will become standard therapeutic innovation priorities rather than experimental technology differentiators.
From 2030-2035, the market will witness sustained expansion from USD 3.58 billion to USD 5.07 billion, representing an addition of USD 1.49 billion or 59% of the decade's growth. This period will be defined by the broadening acceptance of next-generation biologic therapies beyond conventional anti-VEGF platforms, integration of gene therapy approaches across clinical development pipelines, and strengthened diagnostic networks in previously underserved emerging markets.
Sustained-release technology maturation will enable extended treatment interval capabilities approaching multi-month dosing durability, while declining biosimilar costs compress treatment barriers that currently limit anti-VEGF accessibility in resource-constrained healthcare environments.
Where revenue comes from - Now Vs Next (industry-level view)
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | Central retinal vein occlusion | 66.9% | Severe vision loss prevalence |
| Branch retinal vein occlusion | 33.1% | Sectoral retinal involvement | |
| Anti-VEGF therapies | 65.8% | First-line treatment standard | |
| Corticosteroid drugs | 27.6% | Alternative mechanism positioning | |
| Bevacizumab & ranibizumab | 48% | Cost-effective options | |
| Aflibercept | 37% | Enhanced binding affinity | |
| North America markets | 41.2% | Advanced care infrastructure | |
| Future (3-5 yrs) | CRVO treatment platforms | 64-68% | Sustained prevalence leadership |
| BRVO therapeutic expansion | 32-36% | Early detection enhancement | |
| Next-generation anti-VEGF | 68-72% | Extended-duration formulations | |
| Biosimilar anti-VEGF adoption | 22-26% | Emerging market penetration | |
| Sustained-release implants | 15-19% | Injection burden reduction | |
| Gene therapy approaches | 3-7% | Curative modality exploration | |
| Asia Pacific market growth | 32-36% | Infrastructure development momentum |
Retinal Vein Occlusion Treatment Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 2.54 billion |
| Market Forecast (2035) ↑ | USD 5.07 billion |
| Growth Rate ★ | 7.2% CAGR |
| Leading Disease Type → | Central Retinal Vein Occlusion (CRVO) |
| Primary Treatment Modality → | Anti-VEGF Therapies |
The market demonstrates solid fundamentals with central retinal vein occlusion capturing a commanding 66.9% share through proven severe vision impairment patterns and comprehensive anti-VEGF response characteristics.
Anti-VEGF therapy configurations drive primary treatment modality demand at 65.8% share, supported by established clinical evidence infrastructure and guideline recommendation advantages that maintain therapeutic effectiveness across diverse retinal vascular occlusion patient populations.
Distribution concentration remains anchored in retail pharmacies with 42.8% channel dominance and convenient prescription fulfillment ecosystems, while hospital and clinic settings show substantial presence rates driven by intravitreal injection procedure requirements and specialized retinal care delivery models.
Imperatives for Stakeholders in Retinal Vein Occlusion Treatment Market
Design for durability, not just efficacy
- Offer complete therapeutic solutions: anti-VEGF potency optimization + sustained-release formulation development + corticosteroid implant innovation + combination therapy protocols + patient compliance enhancement programs.
- Preconfigured treatment pathways: CRVO intensive management specifications, BRVO early intervention approaches, treatment-naive protocols, and switch therapy algorithms for comprehensive patient outcome optimization.
Regulatory readiness for biosimilar transitions
- Real-time pharmacovigilance monitoring systems, biosimilar equivalence documentation, and supply chain transparency (active pharmaceutical ingredient sourcing, manufacturing facility validation, batch release testing protocols).
Affordability-by-access approach
- Patient assistance program expansion, specialty pharmacy partnership development, insurance prior authorization navigation support, and transparent intravitreal injection cost documentation.
Distribution-focused patient penetration
- Clear retinal specialist network expansion strategies + established vitreoretinal center partnerships (academic medical institutions, community retina practices, ambulatory surgery center coordination); telemedicine screening programs for disease awareness and early referral pathway development.
Segmental Analysis
The market segments by disease type into central retinal vein occlusion and branch retinal vein occlusion, representing the evolution from complete retinal venous obstruction toward sectoral vascular compromise requiring specialized diagnostic recognition and differentiated therapeutic intensity approaches. The treatment modality segmentation divides the market into anti-VEGF therapies (65.8%), corticosteroid drugs (27.6%), and other approaches (6.6%), reflecting distinct therapeutic priorities for vascular endothelial growth factor inhibition versus inflammatory pathway modulation and alternative mechanism exploration.
The end-user segmentation reveals retail pharmacy's leading 42.8% distribution presence, followed by hospitals and clinics at 39.5%, demonstrating varied dispensing channel preferences and intravitreal injection procedure setting requirements. The regional segmentation reveals North America's commanding 41.2% market leadership, followed by Asia Pacific at 29.8% and Europe at 18.3%, demonstrating varied healthcare infrastructure maturity levels and retinal disease management capability development stages.
Which Disease Type will account for the Dominant Share in the Market?

Central retinal vein occlusion commands the leading position in the retinal vein occlusion treatment market with a 66.9% share through proven severe visual impairment characteristics, including complete retinal venous drainage obstruction patterns, extensive macular edema development, and comprehensive therapeutic intervention requirements that enable retinal specialists to achieve vision preservation across varied patient severity levels and ischemic risk profiles.
The segment benefits from clinical community recognition regarding urgent treatment necessity that provides rapid therapeutic initiation, manageable intravitreal injection protocols, and established anti-VEGF response assessment without requiring complex diagnostic stratification.
Advanced optical coherence tomography enables precise macular edema quantification, treatment response monitoring, and integration with existing retinal imaging infrastructure, where therapeutic urgency and visual prognosis implications represent critical patient counseling determinants. Anti-VEGF treatment uptake at 72% within CRVO management captures substantial utilization reflecting clinical guideline alignment and evidence-based therapeutic positioning for macular edema resolution and visual acuity improvement.
Central retinal vein occlusion variants differentiate through proven treatment response predictability, extensive clinical trial evidence foundation, and integration with established retinal vascular disease management algorithms that enhance therapeutic confidence while maintaining optimal injection frequency optimization for diverse patient compliance and healthcare access applications.
Key market characteristics:
- Advanced imaging assessment with optical coherence tomography quantification and fluorescein angiography ischemia evaluation capabilities
- Superior treatment urgency recognition, enabling rapid specialist referral with vision preservation imperative and macular edema intervention timing
- Healthcare infrastructure integration, including retinal specialist consultation coordination, intravitreal injection facility availability, and long-term monitoring protocol establishment for vision outcome optimization applications
Why does BRVO Represent a Significant Share in the Market?
Branch retinal vein occlusion (BRVO) maintains substantial market position with 33.1% share due to its sectoral retinal involvement pattern and favorable visual prognosis characteristics. These vascular occlusions appeal to comprehensive ophthalmologists and retinal specialists recognizing localized macular edema presentations, offering therapeutic intervention through established anti-VEGF protocols and selective treatment approaches.
Market significance is driven by early detection opportunities through routine ophthalmologic examination, emphasizing prompt therapeutic intervention platforms and vision preservation achievement through timely macular edema management in affected retinal sectors.
Which Treatment Type is Relied Upon the Most for Retinal Vein Occlusion?

Anti-VEGF therapy demonstrates treatment modality leadership in the retinal vein occlusion market with a 65.8% share. Growth is attributed to widespread clinical evidence validation and established focus on vascular endothelial growth factor pathway inhibition, macular edema reduction effectiveness, and demonstrated visual acuity improvement that maximizes therapeutic outcomes while maintaining acceptable safety profile standards. Retinal specialists prioritize proven treatment efficacy credibility, guideline recommendation alignment, and integration with established intravitreal injection practice patterns that enable coordinated care delivery experiences across diverse clinical settings.
The segment benefits from substantial randomized controlled trial evidence accumulation and real-world effectiveness data maturity that emphasize anti-VEGF therapy selection for primary therapeutic intervention and predictable outcome achievement applications. Bevacizumab and ranibizumab formulations capture 48% share within the anti-VEGF segment, demonstrating clinical preference for cost-effective therapeutic options with comprehensive clinical experience support and favorable reimbursement accessibility.
Clinical practice guideline updates incorporate anti-VEGF therapy as first-line treatment recommendations for standard care delivery, while biosimilar market entry increases treatment accessibility capabilities that comply with cost-effectiveness requirements and expand patient access across resource-constrained healthcare environments.
What drives Corticosteroid Drug Utilization in Alternative Mechanism Applications?
Corticosteroid drugs capture 27.6% share through comprehensive inflammatory pathway modulation requirements in refractory macular edema management, sustained-release implant technology, and alternative therapeutic mechanism provision.
These treatment approaches demand careful patient selection capable of managing intraocular pressure risk while providing effective anti-inflammatory action and extended treatment duration characteristics, appealing to retinal specialists seeking therapeutic alternatives for anti-VEGF non-responders and patients requiring injection frequency reduction beyond conventional monthly dosing limitations.
Which End User is the Primary Beneficiary of Retinal Vein Occlusion Treatment Methods?
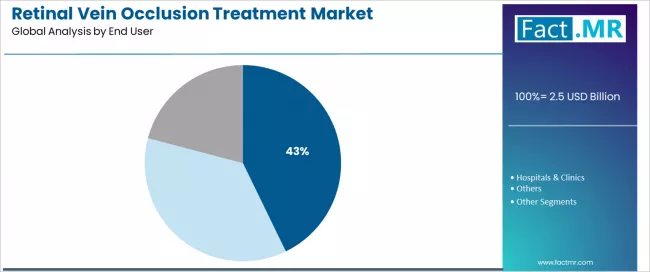
Retail pharmacies establish end-user leadership in the retinal vein occlusion treatment distribution sector with a 42.8% share. This is due to comprehensive prescription fulfillment infrastructure and sustained focus on patient accessibility optimization, medication dispensing efficiency, and convenient service delivery that maximizes patient compliance while maintaining cost-effectiveness standards.
Patients and healthcare providers prioritize prescription accessibility convenience, insurance coordination support, and integration with established pharmacy benefit management systems that enable streamlined medication acquisition across diverse geographic markets. The segment benefits from substantial community pharmacy network density and specialty pharmacy service expertise that emphasize the delivery of refrigerated medication handling for biologic therapeutic preservation and patient counseling support applications.
Local chain pharmacies capture 53% share within retail pharmacy distribution, demonstrating patient preference for established community pharmacy relationships with reliable service delivery and accessible locations. Specialty pharmacy network expansion programs incorporate specialty medication management as standard service offerings for complex therapeutic coordination, while patient assistance program partnerships increase medication access capabilities that address high treatment cost barriers and insurance coverage limitation navigation.
Regional dynamics include:
- Strong presence in community pharmacy settings with comprehensive specialty medication handling capabilities and patient education support
- Increasing adoption of specialty pharmacy mail-order services for convenience enhancement and treatment adherence improvement
- Rising integration with manufacturer patient support programs for co-pay assistance and insurance navigation coordination
How are Hospitals & Clinics Advancing Intravitreal Injection Service Delivery?
Hospitals and clinics capture 39.5% end-user share through specialized intravitreal injection procedure capabilities, comprehensive retinal care infrastructure, and integrated ophthalmology service models.
These healthcare facilities demonstrate on-site medication administration in dedicated procedure rooms and clinic settings, with anti-VEGF injections gaining systematic integration while patient flow optimization drives continued operational efficiency enhancement requiring careful coordination between retinal specialist schedules and pharmaceutical procurement systems.
What establishes North America's Regional Market Leadership in Retinal Vein Occlusion Treatment?

North America establishes regional market leadership in the retinal vein occlusion treatment sector with commanding a 41.2% share due to comprehensive retinal care infrastructure and sustained focus on advanced anti-VEGF therapy adoption, specialized vitreoretinal fellowship training, and established insurance coverage frameworks that maximize treatment accessibility while maintaining clinical excellence standards.
Healthcare providers and pharmaceutical manufacturers prioritize regulatory approval pathway clarity, comprehensive reimbursement coverage availability, and integration with established retinal specialist networks that enables coordinated treatment commercialization across academic and community practice settings.
The region benefits from substantial clinical research infrastructure and retinal imaging technology advancement that emphasizes the development of evidence-based therapeutic platforms for retinal vascular disease intervention and vision preservation applications. USA at 82% within North America demonstrates market concentration through advanced healthcare system integration and comprehensive Medicare coverage supporting anti-VEGF treatment accessibility.
Insurance coverage expansion programs incorporate specialty medication access as standard vision preservation priorities for chronic disease management, while retinal specialist workforce adequacy increases treatment delivery capabilities that meet clinical demand requirements and ensure timely therapeutic intervention across diverse geographic regions including underserved rural communities.
Regional dynamics include:
- Strong concentration in metropolitan areas with comprehensive vitreoretinal centers and subspecialty fellowship-trained retina specialists
- Increasing adoption of ambulatory surgery center models for high-volume intravitreal injection delivery and cost efficiency optimization
- Rising integration with patient registries for real-world outcomes tracking and treatment protocol refinement support
How does Asia Pacific Emerge as Fastest-Growing Regional Market?
Asia Pacific captures 29.8% regional share with accelerating growth momentum through expanding ophthalmic care infrastructure, increasing diabetic retinopathy screening, and biosimilar anti-VEGF adoption.
The region demonstrates rapid healthcare modernization across major urban centers including India at 8.9% and China at 8.5% CAGR, with retinal vein occlusion treatment gaining traction in tertiary eye hospitals and specialized retina clinics while government healthcare initiatives drive continued diagnostic capability development requiring strategic partnerships between international pharmaceutical companies and domestic healthcare providers.
Why does Europe Show Established Retinal Care Framework Leadership?
Europe holds 18.3% regional share with mature market characteristics through comprehensive national health system integration, clinical trial participation excellence, and evidence-based guideline implementation.
Markets across European countries demonstrate established retinal specialist networks and academic ophthalmology center leadership, while regulatory harmonization through European Medicines Agency approval creates opportunities for pharmaceutical manufacturers seeking coordinated market access platforms with systematic reimbursement assessment and health technology evaluation frameworks.
What are the Drivers, Restraints, and Key Trends of the Retinal Vein Occlusion Treatment Market?
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Diabetic population increase & vascular risk factor prevalence (metabolic syndrome, hypertension) | ★★★★★ | Large-scale diabetes epidemic enables higher retinal vein occlusion incidence for therapeutic intervention; systemic vascular disease burden shifting toward comprehensive retinal complication management drives treatment demand across ophthalmology practices. |
| Driver | Aging population demographics & age-related retinal disease (geriatric vision loss, comorbidity prevalence) | ★★★★★ | Drives demand for vision preservation therapeutics and chronic disease management solutions; manufacturers providing durable treatment options gain competitive advantage in elderly patient compliance-challenged segments. |
| Driver | Optical coherence tomography advancement & diagnostic precision (quantitative imaging, biomarker identification) | ★★★★☆ | Retinal specialists demand improved macular edema assessment and treatment monitoring capabilities; imaging technology advancement expanding treatment candidate identification beyond traditional clinical examination limitations. |
| Restraint | High treatment costs & intravitreal injection burden (repeated procedures, patient compliance challenges) | ★★★★★ | Healthcare systems face budget impact scrutiny and treatment adherence difficulties; increases patient dropout rates and affects long-term visual outcome achievement in chronic maintenance therapy scenarios. |
| Restraint | Biosimilar competition & pricing pressure (margin erosion, market share fragmentation) | ★★★☆☆ | Innovator pharmaceutical companies face revenue decline and competitive intensity increases, limiting premium pricing sustainability and affecting research investment in next-generation therapeutic development. |
| Trend | Extended-duration anti-VEGF formulations & port delivery systems (injection frequency reduction, compliance enhancement) | ★★★★★ | Growing clinical expectation for reduced treatment burden beyond monthly intravitreal injections; sustained-release technology advancement becomes core differentiation strategy for future therapeutic paradigm transformation. |
| Trend | Gene therapy development & one-time curative approaches (genetic intervention, permanent efficacy) | ★★★★☆ | Retinal disease management evolving beyond chronic intravitreal injection maintenance toward potential single-administration solutions; gene therapy emergence drives transformative treatment paradigm expectations in specialized patient populations. |
Analysis of the Retinal Vein Occlusion Treatment Market by Key Countries
The retinal vein occlusion treatment market demonstrates robust regional growth dynamics with emerging leaders including India (8.9% CAGR) and China (8.5% CAGR) driving expansion through ophthalmic infrastructure development and biosimilar anti-VEGF adoption acceleration.
Strong performers encompass Japan (7.3% CAGR), USA (6.8% CAGR), and Germany (6.5% CAGR), benefiting from established retinal care networks and comprehensive reimbursement frameworks. Emerging markets feature Brazil (6.2% CAGR) and South Africa (5.8% CAGR), where diagnostic capability improvement and private ophthalmology sector expansion support consistent growth patterns.
Regional synthesis reveals Asian markets leading growth acceleration through comprehensive healthcare modernization positioning and diabetic retinopathy screening expansion, while developed countries demonstrate steady market evolution supported by next-generation biologic innovation and extended-duration formulation development. European markets show solid advancement driven by clinical trial participation and evidence-based guideline implementation frameworks.

| Region/Country | 2025 to 2035 Growth | How to win | What to watch out |
|---|---|---|---|
| India | 8.9% | Focus on biosimilar affordability positioning | Infrastructure disparities; specialist availability |
| China | 8.5% | Lead with domestic biosimilar partnerships | Regulatory complexity; pricing negotiation |
| Japan | 7.3% | Offer reimbursement-optimized protocols | Aging infrastructure; demographic headwinds |
| USA | 6.8% | Push extended-duration innovations | Market saturation; biosimilar erosion |
| Germany | 6.5% | Deliver clinical trial collaboration | Cost containment pressure; HTA requirements |
| Brazil | 6.2% | Provide private sector partnerships | Economic volatility; public system constraints |
| South Africa | 5.8% | Maintain access program development | Resource limitations; specialist concentration |
What drives USA's Advanced Retinal Care Leadership?

The USA establishes comprehensive retinal care leadership through progressive anti-VEGF therapy adoption and documented effectiveness in next-generation biologic implementation across academic vitreoretinal centers and community retina practice networks. The country's 6.8% growth rate reflects innovation in extended-duration anti-VEGF formulation development and port delivery system clinical trial advancement that support continued therapeutic evolution in retinal vein occlusion management despite market maturity.
Growth concentrates in established retina subspecialty practices including private equity-backed retina groups and academic medical center vitreoretinal divisions, where high-volume intravitreal injection services showcase demand for efficient patient throughput that appeals to practice management optimization seeking operational excellence and quality outcome maintenance.
American retinal specialists leverage established fellowship training infrastructure and comprehensive continuing medical education programs, including American Society of Retina Specialists conferences and clinical trial participation networks that create evidence-based practice advancement and therapeutic innovation early adoption. The market benefits from robust specialty pharmacy networks and manufacturer patient assistance program infrastructure that support treatment affordability while maintaining consistent therapeutic adherence and optimal visual outcome achievement.
How does Germany Demonstrate Clinical Trial Excellence Leadership?
Germany's advanced healthcare market demonstrates sophisticated retinal vein occlusion treatment integration with documented effectiveness in clinical research participation and evidence generation through comprehensive university eye clinic networks and retinal specialist collaboration. The country leverages ophthalmology academic expertise and systematic patient registry development to maintain 6.5% growth momentum.
Retinal centers including university hospital eye departments showcase clinical trial recruitment capability where retinal vein occlusion therapeutics integrate with European Medicines Agency regulatory submission support and real-world evidence generation to optimize treatment advancement and ensure health technology assessment compliance under German statutory health insurance reimbursement frameworks.
Retinal specialists prioritize evidence-based treatment protocols and guideline adherence in clinical decision-making, creating demand for clinically validated anti-VEGF therapies with comprehensive health economic evaluation documentation, including cost-effectiveness analysis combined with quality-adjusted life year assessments and budget impact considerations.
The market benefits from established professional society infrastructure and systematic quality assurance programs that provide treatment standardization opportunities and compliance with European Union medical device and pharmaceutical regulations.
Why Does Japan Show Strong Reimbursement Framework Support?
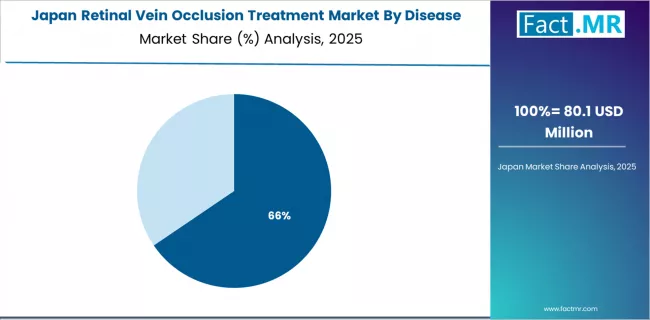
Japan maintains 7.3% CAGR with favorable market characteristics through comprehensive national health insurance coverage, advanced retinal imaging technology utilization, and aging population demographics.
Markets across Japanese healthcare demonstrate systematic ophthalmology subspecialty integration and pharmaceutical reimbursement clarity, while demographic aging trends create sustained opportunities for retinal disease therapeutic manufacturers seeking mature market platforms with predictable pricing frameworks and established treatment protocol adherence patterns supporting consistent market growth trajectories.
What Drives India's Fastest Country-Level Growth?
India establishes fastest country-level growth through progressive ophthalmic care infrastructure expansion and rising diabetic retinopathy incidence, positioning retinal vein occlusion treatment as essential component in vision preservation initiatives across tier-1 metropolitan eye hospitals and emerging regional retina centers.
The country's 8.9% growth rate reflects healthcare accessibility improvements and increasing middle-class insurance coverage expansion that encourage deployment of affordable anti-VEGF options including biosimilar therapeutic alternatives in diverse socioeconomic patient populations.
Growth concentrates in major metropolitan areas including Delhi, Mumbai, Bangalore, and Chennai, where specialized retina clinics showcase adoption of international treatment protocols that appeal to quality-conscious patients seeking fellowship-trained retinal specialist expertise and advanced optical coherence tomography imaging capabilities.
How Does China Advance Biosimilar Anti-VEGF Development?
China captures 8.5% CAGR through expanding research and development infrastructure, domestic biosimilar anti-VEGF drug approval acceleration, and comprehensive ophthalmic care modernization.
The market demonstrates rapid pharmaceutical innovation across major urban centers, with retinal vein occlusion treatment adoption gaining momentum in tertiary eye hospitals and private ophthalmology chains while government healthcare reform initiatives and national essential medicine list inclusion considerations drive continued therapeutic accessibility development requiring careful regulatory navigation and pricing strategy optimization.
Why does Brazil Show Private Ophthalmology Sector Expansion?
Brazil maintains 6.2% CAGR with emerging market characteristics through improving diagnostic capabilities, expanding private ophthalmology infrastructure, and selective public health system integration.
Markets across Brazilian healthcare demonstrate developing retinal subspecialty expertise and increasing optical coherence tomography availability, while economic development creates opportunities for international pharmaceutical manufacturers seeking Latin American market platforms with growing middle-class healthcare consumption and systematic ophthalmology residency training program enhancement supporting gradual retinal care capability maturation.
Europe Market Split by Country

The retinal vein occlusion treatment market in Europe is projected to grow from USD 0.46 billion in 2025 to USD 0.85 billion by 2035, registering a CAGR of 6.4% over the forecast period. Germany is expected to maintain its leadership position with a 26.8% market share in 2025, supported by its advanced ophthalmology infrastructure and comprehensive retinal specialist networks across university eye clinics.
France follows with a 18.9% share in 2025, driven by comprehensive national ophthalmology programs and anti-VEGF therapy integration in hospital-based retinal services. The UK holds a 17.3% share in 2025 through established NHS ophthalmology pathways and national retinal screening program infrastructure.
Italy commands a 14.2% share, while Spain accounts for 11.6% in 2025. The rest of Europe maintains 11.2% of the European market, attributed to increasing retinal disease awareness in Nordic countries and emerging Eastern European healthcare systems implementing subspecialty ophthalmology capability development programs.
Competitive Landscape of the Retinal Vein Occlusion Treatment Market
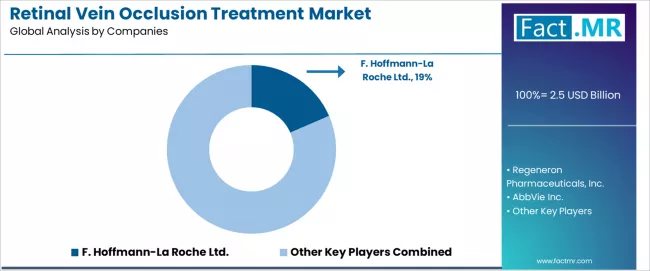
The retinal vein occlusion treatment market exhibits a moderately consolidated competitive structure with approximately 35-50 active players operating across global pharmaceutical development networks and specialized ophthalmology portfolios. F. Hoffmann-La Roche Ltd. maintains market leadership at an 18.5% share, reflecting strong product positioning across established anti-VEGF franchises with sophisticated global commercialization strategies including Lucentis (ranibizumab) market presence.
This competitive landscape demonstrates the specialization of retinal therapeutic manufacturing, where established players leverage clinical evidence generation, regulatory approval expertise, and retinal specialist relationship networks to maintain dominant positions, while emerging biosimilar developers and extended-duration technology innovators create differentiation opportunities through cost-effective therapeutic alternatives and injection burden reduction solutions.
Market leadership is maintained through several critical competitive advantages extending beyond pharmaceutical formulation capabilities and mechanism of action differentiation. Global commercialization infrastructure enables leading players to navigate complex regulatory requirements and access varied market segments including academic vitreoretinal centers, community retina practices, and international ophthalmology networks.
Clinical evidence generation and key opinion leader engagement represent crucial differentiators in retinal therapeutic categories, where pivotal trial execution, real-world evidence accumulation, and guideline influence create prescribing preference among discriminating retinal specialists.
Manufacturing capacity for sterile ophthalmic formulation production, cold chain distribution excellence, and regulatory compliance maintenance separate major pharmaceutical companies from smaller biotechnology firms, while comprehensive medical affairs support addressing clinical education, injection training, and patient assistance programs strengthens market position and supports prescriber loyalty throughout extended product lifecycle management.
The market demonstrates emerging differentiation opportunities in extended-duration formulation categories and gene therapy technologies, where traditional monthly intravitreal injection paradigms face potential disruption from sustained-release innovators and one-time genetic intervention developers offering treatment burden elimination advantages.
Significant competitive advantages persist in established anti-VEGF categories through proven efficacy profiles and comprehensive safety databases. Premium extended-duration platforms with port delivery system technology and multi-month dosing intervals command substantial value propositions through patient compliance enhancement and injection burden reduction.
Next-generation anti-VEGF molecules combining enhanced VEGF binding affinity with optimized pharmacokinetic properties create efficacy differentiation positioning that justifies premium pricing beyond first-generation therapeutic competition. Gene therapy approaches emphasizing single-administration paradigms and potential curative mechanisms generate transformative innovation differentiation and market disruption potential beyond conventional chronic intravitreal injection therapeutic competition.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Established pharmaceutical companies | Anti-VEGF franchises; global distribution; KOL networks | Clinical evidence; regulatory expertise; commercial infrastructure | Biosimilar erosion; innovation speed; extended-duration transition |
| Biosimilar developers | Cost-effective manufacturing; emerging market access; pricing strategies | Affordability positioning; volume scaling; regulatory pathway experience | Brand equity building; KOL relationships; differentiation messaging |
| Extended-duration innovators | Port delivery systems; sustained-release technology; novel formulations | Treatment burden reduction; compliance enhancement; differentiation potential | Clinical validation complexity; regulatory uncertainty; commercialization scale |
| Gene therapy developers | Genetic intervention platforms; one-time administration; curative positioning | Paradigm disruption; premium value capture; transformative potential | Manufacturing complexity; long-term safety unknowns; reimbursement challenges |
| Academic vitreoretinal centers | Clinical research; guideline development; fellowship training | Scientific credibility; patient populations; protocol development capability | Commercial scalability; distribution infrastructure; manufacturing expertise |
Key Players in the Retinal Vein Occlusion Treatment Market
- F. Hoffmann-La Roche Ltd.
- Regeneron Pharmaceuticals, Inc.
- AbbVie Inc.
- Chugai Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Outlook Therapeutics, Inc.
- Kodiak Sciences Inc.
- Aerie Pharmaceuticals Inc.
- Taiwan Liposome Company, Ltd.
- CalciMedica Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Value (USD Million)s (2025) | USD 2.54 billion |
| Disease Type | Central Retinal Vein Occlusion (CRVO), Branch Retinal Vein Occlusion (BRVO) |
| Treatment Type | Anti-VEGF, Corticosteroid Drugs, Others |
| End User | Retail Pharmacy, Hospitals & Clinics, Others |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | USA, India, China, Japan, Germany, Brazil, South Africa, and 15+ additional countries |
| Key Companies Profiled | F. Hoffmann-La Roche Ltd., Regeneron Pharmaceuticals Inc., AbbVie Inc., Chugai Pharmaceutical Co. Ltd., Pfizer Inc., Outlook Therapeutics Inc., Kodiak Sciences Inc., Aerie Pharmaceuticals Inc. |
| Additional Attributes | Dollar sales by disease type and treatment modality categories, regional adoption trends across North America, Asia Pacific, and Europe, competitive landscape with established pharmaceutical companies and emerging biosimilar developers, retinal specialist preferences for proven anti-VEGF efficacy and extended-duration formulation development, integration with hospital ophthalmology departments and retail pharmacy distribution networks, innovations in sustained-release technology and gene therapy approaches, and development of optimized therapeutic platforms with enhanced treatment durability profiles and improved patient compliance characteristics. |
Retinal Vein Occlusion Treatment Market by Segments
-
Disease Type :
- Central Retinal Vein Occlusion (CRVO)
- Ischemic CRVO
- Non-Ischemic CRVO
- Branch Retinal Vein Occlusion (BRVO)
- Major BRVO
- Macular BRVO
- Central Retinal Vein Occlusion (CRVO)
-
Treatment Type :
- Anti-VEGF
- Bevacizumab
- Ranibizumab
- Aflibercept
- Next-Generation Anti-VEGF Agents
- Corticosteroid Drugs
- Intravitreal Triamcinolone
- Dexamethasone Implant
- Fluocinolone Acetonide Implant
- Others
- Laser Photocoagulation
- Emerging Therapies
- Anti-VEGF
-
End User :
- Retail Pharmacy
- Local Chain Pharmacies
- Hospital-Affiliated Pharmacy Outlets
- Online Dispensing Platforms
- Hospitals & Clinics
- Academic Medical Center Ophthalmology Departments
- Community Hospital Eye Clinics
- Ambulatory Surgery Centers
- Others
- Specialty Ophthalmology Clinics
- Vitreoretinal Subspecialty Practices
- Retail Pharmacy
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- India
- China
- Japan
- South Korea
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa
- South Africa
- GCC Countries
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Disease Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Disease Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Disease Type, 2025 to 2035
- Central Retinal Vein Occlusion (CRVO)
- Branch Retinal Vein Occlusion (BRVO)
- Y to o to Y Growth Trend Analysis By Disease Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Disease Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Treatment Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Treatment Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Treatment Type, 2025 to 2035
- Anti-VEGF
- Corticosteriod Drugs
- Others
- Y to o to Y Growth Trend Analysis By Treatment Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Treatment Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End User
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End User, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End User, 2025 to 2035
- Retail Pharmacy
- Hospitals & Clinics
- Others
- Y to o to Y Growth Trend Analysis By End User, 2020 to 2024
- Absolute $ Opportunity Analysis By End User, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
- Y to o to Y Growth Trend Analysis By Region, 2020 to 2024
- Absolute $ Opportunity Analysis By Region, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Disease Type
- By Treatment Type
- By End User
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Disease Type
- By Treatment Type
- By End User
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Disease Type
- By Treatment Type
- By End User
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Disease Type
- By Treatment Type
- By End User
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Disease Type
- By Treatment Type
- By End User
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Disease Type
- By Treatment Type
- By End User
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Disease Type
- By Treatment Type
- By End User
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Disease Type
- By Treatment Type
- By End User
- By Region
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Disease Type
- By Treatment Type
- By End User
- By Region
- Competition Analysis
- Competition Deep Dive
- F. Hoffmann-La Roche Ltd.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Regeneron Pharmaceuticals, Inc.
- AbbVie Inc.
- Chugai Pharmaceutical Co., Ltd.
- Pfizer Inc.
- Outlook Therapeutics, Inc.
- Kodiak Sciences Inc.
- Aerie Pharmaceuticals Inc.
- Taiwan Liposome Company, Ltd.
- CalciMedica Inc.
- F. Hoffmann-La Roche Ltd.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 5: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 9: North America Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 10: North America Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
- Table 13: Latin America Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 14: Latin America Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 15: Latin America Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: Western Europe Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
- Table 18: Western Europe Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 19: Western Europe Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 20: Western Europe Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 21: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: Eastern Europe Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
- Table 23: Eastern Europe Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 24: Eastern Europe Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 25: Eastern Europe Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 26: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 27: East Asia Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
- Table 28: East Asia Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 29: East Asia Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 30: East Asia Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 31: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 32: South Asia and Pacific Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
- Table 33: South Asia and Pacific Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 34: South Asia and Pacific Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 35: South Asia and Pacific Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 36: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 37: Middle East & Africa Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
- Table 38: Middle East & Africa Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 39: Middle East & Africa Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 40: Middle East & Africa Market Value (USD Million) Forecast by Region, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Disease Type
- Figure 6: Global Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Treatment Type
- Figure 9: Global Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by End User
- Figure 12: Global Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 16: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 17: Global Market Attractiveness Analysis by Region
- Figure 18: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 23: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 24: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 25: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 26: North America Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Disease Type
- Figure 29: North America Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Treatment Type
- Figure 32: North America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 33: North America Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 34: North America Market Attractiveness Analysis by End User
- Figure 35: North America Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 36: North America Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 37: North America Market Attractiveness Analysis by Region
- Figure 38: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 39: Latin America Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Disease Type
- Figure 42: Latin America Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 43: Latin America Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 44: Latin America Market Attractiveness Analysis by Treatment Type
- Figure 45: Latin America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 46: Latin America Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 47: Latin America Market Attractiveness Analysis by End User
- Figure 48: Latin America Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 49: Latin America Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 50: Latin America Market Attractiveness Analysis by Region
- Figure 51: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 52: Western Europe Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 53: Western Europe Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 54: Western Europe Market Attractiveness Analysis by Disease Type
- Figure 55: Western Europe Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 56: Western Europe Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 57: Western Europe Market Attractiveness Analysis by Treatment Type
- Figure 58: Western Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 59: Western Europe Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 60: Western Europe Market Attractiveness Analysis by End User
- Figure 61: Western Europe Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 62: Western Europe Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 63: Western Europe Market Attractiveness Analysis by Region
- Figure 64: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 65: Eastern Europe Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 66: Eastern Europe Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 67: Eastern Europe Market Attractiveness Analysis by Disease Type
- Figure 68: Eastern Europe Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 69: Eastern Europe Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 70: Eastern Europe Market Attractiveness Analysis by Treatment Type
- Figure 71: Eastern Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 72: Eastern Europe Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 73: Eastern Europe Market Attractiveness Analysis by End User
- Figure 74: Eastern Europe Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 75: Eastern Europe Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 76: Eastern Europe Market Attractiveness Analysis by Region
- Figure 77: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 78: East Asia Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 79: East Asia Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 80: East Asia Market Attractiveness Analysis by Disease Type
- Figure 81: East Asia Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 82: East Asia Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 83: East Asia Market Attractiveness Analysis by Treatment Type
- Figure 84: East Asia Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 85: East Asia Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 86: East Asia Market Attractiveness Analysis by End User
- Figure 87: East Asia Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 88: East Asia Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 89: East Asia Market Attractiveness Analysis by Region
- Figure 90: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 91: South Asia and Pacific Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 92: South Asia and Pacific Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 93: South Asia and Pacific Market Attractiveness Analysis by Disease Type
- Figure 94: South Asia and Pacific Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 95: South Asia and Pacific Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 96: South Asia and Pacific Market Attractiveness Analysis by Treatment Type
- Figure 97: South Asia and Pacific Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 98: South Asia and Pacific Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 99: South Asia and Pacific Market Attractiveness Analysis by End User
- Figure 100: South Asia and Pacific Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 101: South Asia and Pacific Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 102: South Asia and Pacific Market Attractiveness Analysis by Region
- Figure 103: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 104: Middle East & Africa Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 105: Middle East & Africa Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 106: Middle East & Africa Market Attractiveness Analysis by Disease Type
- Figure 107: Middle East & Africa Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 108: Middle East & Africa Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 109: Middle East & Africa Market Attractiveness Analysis by Treatment Type
- Figure 110: Middle East & Africa Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 111: Middle East & Africa Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 112: Middle East & Africa Market Attractiveness Analysis by End User
- Figure 113: Middle East & Africa Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 114: Middle East & Africa Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 115: Middle East & Africa Market Attractiveness Analysis by Region
- Figure 116: Global Market - Tier Structure Analysis
- Figure 117: Global Market - Company Share Analysis
- FAQs -
How big is the retinal vein occlusion treatment market in 2025?
The global retinal vein occlusion treatment market is estimated to be valued at USD 2.5 billion in 2025.
What will be the size of retinal vein occlusion treatment market in 2035?
The market size for the retinal vein occlusion treatment market is projected to reach USD 5.1 billion by 2035.
How much will be the retinal vein occlusion treatment market growth between 2025 and 2035?
The retinal vein occlusion treatment market is expected to grow at a 7.2% CAGR between 2025 and 2035.
What are the key product types in the retinal vein occlusion treatment market?
The key product types in retinal vein occlusion treatment market are central retinal vein occlusion (crvo) and branch retinal vein occlusion (brvo).
Which treatment type segment to contribute significant share in the retinal vein occlusion treatment market in 2025?
In terms of treatment type, anti-vegf segment to command 65.8% share in the retinal vein occlusion treatment market in 2025.