Artemisinin Combination Therapy Market
Artemisinin Combination Therapy Market Size and Share Forecast Outlook 2025 to 2035
Artemisinin combination therapy market is projected to grow from USD 0.7 billion in 2025 to USD 1.5 billion by 2035, at a CAGR of 8.3%. Artemether + Lumefantrine will dominate with a 3710.0% market share, while public health procurement (global funds, ngos, government tenders) will lead the distribution channel segment with a 4500.0% share.
Artemisinin Combination Therapy Market Forecast and Outlook 2025 to 2035
The global artemisinin combination therapy (ACT) market is projected to reach USD 1.53 billion by 2035, recording an absolute increase of USD 841.0 million over the forecast period. The market, valued at USD 0.69 billion in 2025, is expected to expand at a CAGR of 8.3% between 2025 and 2035.
Quick Stats for Artemisinin Combination Therapy Market
- Artemisinin Combination Therapy Market Value (2025): USD 0.69 billion
- Artemisinin Combination Therapy Market Forecast Value (2035): USD 1.53 billion
- Artemisinin Combination Therapy Market Forecast CAGR: 8.3%
- Leading Type in Artemisinin Combination Therapy Market: Artemether + Lumefantrine
- Key Growth Regions in Artemisinin Combination Therapy Market: Asia Pacific, Africa, and Europe
- Top Players in Artemisinin Combination Therapy Market: Novartis AG, Sanofi S.A., Cipla Ltd., KPC Pharmaceuticals, Fosun Pharmaceutical (Guilin Pharma), Ajanta Pharma, Ipca Laboratories Ltd., Desano Inc., Hovid Berhad, Mylan
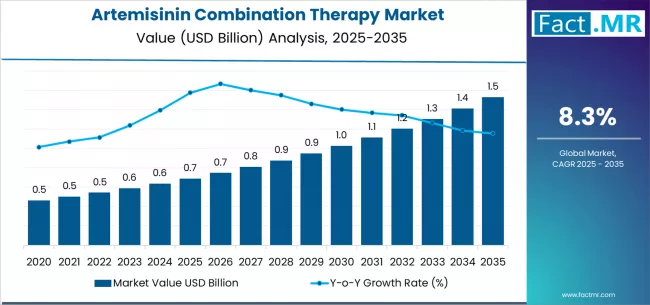
The market is forecast to grow 2.2X, driven by the persistent global malaria burden, rising emphasis on first-line ACT protocols, and ongoing efforts to standardize antimalarial treatment within national malaria control and elimination programs. Artemisinin-based combinations, recommended by the WHO, continue to form the cornerstone of malaria treatment across endemic regions, with fixed-dose combinations and pediatric-friendly dosage formulations increasingly adopted through both public health and private sector channels.
Between 2025 and 2030, the ACT market is projected to expand from USD 0.69 billion to approximately USD 1.03 billion, adding USD 345.0 million in value and accounting for nearly 41% of total forecast growth. This phase will be characterized by increasing demand from Sub-Saharan Africa and Southeast Asia, where malaria remains a leading cause of morbidity and mortality.
Large-scale procurement by national malaria programs, supported by global health funding mechanisms such as the Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM) and the US President’s Malaria Initiative (PMI), will continue to drive bulk purchases of artemisinin-based drugs.
Expanding private retail distribution networks and growing collaboration with NGOs will further strengthen access to ACTs in rural and peri-urban communities. The introduction of dispersible and child-friendly formulations will improve adherence and expand treatment coverage among pediatric populations, a major target group in endemic countries.
From 2030 to 2035, the market is expected to grow from USD 1.03 billion to USD 1.53 billion, adding another USD 0.5 billion and contributing 59% of the decade’s total expansion. This period will be shaped by broader integration of ACTs into preventive and mass drug administration initiatives. Increased adoption of seasonal malaria chemoprevention and community-based delivery models will widen ACT utilization beyond conventional treatment frameworks.
Advancements in combination drug chemistry are expected to yield novel artemisinin derivatives and partner compounds designed to address emerging resistance patterns, particularly in parts of Southeast Asia. Pharmaceutical manufacturers are investing in long-acting formulations and triple-drug combinations to extend treatment durability and counter evolving parasite tolerance. Global health organizations and national governments are also emphasizing supply chain resilience and local pharmaceutical production to ensure consistent availability of high-quality ACTs.
Between 2020 and 2025, the ACT market achieved steady growth supported by increased awareness of standardized malaria treatment protocols and strengthened distribution logistics through public–private partnerships. The widespread transition from monotherapies to combination regimens improved therapeutic outcomes and reduced recrudescence rates, reinforcing ACTs as the global standard of care. Clinical trials during this period demonstrated the continued superiority of ACTs in achieving rapid parasite clearance and minimizing transmission in high-burden regions.
Artemisinin Combination Therapy Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2030, the artemisinin combination therapy market is projected to expand from USD 0.69 billion to USD 1.02 billion, resulting in a value increase of USD 0.33 billion, which represents 39.7% of the total forecast growth for the decade. This phase of development will be shaped by rising demand for WHO-recommended first-line antimalarial treatments and public health procurement programs, product innovation in fixed-dose combination formulations and pediatric dispersible tablets, as well as expanding integration with national malaria elimination strategies and mass drug administration campaigns. Companies are establishing competitive positions through investment in WHO prequalification programs, quality-assured manufacturing facilities, and strategic market expansion across endemic country procurement systems, international donor-funded programs, and travel medicine distribution channels.
From 2030 to 2035, the market is forecast to grow from USD 1.02 billion to USD 1.53 billion, adding another USD 0.51 billion, which constitutes 60.3% of the overall ten-year expansion. This period is expected to be characterized by the expansion of specialized combination therapies, including novel partner drug formulations and triple artemisinin-based combinations tailored for artemisinin-resistant malaria regions, strategic collaborations between pharmaceutical manufacturers and international health organizations for sustainable supply security, and an enhanced focus on quality assurance standards and pharmacovigilance systems for substandard medicine prevention. The growing emphasis on malaria elimination goals and regional certification programs will drive demand for high-efficacy artemisinin combination therapy solutions across diverse epidemiological settings.
Artemisinin Combination Therapy Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 0.69 billion |
| Market Forecast Value (2035) | USD 1.53 billion |
| Forecast CAGR (2025-2035) | 8.3% |
Why is the Artemisinin Combination Therapy Market Experiencing Sustained Growth?
The artemisinin combination therapy market grows by enabling national malaria programs, healthcare providers, and international health organizations to access WHO-recommended antimalarial treatments that support effective parasite clearance while meeting clinical demand for resistance-prevention strategies. Healthcare systems in endemic regions face mounting pressure to implement evidence-based malaria treatment protocols with proven clinical efficacy, with artemisinin-based combination therapies typically providing 95-99% cure rates superior to monotherapy alternatives, making these treatments essential for competitive positioning in tropical disease management and public health intervention categories. The antimalarial drug industry's need for combination formulations that prevent resistance development creates demand for diverse artemisinin-based regimens that can provide superior therapeutic outcomes, maintain consistent efficacy across different parasite populations, and ensure WHO prequalification compliance without compromising affordability or patient adherence standards.
Government initiatives promoting malaria elimination targets and universal health coverage drive adoption in endemic country treatment programs, imported malaria management protocols, and preventive therapy applications, where artemisinin combination therapies have a direct impact on disease control outcomes and mortality reduction. The global health community's growing focus on quality-assured medicines and supply chain integrity further expands market opportunities, with clinical surveillance demonstrating measurable improvements in treatment failure rates, reduced malaria transmission, and enhanced program effectiveness following implementation of WHO-recommended artemisinin combination protocols. However, funding volatility from international donor organizations and the technical requirements for temperature-controlled distribution may limit accessibility among remote endemic communities and developing regions with limited resources for comprehensive pharmaceutical supply chain management and quality control systems.
Segmental Analysis
The market is segmented by type, distribution channel, end user, and region. By type, the market is divided into artemether + lumefantrine, artesunate + amodiaquine, dihydroartemisinin + piperaquine, artesunate + mefloquine, pyronaridine + artesunate, and others.
By distribution channel, the market is categorized into public health procurement, retail pharmacy sales, hospital supply chains, and online/international export channels. By end user, the market includes national malaria programs, private clinics & hospitals, travel & immigration health centers, and pharmacies & NGOs. Regionally, the market is divided into Asia Pacific, Africa, Europe, North America, and Latin America.
By Type, Which Combination Dominates the Artemisinin Combination Therapy Market?
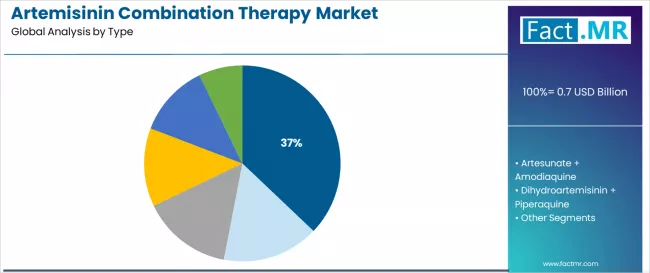
The artemether + lumefantrine segment represents the dominant force in the artemisinin combination therapy market, capturing approximately 37.1% of total market share in 2025. This established combination category encompasses solutions featuring widespread WHO recommendation as first-line treatment across multiple endemic regions, including fixed-dose oral formulations that enable convenient twice-daily dosing and proven efficacy standards across all patient populations.
The artemether + lumefantrine segment's market leadership stems from its superior safety profile and extensive clinical validation, with formulations capable of meeting diverse treatment requirements while maintaining high cure rates and operational reliability across all malaria transmission settings. Within this segment, fixed-dose oral formulations represent 18.0% of the overall market, driven by hospital adoption, travel clinic prescribing preferences, and national program procurement for standardized treatment protocols.
The artesunate + amodiaquine segment maintains substantial market presence with 20.0% market share, serving national malaria programs in West African countries where this combination demonstrates optimal efficacy and cost-effectiveness for first-line treatment protocols. These formulations offer single-daily dosing advantages for improved patient adherence while providing sufficient therapeutic coverage to meet WHO treatment guidelines and regional resistance patterns.
The dihydroartemisinin + piperaquine segment accounts for approximately 14.0% of the market, serving Southeast Asian regions and programs requiring extended post-treatment prophylaxis. The artesunate + mefloquine segment holds 10.0% market share, while pyronaridine + artesunate captures 9.5% market share serving areas with specific resistance concerns, and other combinations account for 9.4% of the market, including emerging triple artemisinin-based therapies and investigational combinations for resistant malaria management.
Key advantages driving the artemether + lumefantrine segment include:
- Extensive WHO prequalification status with proven manufacturing quality that reduces procurement complexity and ensures treatment reliability
- Broad geographic applicability across diverse malaria transmission zones without significant regional resistance patterns
- Proven pediatric formulations delivering age-appropriate dosing while maintaining therapeutic efficacy for vulnerable populations
- Strong safety profile enabling straightforward clinical implementation and regulatory approval across multiple jurisdictions
By Distribution Channel, what is the Leading Segment in the Artemisinin Combination Therapy Market?
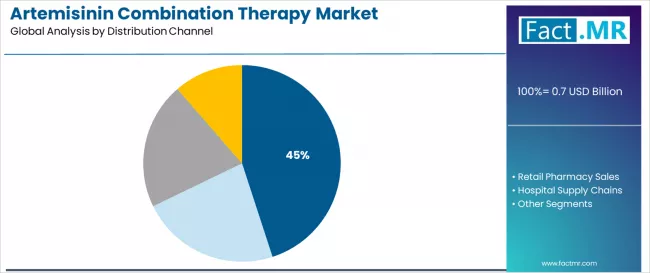
Public health procurement dominates the distribution channel segment with approximately 45.0% market share in 2025, reflecting the critical role of international donor funding and government tender systems in supporting antimalarial drug access worldwide. The public health procurement segment's market leadership is reinforced by substantial Global Fund financing, international NGO purchasing programs, and centralized government procurement systems ensuring affordable treatment access in resource-limited endemic settings. Within this segment, WHO-prequalified suppliers represent 22.0% of total market share, driven by stringent quality requirements for international tender participation and donor-funded program specifications demanding regulatory compliance and manufacturing standards verification.
The retail pharmacy sales segment represents substantial market presence, capturing 28.0% market share through private sector distribution channels serving urban populations, self-medication practices, and out-of-pocket purchasing in endemic countries. This segment benefits from growing middle-class populations in endemic regions seeking immediate treatment access, expanding pharmacy networks in urban centers, and increasing availability of over-the-counter antimalarial formulations in countries with permissive regulatory frameworks.
The hospital supply chains segment accounts for 17.0% market share, serving inpatient malaria management, severe malaria treatment protocols, and referral hospital pharmacy systems. Online and international export channels capture 10.0% market share, serving medical tourism, expatriate populations, and international travel medicine distribution networks connecting endemic and non-endemic country markets.
Key market dynamics supporting distribution channel growth include:
- Public procurement expansion driven by international malaria elimination commitments and donor funding, requiring WHO-prequalified manufacturing facilities in quality-assured supplier networks
- Retail pharmacy modernization trends require branded antimalarial products for consumer confidence and treatment adherence
- Integration of supply chain technologies enabling temperature monitoring and comprehensive product tracking solutions
- Growing emphasis on distribution diversity driving demand for multi-channel access strategies
By End User, what is the Leading Segment in the Artemisinin Combination Therapy Market?
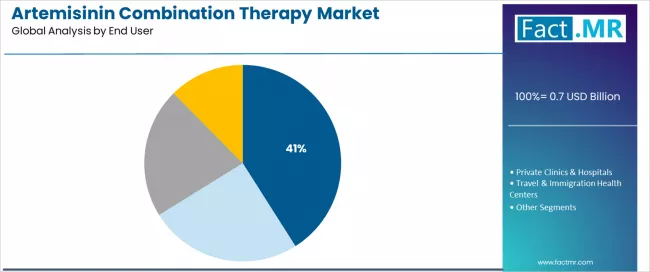
National malaria programs dominate the end user segment with approximately 41.0% market share in 2025, reflecting the central role of government health ministries and centralized disease control initiatives in antimalarial treatment provision across endemic countries.
The national malaria programs segment's market leadership is reinforced by universal health coverage commitments, free treatment policies for confirmed malaria cases, and comprehensive national treatment guidelines mandating artemisinin-based combination therapy as first-line protocols across public health facilities.
The private clinics & hospitals segment represents significant market presence, capturing 30.0% market share through fee-for-service healthcare delivery, private sector malaria diagnosis and treatment services, and urban healthcare facilities serving patients seeking immediate care outside public health systems.
The travel & immigration health centers segment accounts for 18.0% market share, serving pre-travel malaria prophylaxis consultations, imported malaria treatment in non-endemic countries, and specialized travel medicine clinics providing artemisinin combination therapy prescriptions for travelers to malaria-endemic regions.
Pharmacies & NGOs capture 11.0% market share, serving community-based malaria treatment programs, international humanitarian organizations providing emergency healthcare, and retail pharmacy networks in endemic regions offering antimalarial products for self-medication practices.
Key market dynamics supporting end user growth include:
- National program expansion driven by malaria elimination targets and universal health coverage, requiring sustainable artemisinin combination therapy supply systems
- Private healthcare sector growth trends in endemic countries creating demand for quality-assured branded antimalarial products
- Integration of travel medicine protocols enabling comprehensive imported malaria management solutions
- Growing emphasis on end user diversity driving demand for multi-sector distribution strategies
What are the Drivers, Restraints, and Key Trends of the Artemisinin Combination Therapy Market?
The market is driven by three concrete demand factors tied to disease burden and global health priorities. First, persistent malaria transmission in endemic regions creates sustained demand for artemisinin combination therapies, with over 240 million malaria cases annually requiring first-line treatment access, necessitating comprehensive pharmaceutical supply infrastructure. Second, international health initiatives promoting malaria elimination targets and WHO End Malaria strategy goals drive increased procurement of quality-assured artemisinin combinations, with donor organizations allocating substantial funding for antimalarial commodities through 2030. Third, emerging artemisinin resistance in Southeast Asian regions necessitates enhanced surveillance systems and alternative combination therapy development that expands treatment options while addressing efficacy concerns in resistance-affected areas.
Market restraints include supply chain vulnerabilities for artemisinin active pharmaceutical ingredients sourced predominantly from limited agricultural production regions that can cause price volatility and availability concerns, particularly during crop failures or geopolitical disruptions affecting major producing countries. Counterfeit and substandard antimalarial products pose another significant challenge, as falsified artemisinin combinations undermine treatment effectiveness and contribute to resistance development, potentially causing public health program failures and patient mortality. Funding dependency on international donor organizations creates additional market challenges for sustainable supply security, demanding diversified financing mechanisms and domestic resource mobilization strategies to ensure continuous artemisinin combination therapy availability beyond external aid programs.
Key trends indicate accelerated adoption in African endemic countries, particularly Nigeria, Democratic Republic of Congo, and Uganda, where high malaria burden and expanding national program coverage drive comprehensive artemisinin combination therapy deployment. Technology integration trends toward quality assurance systems with mobile authentication platforms, supply chain tracking technologies, and pharmacovigilance reporting mechanisms enable proactive product quality management approaches that optimize treatment outcomes and minimize substandard medicine circulation. However, the market thesis could face disruption if significant advances in next-generation antimalarial compounds or major breakthroughs in malaria vaccine effectiveness reduce reliance on artemisinin-based chemotherapy for malaria treatment and prevention applications.
Analysis of the Artemisinin Combination Therapy Market by Key Country
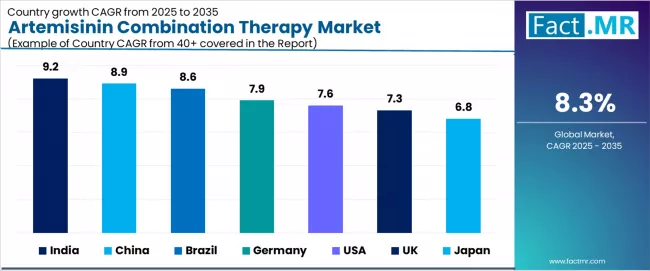
| Country | CAGR (2025-2035) |
|---|---|
| India | 9.2% |
| China | 8.9% |
| Brazil | 8.6% |
| Germany | 7.9% |
| USA | 7.6% |
| UK | 7.3% |
| Japan | 6.8% |
The artemisinin combination therapy market is expanding steadily, with India leading at a 9.2% CAGR through 2035, driven by government malaria-free India mission targeting elimination by 2030, strong artemisinin combination therapy promotion through national vector-borne disease control programs, and expanding domestic pharmaceutical manufacturing capabilities for quality-assured antimalarial production.
China follows at 8.9%, supported by reinforced post-elimination surveillance systems maintaining treatment readiness, substantial artemisinin active pharmaceutical ingredient export capabilities serving global supply chains, and comprehensive malaria importation prevention protocols in border regions.
Brazil records 8.6%, reflecting high malaria incidence in Amazon Basin states, increased WHO-backed funding for malaria control programs, and expanding healthcare infrastructure in endemic municipalities. Germany posts 7.9%, anchored by robust imported malaria case management in tropical medicine centers, higher public awareness regarding travel health precautions, and comprehensive insurance coverage for antimalarial treatments.
The USA advances at 7.6%, emphasizing rising imported malaria cases from travelers and immigrants arriving from Africa and Asia, CDC clinical guidelines recommending artemisinin combinations for severe malaria treatment, and expanding travel medicine clinic networks, while the UK grows at 7.3%, focusing on NHS treatment protocols for imported malaria and substantial contributions to global health funding mechanisms supporting artemisinin combination therapy procurement programs.
Japan demonstrates steady growth at 6.8%, driven by low domestic malaria incidence but consistent use for travel medicine consultations and overseas development assistance programs supporting endemic country malaria control initiatives.
How does India Lead Expansion of the Global Artemisinin Combination Therapy market?
India demonstrates the strongest growth potential in the artemisinin combination therapy market with a CAGR of 9.2% through 2035. The country's leadership position stems from government malaria-free India mission with elimination targets by 2030, comprehensive artemisinin combination therapy promotion through national vector-borne disease control programs and expanding domestic pharmaceutical manufacturing capabilities producing quality-assured antimalarial formulations.
Growth is concentrated in endemic states, including Odisha, Chhattisgarh, Jharkhand, and northeastern regions, where tribal populations and forest-fringe communities face persistent malaria transmission requiring accessible treatment programs. Distribution channels through primary health centers, community health workers, and accredited social health activists expand deployment across remote endemic areas and comprehensive case management initiatives. The country's pharmaceutical manufacturing sector provides indigenous artemisinin combination therapy production capabilities supporting self-reliance in antimalarial supply chains.
Key market factors:
- Treatment demand concentrated in tribal and forest regions with comprehensive malaria surveillance and case management programs
- Public health infrastructure expansion through strengthened primary care facilities and community-based distribution systems
- Comprehensive manufacturing ecosystem, including domestic artemisinin combination therapy production with WHO prequalification capabilities
- Technology integration featuring mobile-based reporting systems, rapid diagnostic test distribution, and quality assurance platforms
How does China Emerge as a Strategic Market for Artemisinin Combination Therapy?
In Yunnan, Guangxi, and border provinces, the maintenance of artemisinin combination therapy stockpiles is continuing across surveillance systems and importation-prevention programs, driven by malaria-free certification achievement and regional cooperation initiatives.
The market demonstrates strong growth momentum with a CAGR of 8.9% through 2035, linked to reinforced post-elimination surveillance maintaining treatment readiness for imported cases, substantial artemisinin active pharmaceutical ingredient export industry serving global antimalarial manufacturers, and comprehensive border health screening protocols preventing malaria reintroduction.
Chinese pharmaceutical companies are maintaining artemisinin combination therapy production capabilities and active ingredient supply chains supporting international malaria control programs while ensuring domestic preparedness for imported malaria management.
The country's agricultural artemisinin production and pharmaceutical manufacturing integration creates persistent supply security for global markets, while increasing emphasis on quality standards drives adoption of Good Manufacturing Practice facilities and regulatory compliance systems.
Key development areas:
- Active pharmaceutical ingredient production and artemisinin cultivation leading global supply chain support
- International export partnerships providing integrated manufacturing services with extensive quality assurance programs
- Technology collaborations between Chinese pharmaceutical companies and international health organizations expanding market reach
- Integration of surveillance technologies and comprehensive imported case management systems
What is the Scope for the Artemisinin Combination Therapy Market in Brazil?
Brazil's market expansion is driven by persistent malaria transmission in Amazon Basin states, including Amazonas, Pará, Acre, and Rondônia, where indigenous populations and mining communities face ongoing infection risk.
The country demonstrates promising growth potential with a CAGR of 8.6% through 2035, supported by high malaria incidence requiring comprehensive treatment access, increased WHO-backed funding supporting national malaria control programs, and expanding healthcare infrastructure in remote endemic municipalities.
Brazilian health authorities face implementation challenges related to geographic accessibility in rainforest regions and illegal mining camp populations requiring innovative distribution strategies beyond fixed health facilities.
Government commitment to malaria elimination and international partnership support creates compelling cases for artemisinin combination therapy adoption, particularly in frontier areas where treatment availability has a direct impact on disease control outcomes.
Market characteristics:
- Amazon region malaria control showing treatment access expansion with mobile health teams and community health agent networks
- Regional program coordination trends focused on cross-border initiatives with neighboring endemic countries
- Future projections indicate the need for enhanced surveillance systems and targeted intervention strategies
- Growing emphasis on indigenous population health and culturally appropriate treatment delivery models
How will Treatment for Imported Malaria drive Artemisinin Combination Therapy Prospects in Germany?
The German market leads in imported malaria management based on comprehensive tropical medicine center networks and specialized infectious disease expertise for returning travelers and immigrants. The country shows strong potential with a CAGR of 7.9% through 2035, driven by robust imported malaria case management protocols in university hospitals, higher public awareness regarding pre-travel health consultations and malaria risk, and comprehensive health insurance coverage for antimalarial treatment costs.
German healthcare providers maintain artemisinin combination therapy procurement for severe malaria treatment in intensive care settings and outpatient management of uncomplicated imported cases, particularly from travelers returning from Sub-Saharan Africa. Technology deployment channels through established tropical medicine institutes and travel health clinic networks expand coverage across major cities with international airports and immigrant health services.
Leading market segments:
- Tropical medicine centers and infectious disease departments implementing comprehensive imported malaria protocols
- Travel clinic partnerships with pharmaceutical distributors achieving standard artemisinin combination therapy availability
- Strategic collaborations between healthcare providers and international health organizations supporting global malaria initiatives
- Focus on clinical guideline adherence and treatment outcome monitoring
What are the prospects for the Artemisinin Combination Therapy in USA?
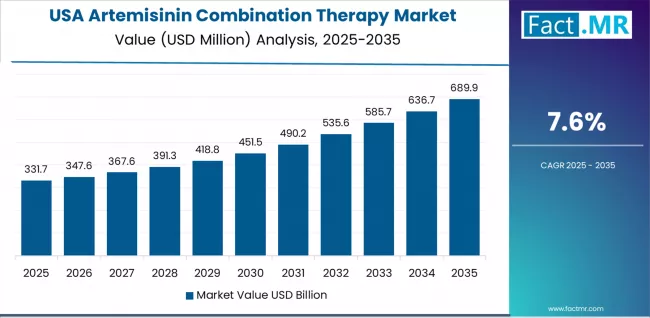
In major metropolitan areas including New York, Atlanta, Houston, and Los Angeles, healthcare providers are implementing artemisinin combination therapy protocols for imported malaria management serving returning travelers, immigrants, and refugees from endemic regions, with documented case management studies demonstrating effective treatment outcomes through CDC-recommended artemisinin-based regimens.
The market shows strong growth potential with a CAGR of 7.6% through 2035, linked to rising imported malaria cases from increasing international travel to Africa and Asia, CDC clinical guidelines specifically recommending artemisinin combinations for severe malaria treatment protocols, and expanding travel medicine clinic networks providing pre-travel consultations and post-travel fever evaluation services.
Healthcare providers maintain artemisinin combination therapy access through hospital pharmacies and specialized infectious disease departments supporting prompt treatment initiation for suspected malaria cases. The country's public health surveillance system creates persistent demand for quality-assured antimalarial products and comprehensive treatment protocols that integrate with notifiable disease reporting frameworks.
Market development factors:
- Academic medical centers and infectious disease specialists leading imported malaria management across USA
- CDC guidance programs providing treatment protocol support for healthcare provider education
- Strategic partnerships between pharmaceutical importers and specialty distributors ensuring artemisinin combination therapy availability
- Emphasis on severe malaria treatment capabilities and intravenous artesunate access
How does Commitment toward Upholding Global Healthcare Standards Reinforce Scope for Artemisinin Combination Therapy in the UK?
The UK's artemisinin combination therapy market demonstrates sophisticated integration of imported malaria treatment protocols and substantial international development funding supporting endemic country procurement programs, with comprehensive NHS guidelines ensuring standardized artemisinin-based treatment for returning travelers diagnosed with malaria across primary care and hospital settings.
The country maintains steady growth momentum with a CAGR of 7.3% through 2035, driven by NHS treatment protocols for imported malaria incorporating artemisinin combinations as first-line therapy, expanding UK Aid funding contributions supporting Global Fund malaria programs and direct bilateral assistance for artemisinin combination therapy procurement, and comprehensive travel health services integrated with primary care practices and specialist tropical medicine units.
Major healthcare centers, including London Hospital for Tropical Diseases, Liverpool School of Tropical Medicine, and regional infectious disease units, showcase advanced imported malaria management where artemisinin combinations integrate seamlessly with rapid diagnostic capabilities and specialist consultation services.
Key market characteristics:
- NHS imported malaria management driving standardized artemisinin combination therapy protocols with clinical effectiveness monitoring
- International development partnerships enabling substantial procurement funding for endemic country programs through UK government aid
- Technology collaboration between UK pharmaceutical companies and global health organizations expanding access initiatives
- Emphasis on travel health promotion and malaria prevention education programs
How will Overseas Assistance Programs Influence the Market’s Outcome in Japan?
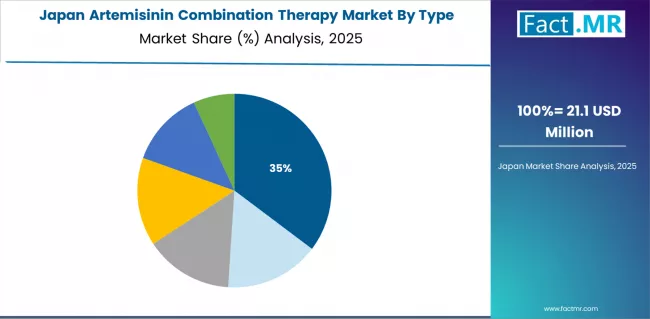
Japan's artemisinin combination therapy market demonstrates limited domestic clinical demand but significant engagement through international health cooperation and travel medicine applications, characterized by comprehensive overseas development assistance supporting malaria-endemic countries and specialized tropical medicine centers serving returning travelers and expatriate populations.
The country shows steady growth momentum with a CAGR of 6.8% through 2035, driven by low domestic malaria incidence requiring minimal treatment volumes but consistent use for travel medicine consultations and aging traveler demographics seeking comprehensive pre-travel health services. Japan's emphasis on international health security and development cooperation creates requirements for quality-assured antimalarial product procurement supporting bilateral assistance programs and multilateral funding contributions to global malaria initiatives.
The market benefits from established partnerships between Japanese pharmaceutical companies and international health organizations, creating comprehensive supply chain support for endemic country programs while maintaining domestic treatment readiness for imported cases.
Key market characteristics:
- Travel medicine centers and infectious disease departments maintaining artemisinin combination therapy access for imported case management
- Overseas development assistance programs enabling procurement support for partner countries through Japanese government funding
- Technology collaboration between Japanese organizations and global health initiatives expanding market engagement
- Emphasis on international health security and comprehensive disease surveillance methodologies
Competitive Landscape of the Artemisinin Combination Therapy Market
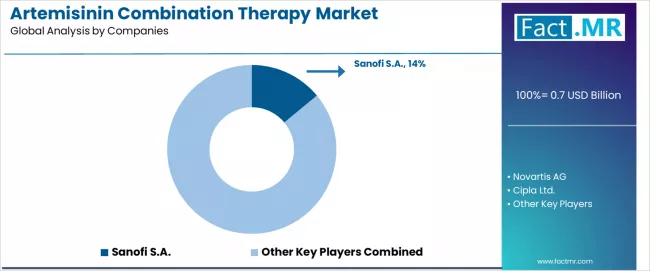
The artemisinin combination therapy market features approximately 10-15 meaningful players with moderate concentration, where the top three companies control roughly 40-45% of global market share through established WHO-prequalified product portfolios and extensive international procurement relationships. Competition centers on quality assurance credentials, WHO prequalification status, and competitive tender pricing rather than brand differentiation alone.
Market leaders include Novartis AG, holding approximately 18.5% global market share, maintaining competitive advantages through Coartem (artemether-lumefantrine) brand leadership with extensive clinical validation, WHO prequalification status, and comprehensive global distribution networks serving both public health procurement programs and private sector markets, creating established supplier relationships with international health organizations.
Sanofi S.A. follows with 14.0% market share through ASAQ (artesunate-amodiaquine) fixed-dose combination products serving West African national programs, while Cipla Ltd. captures 10.5% market share leveraging cost-competitive manufacturing capabilities and quality-assured production facilities serving international tender markets and generic antimalarial supply chains.
Challengers encompass KPC Pharmaceuticals with 8.5% market share competing through dihydroartemisinin-piperaquine combinations serving Southeast Asian markets, Fosun Pharmaceutical (Guilin Pharma) holding 8.0% market share through artesunate active ingredient production and combination product manufacturing, and Ajanta Pharma capturing 7.5% market share offering diverse artemisinin combination portfolios across multiple partner drug formulations.
Antimalarial specialists, including Ipca Laboratories Ltd. with 6.5% market share focusing on WHO-prequalified artemether-lumefantrine products, Desano Inc. holding 6.0% market share through specialized combination formulations, and Hovid Berhad capturing 5.5% market share serving Southeast Asian regional markets, focus on specific combination types or geographic regions, offering differentiated capabilities in pediatric formulations, dispersible tablet technologies, and regulatory compliance across multiple jurisdictions.
Generic manufacturers and emerging pharmaceutical companies create competitive pressure through cost-effective production and WHO prequalification achievement, particularly manufacturers based in India and China where established active pharmaceutical ingredient supply chains provide advantages in raw material access and manufacturing cost structures.
Market dynamics favor companies that combine WHO prequalification credentials with competitive pricing strategies and comprehensive technical support services addressing regulatory requirements, quality assurance documentation, and pharmacovigilance reporting obligations throughout international procurement processes.
Strategic partnerships between pharmaceutical manufacturers and international health organizations for long-term supply agreements, along with investments in manufacturing capacity expansion and quality system enhancements, characterize the competitive evolution as market participants seek to strengthen positions in donor-funded procurement programs and emerging private sector distribution channels across endemic country markets.
Key Players in the Artemisinin Combination Therapy Market
- Novartis AG
- Sanofi S.A.
- Cipla Ltd.
- KPC Pharmaceuticals
- Fosun Pharmaceutical (Guilin Pharma)
- Ajanta Pharma
- Ipca Laboratories Ltd.
- Desano Inc.
- Hovid Berhad
- Mylan
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Value (USD Million)s | USD 0.69 billion |
| Type | Artemether + Lumefantrine (Fixed-dose oral formulations), Artesunate + Amodiaquine, Dihydroartemisinin + Piperaquine, Artesunate + Mefloquine, Pyronaridine + Artesunate, Others |
| Distribution Channel | Public health procurement (WHO-prequalified suppliers), Retail pharmacy sales, Hospital supply chains, Online/International export channels |
| End User | National malaria programs, Private clinics & hospitals, Travel & immigration health centers, Pharmacies & NGOs |
| Regions Covered | Asia Pacific, Africa, Europe, North America, Latin America |
| Country Covered | India, China, Brazil, USA, Germany, UK, Japan, and 40+ countries |
| Key Companies Profiled | Novartis AG, Sanofi S.A., Cipla Ltd., KPC Pharmaceuticals, Fosun Pharmaceutical (Guilin Pharma), Ajanta Pharma, Ipca Laboratories Ltd., Desano Inc., Hovid Berhad, Mylan |
| Additional Attributes | Dollar sales by type and distribution channel categories, regional adoption trends across Asia Pacific, Africa, and Europe, competitive landscape with WHO-prequalified manufacturers and generic pharmaceutical companies, formulation specifications and quality standards, integration with national malaria control programs and international health initiatives. |
Artemisinin Combination Therapy Market by Segments
-
Type :
- Artemether + Lumefantrine
- Artesunate + Amodiaquine
- Dihydroartemisinin + Piperaquine
- Artesunate + Mefloquine
- Pyronaridine + Artesunate
- Others
-
Distribution Channel :
- Public Health Procurement (Global Funds, NGOs, Government Tenders)
- Retail Pharmacy Sales
- Hospital Supply Chains
- Online/International Export Channels
-
End User :
- National Malaria Programs
- Private Clinics & Hospitals
- Travel & Immigration Health Centers
- Pharmacies & NGOs
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Africa
- Nigeria
- South Africa
- Democratic Republic of Congo
- Uganda
- Rest of Sub-Saharan Africa
- North Africa
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Chile
- Rest of Latin America
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Type, 2025 to 2035
- Artemether + Lumefantrine
- Artesunate + Amodiaquine
- Dihydroartemisinin + Piperaquine
- Artesunate + Mefloquine
- Pyronaridine + Artesunate
- Others
- Y to o to Y Growth Trend Analysis By Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Distribution Channel
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Distribution Channel, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Distribution Channel, 2025 to 2035
- Public Health Procurement (Global Funds, NGOs, Government Tenders)
- Retail Pharmacy Sales
- Hospital Supply Chains
- Online/International Export Channels
- Y to o to Y Growth Trend Analysis By Distribution Channel, 2020 to 2024
- Absolute $ Opportunity Analysis By Distribution Channel, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End User
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End User, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End User, 2025 to 2035
- National Malaria Programs
- Private Clinics & Hospitals
- Travel & Immigration Health Centers
- Pharmacies & NGOs
- Y to o to Y Growth Trend Analysis By End User, 2020 to 2024
- Absolute $ Opportunity Analysis By End User, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Type
- By Distribution Channel
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Distribution Channel
- By End User
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Type
- By Distribution Channel
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Distribution Channel
- By End User
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Type
- By Distribution Channel
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Distribution Channel
- By End User
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Type
- By Distribution Channel
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Distribution Channel
- By End User
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Type
- By Distribution Channel
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Distribution Channel
- By End User
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Type
- By Distribution Channel
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Distribution Channel
- By End User
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Type
- By Distribution Channel
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Distribution Channel
- By End User
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Distribution Channel
- By End User
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Type
- By Distribution Channel
- By End User
- Competition Analysis
- Competition Deep Dive
- Sanofi S.A.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Novartis AG
- Cipla Ltd.
- KPC Pharmaceuticals
- Fosun Pharmaceutical (Guilin Pharma)
- Ajanta Pharma
- Ipca Laboratories Ltd.
- Desano Inc.
- Hovid Berhad
- Mylan
- Sanofi S.A.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by End User, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by End User, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Type
- Figure 6: Global Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Distribution Channel
- Figure 9: Global Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by End User
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Type
- Figure 26: North America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Distribution Channel
- Figure 29: North America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by End User
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Type
- Figure 36: Latin America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Distribution Channel
- Figure 39: Latin America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by End User
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Type
- Figure 46: Western Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Distribution Channel
- Figure 49: Western Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by End User
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Type
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Distribution Channel
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by End User
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Type
- Figure 66: East Asia Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Distribution Channel
- Figure 69: East Asia Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by End User
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Type
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Distribution Channel
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by End User
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Type, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Type
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Distribution Channel
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by End User, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by End User
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the artemisinin combination therapy market in 2025?
The global artemisinin combination therapy market is estimated to be valued at USD 0.7 billion in 2025.
What will be the size of artemisinin combination therapy market in 2035?
The market size for the artemisinin combination therapy market is projected to reach USD 1.5 billion by 2035.
How much will be the artemisinin combination therapy market growth between 2025 and 2035?
The artemisinin combination therapy market is expected to grow at a 8.3% CAGR between 2025 and 2035.
What are the key product types in the artemisinin combination therapy market?
The key product types in artemisinin combination therapy market are artemether + lumefantrine , artesunate + amodiaquine, dihydroartemisinin + piperaquine, artesunate + mefloquine, pyronaridine + artesunate and others.
Which distribution channel segment to contribute significant share in the artemisinin combination therapy market in 2025?
In terms of distribution channel, public health procurement (global funds, ngos, government tenders) segment to command 4500.0% share in the artemisinin combination therapy market in 2025.