Anti-Venom Market
Anti-Venom Market Size and Share Forecast Outlook 2025 to 2035
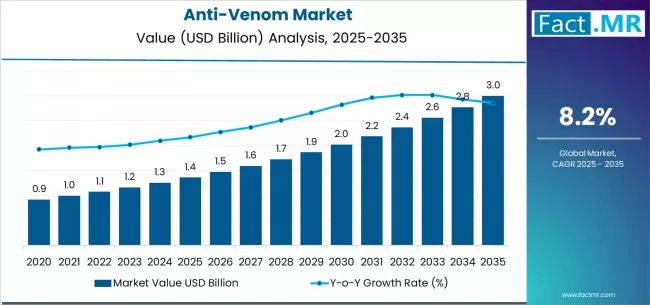
Anti-venom market is projected to grow from USD 1.4 billion in 2025 to USD 3.0 billion by 2035, at a CAGR of 8.2%. Snake Anti-Venom will dominate with a 5080.0% market share, while polyvalent will lead the type segment with a 6710.0% share.
Anti-Venom Market Forecast and Outlook 2025 to 2035
The global anti-venom market is projected to reach USD 3.0 billion by 2035, recording an absolute increase of USD 1.64 billion over the forecast period. The market is valued at USD 1.36 billion in 2025 and is set to rise at a CAGR of 8.2% during the assessment period.
Quick Stats for Anti-Venom Market
- Anti-Venom Market Value (2025): USD 1.36 billion
- Anti-Venom Market Forecast Value (2035): USD 3.0 billion
- Anti-Venom Market Forecast CAGR: 8.2%
- Leading Species Type in Anti-Venom Market: Snake Anti-Venom
- Key Growth Regions in Anti-Venom Market: Asia Pacific, North America, and Europe
- Top Players in Anti-Venom Market: Bharat Serums and Vaccines Ltd., CSL Limited, Boehringer Ingelheim, Pfizer Inc., Merck & Co. Inc., MicroPharm Limited, Incepta Pharmaceuticals Ltd., Haffkine Bio-Pharma Corp. Ltd., South African Vaccine Producers (Pty) Ltd., Sigma Aldrich (Merck Group)
The market is expected to grow 2.2X during the same period, supported by increasing incidence of venomous snakebites in tropical and subtropical regions, rising awareness about timely anti-venom administration, and expanding healthcare infrastructure in emerging economies worldwide, driving demand for specialized anti-venom products and increasing investments in venom research and production facility development globally.
The healthcare sector faces mounting pressure to reduce snakebite mortality rates while meeting stringent quality standards for anti-venom products, with modern anti-venom formulations providing documented survival rate improvements of 80-95% when administered promptly compared to delayed treatment scenarios.
Rising awareness campaigns by the World Health Organization and expanding access to emergency medical services across rural areas create substantial opportunities for anti-venom manufacturers and healthcare providers. However, high production costs for species-specific formulations and technical challenges in maintaining cold chain logistics across remote regions may pose obstacles to market expansion.
The snake anti-venom segment dominates market activity with approximately 50.8% share in 2024, driven by the extensive prevalence of venomous snake species and high mortality rates associated with snakebites across tropical regions worldwide. Healthcare providers and government health programs increasingly recognize the critical importance of anti-venom stockpiling, with typical treatment protocols requiring immediate administration within 4-6 hours of envenomation to prevent permanent tissue damage and fatalities.
The monovalent anti-venom segment demonstrates robust growth potential with a 9.5% CAGR, supported by increasing demand for species-specific formulations and enhanced efficacy requirements in specialized treatment protocols. Hospitals represent the most dynamic end-use application with strong adoption rates, driven by expanding emergency care infrastructure and standardized snakebite treatment protocols for critical care operations.
Regional dynamics show Asia Pacific maintaining significant market presence with substantial share in 2024, supported by high snakebite incidence rates and government-backed anti-venom procurement programs across India, China, and Southeast Asian markets.
North America demonstrates strong research and development activity driven by advanced biotechnology capabilities and regulatory support for anti-venom innovation, while Europe emphasizes quality standards and specialized production technologies. India leads country-level growth at 9.7% CAGR through extensive rural healthcare expansion and government snakebite mitigation initiatives, followed by China at 9.2% supported by domestic manufacturing capabilities.
The competitive landscape features moderate concentration with Bharat Serums and Vaccines Ltd. holding significant market share, while established players including CSL Limited, Boehringer Ingelheim, and Pfizer Inc. compete through comprehensive product portfolios and advanced biotechnology capabilities across diverse therapeutic applications.
Anti-Venom Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the anti-venom market is projected to expand from USD 1.36 billion to USD 1.86 billion, resulting in a value increase of USD 0.5 billion, which represents 30.5% of the total forecast growth for the period. This phase of development will be shaped by rising incidence of venomous bites in rural and agricultural regions, product innovation in monovalent and polyvalent anti-venom formulations, as well as expanding integration with emergency healthcare systems and government procurement programs. Companies are establishing competitive positions through investment in specialized biotechnology manufacturing capabilities, advanced purification technologies, and strategic market expansion across hospital, clinic, and institutional applications.
From 2029 to 2035, the market is forecast to grow from USD 1.86 billion to USD 3.0 billion, adding another USD 1.14 billion, which constitutes 69.5% of the overall expansion. This period is expected to be characterized by the expansion of specialized anti-venom applications, including next-generation recombinant antibody therapies and species-specific formulations tailored for regional venom profiles, strategic collaborations between pharmaceutical manufacturers and research institutions, and an enhanced focus on accessibility and affordability initiatives. The growing emphasis on WHO's snakebite roadmap 2030 and healthcare infrastructure development in endemic regions will drive demand for comprehensive anti-venom solutions across diverse geographic applications.
Anti-Venom Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 1.36 billion |
| Market Forecast Value (2035) | USD 3.0 billion |
| Forecast CAGR (2025-2035) | 8.2% |
Why is the Anti-Venom Market Growing?
The anti-venom market grows by enabling healthcare providers, government health programs, and medical facilities to deliver life-saving treatments while accessing advanced biotechnology formulations without substantial in-house research and development requirements.
Healthcare systems and emergency medical services face mounting pressure to reduce snakebite mortality and morbidity rates while managing complex envenomation cases across diverse geographic regions, with modern anti-venom products typically providing 80-95% survival rates when administered within critical time windows compared to untreated alternatives, making anti-venom stockpiling essential for emergency preparedness and public health positioning.
The healthcare industry's need for species-specific treatment capabilities and rapid-acting formulations creates demand for comprehensive anti-venom solutions that can provide superior efficacy, maintain consistent quality standards, and ensure reliable availability without compromising patient outcomes or healthcare system performance metrics.
Government initiatives promoting snakebite prevention and treatment accessibility drive adoption in rural healthcare facilities, emergency medical services, and specialized toxicology centers, where anti-venom availability has a direct impact on mortality reduction and community health outcomes.
The World Health Organization's inclusion of snakebite envenoming on its list of neglected tropical diseases in 2017 has catalyzed increased funding and policy attention, creating momentum for anti-venom market expansion.
Production complexity constraints for polyvalent formulations and the technical requirements for maintaining temperature-controlled distribution networks may limit accessibility among remote healthcare facilities and developing regions with limited financial resources for sophisticated cold chain infrastructure investments.
Segmental Analysis
The market is segmented by species, type, mode of action, end use, and region. By species, the market is divided into snake anti-venom, scorpion anti-venom, spider anti-venom, and others. Based on type, the market is categorized into polyvalent and monovalent.
By mode of action, the market includes neurotoxic, cytotoxic, haemotoxic, cardiotoxic, and myotoxic & others. By end use, the market encompasses hospitals, clinics, ambulatory surgical centers, and others. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
By Species, why does the Snake Anti-Venom Segment Account for a Dominant Market Share?
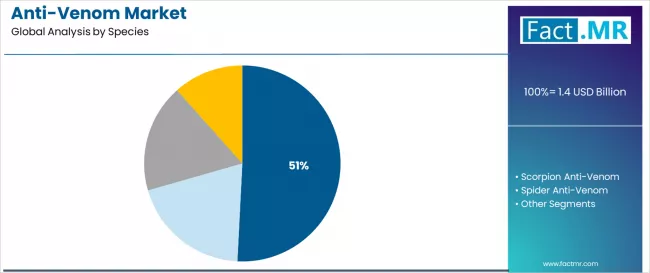
The snake anti-venom segment represents the dominant force in the anti-venom market, capturing approximately 50.8% of total market share in 2025. This established species category encompasses solutions featuring broad-spectrum snake venom neutralization and species-specific formulations, including advanced polyvalent products and specialized monovalent treatments that enable superior therapeutic outcomes and mortality reduction across all snakebite envenomation cases.
The snake anti-venom segment's market leadership stems from its critical role in addressing the highest incidence of venomous encounters globally, with solutions capable of neutralizing diverse snake venoms while maintaining consistent efficacy standards and safety profiles across all treatment environments.
The scorpion anti-venom segment maintains a substantial 26.5% market share, serving healthcare providers who require specialized formulations with enhanced neutralizing capabilities for scorpion sting treatments and pediatric care applications. These solutions offer rapid-acting therapeutic effects for scorpion envenomation while providing sufficient specificity to address regional scorpion species variations.
The scorpion anti-venom segment demonstrates the fastest growth trajectory with a CAGR of 9.1% from 2025 to 2035, driven by increasing scorpion sting incidence rates in arid regions and growing awareness of treatment protocols. Within the snake anti-venom segment, common cobra-specific formulations represent 23.4% of the category, reflecting the prevalence of cobra species in endemic regions.
The spider anti-venom segment accounts for 13.8% market share, addressing treatments for widow spider and funnel-web spider envenomations. The others segment, encompassing fish, insect, and marine creature anti-venoms, maintains 8.9% market share, serving specialized treatment requirements for jellyfish stings, stonefish envenomations, and hymenoptera allergic reactions.
Key therapeutic advantages driving the snake anti-venom segment include:
- Advanced neutralizing technology with multi-species coverage that enhances survival rates and ensures consistent therapeutic performance across diverse snakebite scenarios
- Established clinical protocols allowing streamlined treatment administration across different healthcare settings without extensive specialized training requirements
- Enhanced compatibility features enabling diverse formulation configurations while maintaining patient safety and therapeutic reliability
- Superior efficacy profiles providing optimal clinical outcomes for various snakebite envenomation presentations and severity levels
By Type, what makes the Polyvalent Segment Account for the Largest Market Share?
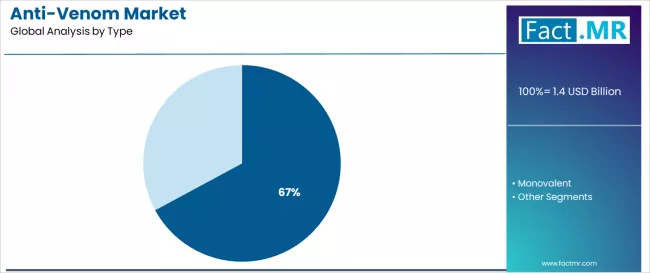
Polyvalent anti-venom dominates the anti-venom market with approximately 67.1% market share in 2025, reflecting the critical need for broad-spectrum protection against multiple venomous species in emergency treatment scenarios worldwide. The polyvalent segment's market leadership is reinforced by practical treatment requirements in resource-limited settings, where snake species identification remains challenging, and healthcare facilities require comprehensive coverage capabilities to address diverse envenomation cases across geographic regions.
Within the polyvalent segment, broad-spectrum snake group neutralizers account for 30.6% of the category, representing formulations designed to neutralize venoms from multiple related snake species within the same taxonomic group. These products prove particularly valuable in regions with high biodiversity and overlapping snake species distributions.
The monovalent segment represents 32.9% market share, demonstrating the strongest growth trajectory with a CAGR of 9.5% from 2025 to 2035, driven by increasing emphasis on species-specific treatments, enhanced efficacy requirements, and reduced adverse reaction profiles.
This segment benefits from growing clinical evidence supporting targeted therapy approaches that require precise species identification, optimized neutralizing capacity, and reduced protein load compared to polyvalent alternatives in well-equipped medical facilities.
Within the monovalent segment, species-specific cobra serum formulations account for 21.5% of the category, reflecting the high prevalence of cobra envenomations and the therapeutic advantages of targeted treatment approaches in tertiary care centers with diagnostic capabilities.
Key market dynamics supporting type segmentation growth include:
- Polyvalent expansion driven by emergency treatment requirements and practical limitations in snake identification, requiring comprehensive coverage solutions in rural healthcare settings
- Monovalent advancement trends requiring specialized production facilities and advanced purification systems for enhanced efficacy and safety differentiation
- Integration of immunological testing technologies enabling rapid species identification and appropriate anti-venom selection in equipped facilities
- Growing emphasis on adverse reaction reduction driving demand for specialized, purified formulations without traditional serum sickness limitations
By Mode of Action, How Do Neurotoxic Anti-Venoms Account for Significant Market Share?
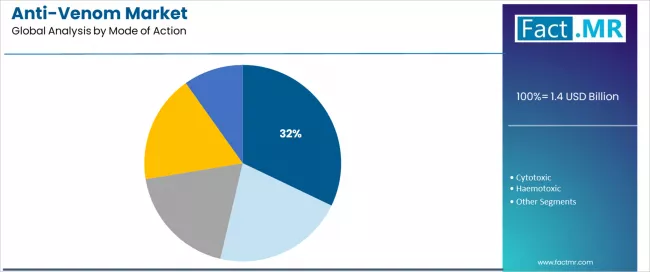
Neurotoxic anti-venoms represent the leading mode of action category in the anti-venom market with approximately 32.1% market share in 2025, reflecting the extensive prevalence of neurotoxic snake species including cobras, kraits, and coral snakes across tropical and subtropical regions.
The neurotoxic segment demonstrates consistent demand driven by high mortality rates associated with respiratory paralysis, urgent treatment requirements, and the critical importance of prompt anti-venom administration in preventing fatal outcomes.
The cytotoxic anti-venom segment maintains 26.5% market share, addressing tissue-destroying venom effects from vipers, cobras, and certain other species that cause extensive local necrosis and systemic complications. This segment demonstrates an 8.4% CAGR from 2025 to 2035, supported by increasing recognition of long-term morbidity prevention and reconstructive surgery cost reduction through timely anti-venom treatment.
The haemotoxic segment accounts for 18.2% market share with a 7.9% CAGR, focusing on treatments for venom-induced coagulopathies and bleeding disorders common in viper envenomations. The cardiotoxic segment holds 12.0% market share with a 7.7% CAGR, addressing cardiac complications from scorpion stings and certain snake venoms. The myotoxic and others category encompasses 11.1% market share with a 7.5% CAGR, treating muscle-damaging venoms and less-common venom mechanisms.
The neurotoxic segment emerges as the highest-growth category with an 8.8% CAGR from 2025 to 2035, driven by increasing snakebite fatalities from neurotoxic species in agricultural regions and expanding healthcare infrastructure capable of supporting ventilatory support during treatment protocols.
Key mode of action dynamics include:
- Neurotoxic anti-venom expansion accelerating in endemic regions with emphasis on respiratory support capabilities and intensive care integration
- Cytotoxic treatment advancement driving demand for early intervention protocols and tissue preservation in rural and semi-urban healthcare facilities
- Haemotoxic anti-venom modernization requiring specialized laboratory monitoring and comprehensive coagulation management in equipped hospitals
- Cardiotoxic formulation development prioritizing pediatric treatment protocols and rapid-acting formulations for scorpion envenomation management
By End Use, why do Hospitals Account for a Significant Market Share?
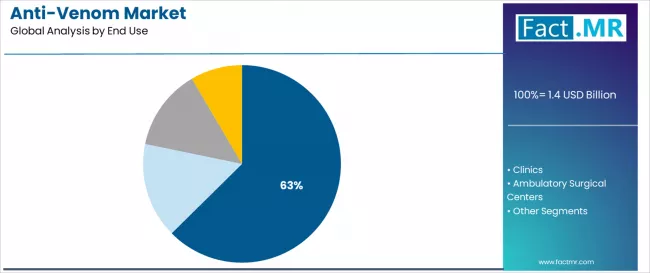
Hospitals represent the leading end-use application in the anti-venom market with approximately 62.6% market share in 2024, reflecting the critical role of hospital emergency departments and intensive care units in managing severe envenomation cases requiring comprehensive medical support.
The hospitals segment demonstrates consistent demand driven by availability of intensive care facilities, ventilatory support equipment, and specialized medical personnel capable of managing complex envenomation presentations and adverse reactions.
The clinics segment maintains substantial market presence with 25.7% share and emerges as the fastest-growing end-use category with a CAGR of 9.0% from 2025 to 2035, driven by expanding primary healthcare infrastructure in rural endemic regions and government initiatives to decentralize anti-venom access to community health centers.
Clinics provide critical first-line treatment capabilities in areas where hospital access remains limited, enabling timely anti-venom administration that significantly improves patient outcomes.
The ambulatory surgical centers segment accounts for 7.4% market share with an 8.2% CAGR, serving patients requiring post-envenomation surgical interventions including fasciotomy procedures and reconstructive surgeries following cytotoxic venom damage. The others category, encompassing emergency medical services, mobile health units, and military medical facilities, holds 4.3% market share with a 7.6% CAGR.
Key end-use dynamics include:
- Hospital emergency departments accelerating anti-venom protocol standardization across major medical centers with emphasis on rapid administration and intensive monitoring capabilities
- Clinic expansion driving accessibility improvements in high-incidence rural regions through government procurement programs and healthcare worker training initiatives
- Ambulatory surgical center integration requiring specialized post-envenomation care protocols and reconstructive surgery capabilities for bite complication management
- Mobile health units and emergency services prioritizing portable anti-venom storage solutions and field administration protocols in remote areas with limited healthcare infrastructure
What are the Drivers, Restraints, and Key Trends of the Anti-Venom Market?
The market is driven by three concrete demand factors tied to public health outcomes and healthcare accessibility. First, increasing incidence of venomous bites and stings creates growing demand for anti-venom products, with the World Health Organization estimating 5.4 million snakebites annually resulting in 81,000-138,000 deaths worldwide, requiring comprehensive anti-venom availability and distribution networks.
Second, government initiatives through WHO's snakebite roadmap 2030 and national snakebite management programs drive increased procurement of anti-venom products, with many countries implementing centralized procurement systems and strategic stockpiling programs to ensure emergency preparedness.
Third, technological advancements in recombinant antibody production, improved purification techniques, and next-generation immunization protocols enable more effective and safer anti-venom formulations that improve clinical outcomes while reducing adverse reactions and treatment complications.
Market restraints include high production costs and limited manufacturing capacity for specialized anti-venom formulations that can challenge market participants in developing affordable treatment options, particularly in low-income endemic regions where snakebite burden remains highest and healthcare budgets face severe constraints.
Cold chain infrastructure requirements for anti-venom storage and distribution pose another significant challenge, as products typically require refrigerated storage at 2-8°C, potentially affecting availability and quality in remote areas with unreliable electricity supply.
Limited awareness among rural populations regarding timely treatment seeking and widespread use of traditional healers create additional barriers to effective anti-venom utilization, demanding ongoing investment in community education programs and healthcare worker training initiatives.
Key trends indicate accelerated adoption in Asia-Pacific markets, particularly India, China, and Southeast Asian countries, where agricultural activities and tropical climates drive high snakebite incidence rates and comprehensive anti-venom procurement programs.
Technology evolution trends toward recombinant anti-venom development, oligoclonal antibody therapies, and advanced immunization techniques using synthetic peptides enable more efficient production approaches that optimize therapeutic efficacy and minimize manufacturing costs and adverse reaction risks.
However, the market thesis could face disruption if significant advances in venom-targeting small molecule therapeutics or major breakthroughs in rapid-acting synthetic inhibitors reduce reliance on traditional antibody-based anti-venom products.
Analysis of the Anti-Venom Market by Key Country
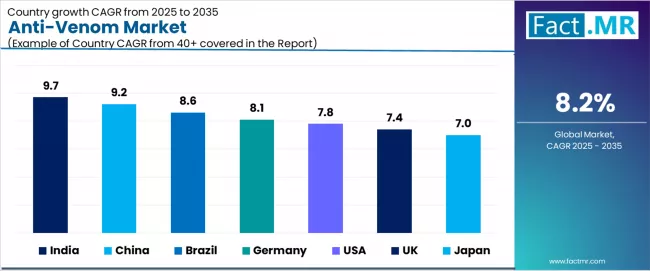
| Country | CAGR (2025-2035) |
|---|---|
| India | 9.7% |
| China | 9.2% |
| Brazil | 8.6% |
| Germany | 8.1% |
| USA | 7.8% |
| UK | 7.4% |
| Japan | 7.0% |
The global anti-venom market is expanding steadily, with India leading at a 9.7% CAGR through 2035, driven by high snakebite incidence in agricultural regions, government-backed National Action Plan on Snakebite Envenoming (NAP-SE), and domestic manufacturing capabilities.
China follows at 9.2%, supported by expanding rural healthcare infrastructure, traditional medicine integration, and biotechnology research investments. Brazil records 8.6%, reflecting public health initiatives and established anti-venom production facilities. Germany grows at 8.1%, anchored by advanced biotechnology research and pharmaceutical manufacturing excellence.
The USA advances at 7.8%, leveraging regulatory support for rare disease therapeutics and academic research programs. The UK posts 7.4%, focusing on tropical medicine research and international health programs, while Japan grows steadily at 7.0%, emphasizing quality manufacturing standards and regional export capabilities.
How does India Lead in the Global Anti-Venom Market?
India demonstrates the strongest growth potential in the anti-venom market with a CAGR of 9.7% through 2035. The country's leadership position stems from extremely high snakebite burden with an estimated 2.7 million envenomations annually, comprehensive National Action Plan on Snakebite Envenoming (NAP-SE) implementation, and well-established domestic anti-venom manufacturing infrastructure driving the adoption of polyvalent and monovalent anti-venom solutions.
Growth is concentrated in rural agricultural regions and high-incidence states, including West Bengal, Uttar Pradesh, Bihar, Odisha, and Andhra Pradesh, where healthcare providers and government health programs are implementing standardized snakebite treatment protocols for enhanced mortality reduction and disability prevention.
Distribution channels through government procurement systems, primary health centers, and district hospitals expand access across rural communities and tribal areas. The country's Ministry of Health and Family Welfare provides policy support for anti-venom accessibility, including comprehensive procurement guidelines, quality assurance programs, and healthcare worker training initiatives.
Key market factors:
- Rural healthcare expansion concentrated in high-incidence agricultural regions with comprehensive snakebite management programs and community awareness initiatives
- Government support through NAP-SE implementation, centralized procurement systems, and free anti-venom distribution at public healthcare facilities
- Comprehensive manufacturing ecosystem including established producers like Bharat Serums and Vaccines, Haffkine Bio-Pharma, and Premium Serums with proven production capabilities
- Technology integration featuring advanced purification techniques, quality control systems, and species-specific formulation development programs
Why does China Emerge as a High-Volume Growth Market?
In major agricultural regions and rural provinces including Guangdong, Guangxi, Yunnan, and Sichuan, the adoption of comprehensive anti-venom treatment protocols is accelerating across township hospitals and county health facilities, driven by healthcare modernization and government-backed snakebite management programs.
The market demonstrates strong growth momentum with a CAGR of 9.2% through 2035, linked to comprehensive rural healthcare infrastructure development and increasing focus on traditional Chinese medicine integration with modern anti-venom therapy.
Chinese healthcare authorities are implementing standardized treatment guidelines and procurement systems to enhance snakebite case management while meeting growing demand in agricultural and tea-plantation regions.
The country's biotechnology development initiatives create ongoing demand for domestically produced anti-venom formulations, while increasing emphasis on quality standards drives adoption of advanced manufacturing technologies.
Key development areas:
- Rural healthcare facilities and township hospitals leading anti-venom adoption with comprehensive snakebite treatment protocols and emergency response systems
- Government procurement programs providing subsidized anti-venom distribution with expanding coverage rates across endemic provinces
- Technology partnerships between Chinese pharmaceutical manufacturers and research institutions expanding domestic production capabilities
- Integration of traditional medicine approaches with modern anti-venom therapy and development of combination treatment protocols
How does the Market for Anti-Venom Look in the USA?
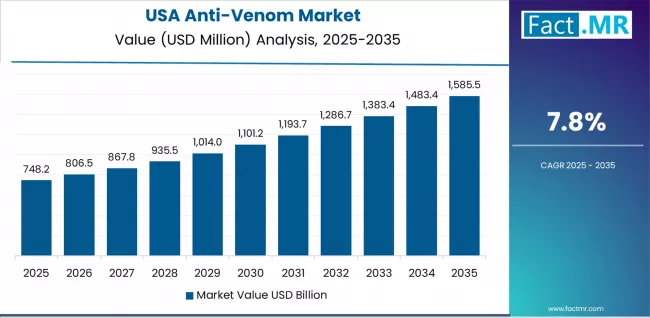
The USA’s market expansion is driven by research and development activities in academic medical centers, investment in rare disease therapeutics by pharmaceutical companies, and comprehensive regulatory frameworks supporting anti-venom innovation. The country demonstrates steady growth potential with a CAGR of 7.8% through 2035, supported by FDA orphan drug designation programs and National Institutes of Health research funding for venom therapeutics.
Healthcare providers face implementation challenges related to regional snake species variation and limited domestic manufacturing capacity, requiring strategic importation agreements and collaboration with international anti-venom producers. However, growing outdoor recreational activities and climate change-related snake range expansion create evolving treatment needs for anti-venom products, particularly in southern states where venomous snake encounters impact public health outcomes.
Market characteristics:
- Academic medical centers and toxicology research institutions showing robust activity with substantial investment in novel anti-venom development and clinical trials
- Regional procurement initiatives focused on southeastern and southwestern states with higher venomous snake populations and bite incidence rates
- Future projections indicate growing need for coral snake anti-venom alternatives and development of recombinant antibody therapies for domestic species
- Growing emphasis on wilderness medicine and emergency medical services training in snakebite management and anti-venom administration protocols
How does Germany Demonstrates Biotechnology Excellence in the Anti-Venom Sector?
The market for anti-venom in Germany leads in advanced anti-venom research and development based on pharmaceutical biotechnology capabilities and precision manufacturing technologies for enhanced product quality and therapeutic efficacy. The country shows strong potential with a CAGR of 8.1% through 2035, driven by academic research partnerships, contract manufacturing services for international markets, and export-oriented production facilities in major pharmaceutical regions, including North Rhine-Westphalia, Bavaria, Baden-Württemberg, and Hesse.
German pharmaceutical companies are advancing recombinant antibody development and oligoclonal therapy research for next-generation anti-venom products, particularly targeting tropical disease applications demanding sophisticated production capabilities. Technology development through university research programs and biotechnology companies expands innovation across purification technologies and formulation optimization.
Leading market segments:
- Pharmaceutical research and contract manufacturing facilities implementing advanced anti-venom production technologies and quality assurance systems
- Biotechnology partnerships with international health organizations, achieving high-purity formulations and reduced adverse reaction profiles
- Strategic collaborations between German pharmaceutical companies and endemic country health authorities expanding market access and technology transfer
- Focus on recombinant antibody platforms and next-generation therapeutic development for improved efficacy and safety profiles
How will Emphasis on Tropical Medicine Research Determine the Anti-Venom Market’s Trajectory in the UK?
In London, Liverpool, Oxford, and other major academic centers, research institutions are implementing comprehensive anti-venom research programs to advance treatment protocols and improve accessibility in endemic regions, with documented contributions showing substantial impact on global snakebite management and WHO policy development.
The market shows steady growth potential with a CAGR of 7.4% through 2035, linked to ongoing tropical medicine research, international development programs, and collaboration with endemic country health systems.
Research institutions and public health organizations are advancing evidence-based treatment guidelines and anti-venom efficacy studies to enhance clinical outcomes while supporting global health initiatives. The country's established academic infrastructure creates ongoing contributions to anti-venom development and international capacity building programs that support endemic country manufacturing capabilities.
Market development factors:
- Academic medical centers and tropical medicine institutes leading anti-venom research initiatives across UK with emphasis on treatment protocol optimization
- International development funding providing support for endemic country anti-venom procurement and healthcare infrastructure development
- Strategic partnerships between British research institutions and WHO programs expanding technical assistance and guideline development
- Emphasis on regulatory harmonization and quality standards advancement across international anti-venom production facilities
How does Japan show Manufacturing Quality Leadership?
Japan's anti-venom market demonstrates sophisticated production capabilities focused on quality excellence and precision manufacturing optimization, with documented expertise in pharmaceutical manufacturing achieving substantial improvement in product purity and consistency across biological therapeutics production.
The country maintains steady growth momentum with a CAGR of 7.0% through 2035, driven by pharmaceutical companies' emphasis on quality assurance and continuous process improvement methodologies that align with Japanese manufacturing standards applied to biological products.
Major pharmaceutical production regions, including Kanto, Kansai, Chubu, and Kyushu, showcase advanced biotechnology manufacturing where anti-venom production integrates seamlessly with comprehensive quality control systems and regulatory compliance programs.
Key market characteristics:
- Pharmaceutical manufacturers driving advanced production technologies with emphasis on purity standards and consistency optimization across biological product portfolios
- Quality partnerships enabling high manufacturing compliance with comprehensive validation programs and international regulatory standards
- Technology collaboration between Japanese pharmaceutical companies and endemic country health authorities expanding export capabilities and technical assistance
- Emphasis on manufacturing efficiency requirements and continuous quality improvement methodologies for biological therapeutics
Will Emphasis on Public Health and Awareness Programs Widen Prospects for Anti-Venom in Brazil?
In major endemic regions including São Paulo, Minas Gerais, Bahia, and Pará, the implementation of comprehensive snakebite treatment protocols is expanding across public health facilities and rural healthcare posts, driven by government-backed anti-venom production and distribution programs.
The market demonstrates strong growth potential with a CAGR of 8.6% through 2035, linked to comprehensive public health initiatives and domestic anti-venom manufacturing through Instituto Butantan, Fundação Ezequiel Dias, and other public research institutions.
Brazilian health authorities are implementing standardized treatment protocols and ensuring free anti-venom availability through the Unified Health System (SUS) while meeting growing demand in agricultural and rainforest regions. The country's established production infrastructure creates ongoing anti-venom supply for domestic needs and regional export markets, while increasing emphasis on species-specific formulations drives product portfolio expansion.
Key development areas:
- Public health facilities and rural healthcare posts leading anti-venom distribution with comprehensive snakebite management programs and emergency protocols
- Government production facilities providing free anti-venom distribution through national health system with high accessibility rates
- Technology development through public research institutions expanding formulation capabilities and regional venom characterization programs
- Integration of community health worker training and public awareness campaigns for timely treatment seeking behavior
Europe Market Split by Country
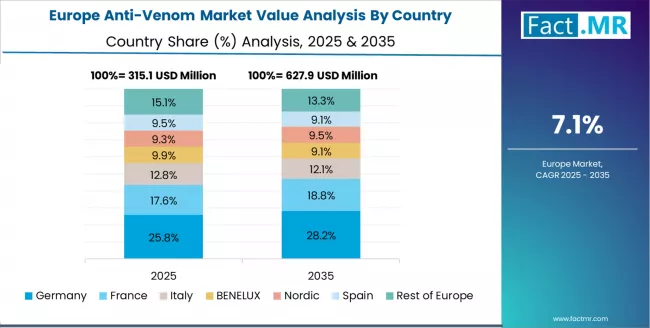
The anti-venom market in Europe is projected to grow from USD 331.0 million in 2025 to USD 729.0 million by 2035, registering a CAGR of 8.2% over the forecast period. Germany is expected to maintain its leadership position with a 28.5% market share in 2025, declining slightly to 27.8% by 2035, supported by its extensive pharmaceutical biotechnology infrastructure, advanced research capabilities, and contract manufacturing networks serving international markets.
The UK follows with a 22.4% share in 2025, projected to reach 22.8% by 2035, driven by comprehensive tropical medicine research programs at institutions including Liverpool School of Tropical Medicine and London School of Hygiene & Tropical Medicine. France holds a 19.6% share in 2025, expected to maintain 19.5% by 2035 through Institut Pasteur research activities and pharmaceutical manufacturing capabilities.
Italy commands a 12.2% share, while Spain accounts for 9.5% in 2025. The Rest of Europe region is anticipated to gain momentum, expanding its collective share from 7.8% to 8.2% by 2035, attributed to increasing anti-venom research collaboration in Nordic countries and emerging contract manufacturing capabilities in Eastern European pharmaceutical facilities.
How does Research Excellence Determine Anti-Venom Development in Japan?
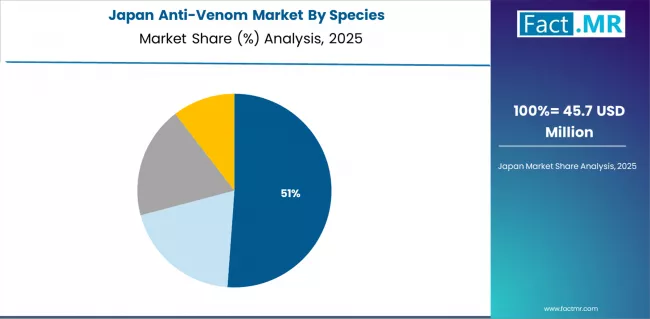
The Japanese anti-venom market demonstrates a mature and quality-focused landscape, characterized by sophisticated pharmaceutical manufacturing capabilities and precision quality control systems supporting export-oriented production for Asian regional markets and research collaborations with endemic countries.
Japan's emphasis on manufacturing excellence and regulatory compliance drives demand for high-quality biological therapeutics that support comprehensive quality assurance initiatives and international regulatory requirements in pharmaceutical operations.
The market benefits from strong partnerships between pharmaceutical manufacturers, academic research institutions, and international health organizations, creating comprehensive quality ecosystems that prioritize production consistency, regulatory compliance excellence, and technical precision programs.
Pharmaceutical manufacturing centers in major industrial regions showcase advanced biotechnology production capabilities where anti-venom manufacturing achieves quality improvements through integrated process control and continuous validation programs.
How is Academic Research Promoting Anti-Venom Formulations Innovation in South Korea?
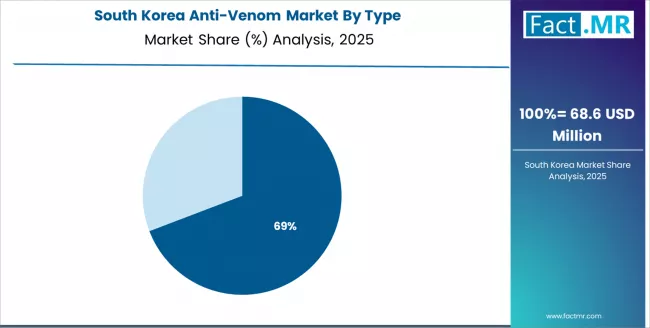
The South Korean anti-venom market is characterized by growing research emphasis from academic institutions and biotechnology companies, with increasing investment in recombinant antibody development and next-generation anti-venom technologies that complement the country's advanced biopharmaceutical manufacturing capabilities.
The market is demonstrating a growing focus on tropical disease research collaboration and export-oriented production potential, as Korean pharmaceutical companies increasingly engage with international health organizations and endemic country partnerships for technology transfer and manufacturing capacity building.
Local biotechnology companies and research institutions are advancing novel anti-venom formulation technologies through strategic collaborations with global pharmaceutical companies, offering specialized research services including venom characterization, antibody engineering, and preclinical development programs.
The competitive landscape shows increasing integration of Korean pharmaceutical manufacturing expertise with international anti-venom development initiatives, creating collaborative models that combine advanced biotechnology capabilities with global health priority alignment and emerging market access opportunities.
Competitive Landscape of the Anti-Venom Market
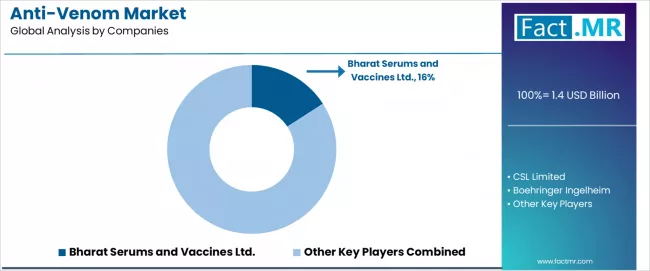
The anti-venom market features approximately 15-25 meaningful players with moderate concentration, where the top three companies control roughly 35-45% of global market share through established production facilities, regulatory approvals, and government procurement relationships.
Competition centers on product efficacy, safety profiles, and production capacity rather than price competition alone, as anti-venom products typically face limited competition within specific geographic markets due to regulatory barriers and species-specific formulation requirements.
Market leaders include Bharat Serums and Vaccines Ltd., which maintains the largest global market share at approximately 15.9% through comprehensive polyvalent and monovalent anti-venom portfolios, extensive distribution networks across endemic regions, and ongoing research partnerships with Indian Institute of Science for next-generation anti-venom development.
CSL Limited holds significant market position through diversified anti-venom products addressing snake, spider, and marine envenomations, with significant strength in Australian and Asia-Pacific markets supported by advanced biotechnology manufacturing capabilities and quality assurance systems.
Challengers encompass established pharmaceutical companies including Boehringer Ingelheim, Pfizer Inc., and Merck & Co. Inc., which compete through investment in toxin-neutralizing antibody research, rare disease therapeutic portfolios, and regulatory expertise in biological product development. Specialized anti-venom producers including MicroPharm Limited focus on monoclonal antibody development and novel immunotherapy approaches, offering differentiated capabilities in species-specific formulations and reduced adverse reaction profiles.
Regional producers create important market dynamics through government-linked manufacturing facilities and public health-oriented production models, particularly in high-burden countries including India (Haffkine Bio-Pharma Corp. Ltd.), Bangladesh (Incepta Pharmaceuticals Ltd.), South Africa (South African Vaccine Producers), and Brazil (Instituto Butantan, Fundação Ezequiel Dias).
These public sector manufacturers often prioritize accessibility and affordability over profit maximization, competing through government procurement contracts, subsidized pricing programs, and regional distribution networks.
Market dynamics favor companies that combine reliable production capabilities with comprehensive quality assurance systems, regulatory compliance expertise, and established relationships with government health authorities and international procurement agencies including WHO, UNICEF, and Pan American Health Organization.
Global Anti-Venom Market — Stakeholder Contribution Framework
Anti-venom solutions represent a critical life-saving therapeutic intervention that enables healthcare providers, government health programs, and emergency medical services to deliver effective envenomation treatment without substantial in-house research and production investment, typically providing 80-95% survival rates when administered promptly compared to untreated cases while ensuring reduced mortality and permanent disability.
With the market projected to grow from USD 1.36 billion in 2025 to USD 3.0 billion by 2035 at an 8.2% CAGR, these solutions offer compelling advantages—superior therapeutic efficacy, enhanced patient outcomes, and accessibility improvements—making them essential for snake anti-venom applications (50.8% market share), hospital settings (62.6% share), and diverse healthcare applications seeking reliable envenomation treatment solutions. Scaling market penetration and product accessibility requires coordinated action across public health policy, pharmaceutical regulation, anti-venom manufacturers, healthcare providers, and international health organizations.
How Governments Could Spur Local Development and Adoption?
- Public Health Programs: Include anti-venom availability in national essential medicines lists, providing targeted procurement funding for public healthcare facilities and supporting domestic manufacturers through development grants and production subsidies.
- Tax Policy & Investment Support: Implement tax exemptions for anti-venom imports and local production, provide financial incentives for pharmaceutical companies investing in anti-venom manufacturing capabilities, and establish favorable regulatory frameworks that encourage domestic production over complete import dependence.
- Regulatory Framework Development: Create streamlined approval processes for anti-venom registrations across therapeutic categories, establish clear quality standards and testing protocols for biological products, and develop regional harmonization initiatives that facilitate cross-border anti-venom distribution and emergency stockpiling.
- Skills Development & Training: Fund medical education programs for healthcare workers, emergency medical technicians, and rural health practitioners. Invest in technology transfer initiatives that bridge pharmaceutical biotechnology with endemic country manufacturing capabilities and quality assurance systems.
- Market Access & Competition: Establish centralized procurement systems that ensure consistent anti-venom availability for public health facilities, support cold chain infrastructure development through logistics improvement programs, and create regulatory environments that encourage innovation in anti-venom formulations while maintaining quality standards.
How Industry Bodies Could Support Market Development?
- Quality Standards & Certification: Define standardized testing methodologies for anti-venom products across species categories, establish universal potency assessment and safety testing protocols, and create certification programs for manufacturing quality that healthcare providers and procurement agencies can rely on.
- Market Education & Best Practices: Lead public health messaging that demonstrates anti-venom treatment advantages, emphasizing improved survival rates, reduced disability outcomes, and superior healthcare cost-effectiveness compared to delayed treatment or traditional medicine alternatives.
- Technology Integration Standards: Develop interoperability standards for anti-venom supply chain systems, establish cold chain monitoring guidelines, and create information sharing frameworks for adverse reaction monitoring, ensuring seamless integration across different healthcare environments and regulatory requirements.
- Professional Development: Deliver certification programs for healthcare providers, pharmacists, and emergency medical personnel on optimizing anti-venom administration, adverse reaction management, and envenomation case assessment in diverse clinical settings.
How Manufacturers and Biotechnology Companies Could Strengthen the Ecosystem?
- Advanced Product Development: Develop next-generation anti-venom formulations with enhanced recombinant antibody capabilities, improved safety profiles, and species-specific characteristics that enhance therapeutic reliability while reducing production complexity and adverse reaction rates.
- Quality Assurance Systems: Provide comprehensive manufacturing quality programs that integrate batch testing, stability monitoring, potency verification, and cold chain validation, enabling healthcare systems to maximize treatment effectiveness and patient safety outcomes.
- Distribution & Support Networks: Offer flexible cold chain solutions for healthcare facilities and rural health posts, including temperature monitoring consultation services, storage equipment support programs, and emergency resupply pathways that keep anti-venom products accessible across endemic regions.
- Research & Development Networks: Build comprehensive biotechnology research capabilities, collaborative venom characterization programs, and formulation optimization systems that ensure anti-venom technologies maintain high efficacy standards and consistent safety profiles across diverse envenomation scenarios.
How Healthcare Providers and Distributors Could Navigate Treatment Access?
- Diversified Product Portfolios: Expand anti-venom availability across snake anti-venom applications (50.8% species dominance), hospital operations (62.6% share), and clinic settings, with particular focus on monovalent formulations (9.5% CAGR) and specialized solutions for neurotoxic envenomations.
- Geographic Service Development: Establish distribution capabilities in high-incidence markets like India (9.7% CAGR) and China (9.2% CAGR), while strengthening presence in established markets like Brazil (8.6% CAGR) and Germany (8.1% CAGR) through regional partnerships and local storage facilities.
- Technology-Enabled Distribution: Implement advanced cold chain monitoring systems with real-time temperature tracking, automated inventory management, and predictive restocking protocols that differentiate service offerings and improve product availability and quality assurance.
- Flexible Access Models: Develop government procurement partnerships, emergency stockpiling arrangements, and subsidized distribution programs that accommodate varying healthcare system needs, from well-equipped tertiary hospitals to resource-limited rural health posts.
How Investors and Financial Enablers Could Unlock Value?
- Manufacturing Capacity Financing: Provide growth capital for established anti-venom manufacturers to expand production capabilities and geographic reach, particularly in endemic countries with growing domestic demand and regional export potential.
- Innovation Investment: Support biotechnology startups developing recombinant anti-venom technologies, oligoclonal antibody therapies, and novel immunization approaches that enhance therapeutic efficacy and reduce production costs.
- Regional Production Development: Finance manufacturing facility establishment in high-burden regions, supporting localization initiatives that reduce dependence on imports while maintaining international quality standards and WHO prequalification status.
- Supply Chain Infrastructure: Invest in cold chain logistics networks, regional distribution centers, and emergency stockpile facilities that create resilient anti-venom supply systems, improve product accessibility, and enhance emergency preparedness across endemic regions.
Key Players in the Anti-Venom Market
- Bharat Serums and Vaccines Ltd.
- CSL Limited
- Boehringer Ingelheim
- Pfizer Inc.
- Merck & Co., Inc.
- MicroPharm Limited
- Incepta Pharmaceuticals Ltd.
- Haffkine Bio-Pharma Corp. Ltd.
- South African Vaccine Producers (Pty) Ltd.
- Sigma Aldrich (Merck Group)
- Instituto Butantan
- Fundação Ezequiel Dias (FUNED)
- Vins Bioproducts Limited
- Premium Serums and Vaccines Pvt. Ltd.
- Serum Biotech Ltd.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Value (USD Million)s | USD 1.36 Billion |
| Species | Snake Anti-Venom, Scorpion Anti-Venom, Spider Anti-Venom, Others |
| Type | Polyvalent, Monovalent |
| Mode of Action | Neurotoxic, Cytotoxic, Haemotoxic, Cardiotoxic, Myotoxic & Others |
| End Use | Hospitals, Clinics, Ambulatory Surgical Centers, Others |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Country Covered | India, China, Brazil, Germany, USA, UK, Japan, and 40+ countries |
| Key Companies Profiled | Bharat Serums and Vaccines Ltd., CSL Limited, Boehringer Ingelheim, Pfizer Inc., Merck & Co. Inc., MicroPharm Limited, Incepta Pharmaceuticals Ltd., Haffkine Bio-Pharma Corp. Ltd., South African Vaccine Producers (Pty) Ltd., Sigma Aldrich (Merck Group), Instituto Butantan, Fundação Ezequiel Dias, Vins Bioproducts Limited, Premium Serums and Vaccines Pvt. Ltd., Serum Biotech Ltd. |
| Additional Attributes | Dollar sales by species and type categories, regional adoption trends across Asia Pacific, North America, and Latin America, competitive landscape with anti-venom manufacturers and biotechnology companies, product specifications and efficacy requirements, integration with emergency healthcare systems and government procurement programs, innovations in anti-venom biotechnology and purification systems, and development of specialized formulations with enhanced safety and therapeutic optimization capabilities. |
Anti-Venom Market by Segments
-
Species :
- Snake Anti-Venom
- Scorpion Anti-Venom
- Spider Anti-Venom
- Others
-
Type :
- Polyvalent
- Monovalent
-
Mode of Action :
- Neurotoxic
- Cytotoxic
- Haemotoxic
- Cardiotoxic
- Myotoxic & Others
-
End Use :
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Others
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Value (USD Million)ed Arab Emirates
- Turkey
- South Africa
- Other GCC Countries
- Other African Union
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Species
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Species, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Species, 2025 to 2035
- Snake Anti-Venom
- Scorpion Anti-Venom
- Spider Anti-Venom
- Others
- Y to o to Y Growth Trend Analysis By Species, 2020 to 2024
- Absolute $ Opportunity Analysis By Species, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Type, 2025 to 2035
- Polyvalent
- Monovalent
- Y to o to Y Growth Trend Analysis By Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Mode of Action
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Mode of Action, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Mode of Action, 2025 to 2035
- Neurotoxic
- Cytotoxic
- Haemotoxic
- Cardiotoxic
- Myotoxic & Others
- Y to o to Y Growth Trend Analysis By Mode of Action, 2020 to 2024
- Absolute $ Opportunity Analysis By Mode of Action, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Others
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Species
- By Type
- By Mode of Action
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Species
- By Type
- By Mode of Action
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Species
- By Type
- By Mode of Action
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Species
- By Type
- By Mode of Action
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Species
- By Type
- By Mode of Action
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Species
- By Type
- By Mode of Action
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Species
- By Type
- By Mode of Action
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Species
- By Type
- By Mode of Action
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Species
- By Type
- By Mode of Action
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Species
- By Type
- By Mode of Action
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Species
- By Type
- By Mode of Action
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Species
- By Type
- By Mode of Action
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Species
- By Type
- By Mode of Action
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Species
- By Type
- By Mode of Action
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Species
- By Type
- By Mode of Action
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Species
- By Type
- By Mode of Action
- By End Use
- Competition Analysis
- Competition Deep Dive
- Bharat Serums and Vaccines Ltd.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- CSL Limited
- Boehringer Ingelheim
- Pfizer Inc.
- Merck & Co., Inc.
- MicroPharm Limited
- Incepta Pharmaceuticals Ltd.
- Haffkine Bio-Pharma Corp. Ltd.
- South African Vaccine Producers (Pty) Ltd.
- Sigma Aldrich (Merck Group)
- Instituto Butantan
- Fundação Ezequiel Dias (FUNED)
- Vins Bioproducts Limited
- Premium Serums and Vaccines Pvt. Ltd.
- Serum Biotech Ltd.
- Bharat Serums and Vaccines Ltd.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Species, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Mode of Action, 2020 to 2035
- Table 5: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Species, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 9: North America Market Value (USD Million) Forecast by Mode of Action, 2020 to 2035
- Table 10: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Species, 2020 to 2035
- Table 13: Latin America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 14: Latin America Market Value (USD Million) Forecast by Mode of Action, 2020 to 2035
- Table 15: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: Western Europe Market Value (USD Million) Forecast by Species, 2020 to 2035
- Table 18: Western Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 19: Western Europe Market Value (USD Million) Forecast by Mode of Action, 2020 to 2035
- Table 20: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 21: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: Eastern Europe Market Value (USD Million) Forecast by Species, 2020 to 2035
- Table 23: Eastern Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 24: Eastern Europe Market Value (USD Million) Forecast by Mode of Action, 2020 to 2035
- Table 25: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 26: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 27: East Asia Market Value (USD Million) Forecast by Species, 2020 to 2035
- Table 28: East Asia Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 29: East Asia Market Value (USD Million) Forecast by Mode of Action, 2020 to 2035
- Table 30: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 31: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 32: South Asia and Pacific Market Value (USD Million) Forecast by Species, 2020 to 2035
- Table 33: South Asia and Pacific Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 34: South Asia and Pacific Market Value (USD Million) Forecast by Mode of Action, 2020 to 2035
- Table 35: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 36: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 37: Middle East & Africa Market Value (USD Million) Forecast by Species, 2020 to 2035
- Table 38: Middle East & Africa Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 39: Middle East & Africa Market Value (USD Million) Forecast by Mode of Action, 2020 to 2035
- Table 40: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Species, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Species, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Species
- Figure 6: Global Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Type
- Figure 9: Global Market Value Share and BPS Analysis by Mode of Action, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Mode of Action, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Mode of Action
- Figure 12: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by End Use
- Figure 15: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 16: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 17: Global Market Attractiveness Analysis by Region
- Figure 18: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 23: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 24: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 25: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 26: North America Market Value Share and BPS Analysis by Species, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Species, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Species
- Figure 29: North America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Type
- Figure 32: North America Market Value Share and BPS Analysis by Mode of Action, 2025 and 2035
- Figure 33: North America Market Y to o to Y Growth Comparison by Mode of Action, 2025 to 2035
- Figure 34: North America Market Attractiveness Analysis by Mode of Action
- Figure 35: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 36: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 37: North America Market Attractiveness Analysis by End Use
- Figure 38: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 39: Latin America Market Value Share and BPS Analysis by Species, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Species, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Species
- Figure 42: Latin America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 43: Latin America Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 44: Latin America Market Attractiveness Analysis by Type
- Figure 45: Latin America Market Value Share and BPS Analysis by Mode of Action, 2025 and 2035
- Figure 46: Latin America Market Y to o to Y Growth Comparison by Mode of Action, 2025 to 2035
- Figure 47: Latin America Market Attractiveness Analysis by Mode of Action
- Figure 48: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 49: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 50: Latin America Market Attractiveness Analysis by End Use
- Figure 51: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 52: Western Europe Market Value Share and BPS Analysis by Species, 2025 and 2035
- Figure 53: Western Europe Market Y to o to Y Growth Comparison by Species, 2025 to 2035
- Figure 54: Western Europe Market Attractiveness Analysis by Species
- Figure 55: Western Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 56: Western Europe Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 57: Western Europe Market Attractiveness Analysis by Type
- Figure 58: Western Europe Market Value Share and BPS Analysis by Mode of Action, 2025 and 2035
- Figure 59: Western Europe Market Y to o to Y Growth Comparison by Mode of Action, 2025 to 2035
- Figure 60: Western Europe Market Attractiveness Analysis by Mode of Action
- Figure 61: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 62: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 63: Western Europe Market Attractiveness Analysis by End Use
- Figure 64: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 65: Eastern Europe Market Value Share and BPS Analysis by Species, 2025 and 2035
- Figure 66: Eastern Europe Market Y to o to Y Growth Comparison by Species, 2025 to 2035
- Figure 67: Eastern Europe Market Attractiveness Analysis by Species
- Figure 68: Eastern Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 69: Eastern Europe Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 70: Eastern Europe Market Attractiveness Analysis by Type
- Figure 71: Eastern Europe Market Value Share and BPS Analysis by Mode of Action, 2025 and 2035
- Figure 72: Eastern Europe Market Y to o to Y Growth Comparison by Mode of Action, 2025 to 2035
- Figure 73: Eastern Europe Market Attractiveness Analysis by Mode of Action
- Figure 74: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 75: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 76: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 77: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 78: East Asia Market Value Share and BPS Analysis by Species, 2025 and 2035
- Figure 79: East Asia Market Y to o to Y Growth Comparison by Species, 2025 to 2035
- Figure 80: East Asia Market Attractiveness Analysis by Species
- Figure 81: East Asia Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 82: East Asia Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 83: East Asia Market Attractiveness Analysis by Type
- Figure 84: East Asia Market Value Share and BPS Analysis by Mode of Action, 2025 and 2035
- Figure 85: East Asia Market Y to o to Y Growth Comparison by Mode of Action, 2025 to 2035
- Figure 86: East Asia Market Attractiveness Analysis by Mode of Action
- Figure 87: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 88: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 89: East Asia Market Attractiveness Analysis by End Use
- Figure 90: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 91: South Asia and Pacific Market Value Share and BPS Analysis by Species, 2025 and 2035
- Figure 92: South Asia and Pacific Market Y to o to Y Growth Comparison by Species, 2025 to 2035
- Figure 93: South Asia and Pacific Market Attractiveness Analysis by Species
- Figure 94: South Asia and Pacific Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 95: South Asia and Pacific Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 96: South Asia and Pacific Market Attractiveness Analysis by Type
- Figure 97: South Asia and Pacific Market Value Share and BPS Analysis by Mode of Action, 2025 and 2035
- Figure 98: South Asia and Pacific Market Y to o to Y Growth Comparison by Mode of Action, 2025 to 2035
- Figure 99: South Asia and Pacific Market Attractiveness Analysis by Mode of Action
- Figure 100: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 101: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 102: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 103: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 104: Middle East & Africa Market Value Share and BPS Analysis by Species, 2025 and 2035
- Figure 105: Middle East & Africa Market Y to o to Y Growth Comparison by Species, 2025 to 2035
- Figure 106: Middle East & Africa Market Attractiveness Analysis by Species
- Figure 107: Middle East & Africa Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 108: Middle East & Africa Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 109: Middle East & Africa Market Attractiveness Analysis by Type
- Figure 110: Middle East & Africa Market Value Share and BPS Analysis by Mode of Action, 2025 and 2035
- Figure 111: Middle East & Africa Market Y to o to Y Growth Comparison by Mode of Action, 2025 to 2035
- Figure 112: Middle East & Africa Market Attractiveness Analysis by Mode of Action
- Figure 113: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 114: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 115: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 116: Global Market - Tier Structure Analysis
- Figure 117: Global Market - Company Share Analysis
- FAQs -
How big is the anti-venom market in 2025?
The global anti-venom market is estimated to be valued at USD 1.4 billion in 2025.
What will be the size of anti-venom market in 2035?
The market size for the anti-venom market is projected to reach USD 3.0 billion by 2035.
How much will be the anti-venom market growth between 2025 and 2035?
The anti-venom market is expected to grow at a 8.2% CAGR between 2025 and 2035.
What are the key product types in the anti-venom market?
The key product types in anti-venom market are snake anti-venom, scorpion anti-venom, spider anti-venom and others.
Which type segment to contribute significant share in the anti-venom market in 2025?
In terms of type, polyvalent segment to command 6710.0% share in the anti-venom market in 2025.