Lysosomal Acid Lipase Deficiency Treatment Market
Lysosomal Acid Lipase Deficiency Treatment Market Size and Share Forecast Outlook 2025 to 2035
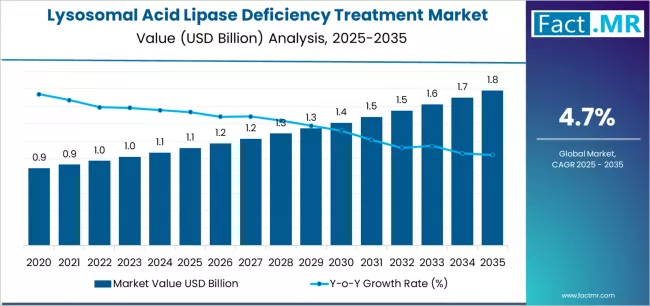
Lysosomal acid lipase deficiency treatment market is projected to grow from USD 1.1 billion in 2025 to USD 1.8 billion by 2035, at a CAGR of 4.7%. Wolman Disease will dominate with a 61.3% market share, while enzyme replacement therapy (ert) will lead the treatment type segment with a 78.4% share.
Lysosomal Acid Lipase Deficiency Treatment Market Forecast and Outlook 2025 to 2035
The global lysosomal acid lipase deficiency treatment market is set to grow from USD 1.12 billion in 2025 to USD 1.78 billion by 2035, adding USD 0.66 billion in new revenue and advancing at a CAGR of 4.7%.
Growth is driven by rising newborn screening protocol implementation, escalating rare disease awareness initiatives, and expanding genetic testing capabilities in emerging economies seeking comprehensive metabolic disorder management solutions.
Wolman disease holds a 61.3% share in 2025, favored in clinical treatment environments for its early detection opportunities, established diagnostic pathways, and comprehensive therapeutic protocols addressing diverse patient severity levels.
Infantile-onset Wolman disease represents 35.7% within the indication segment and remains critical in neonatal intensive care settings where early intervention necessity and genetic screening advancement match clinical treatment requirements. Cholesteryl ester storage disease at 4.9% CAGR is accelerating among late-onset patient segments as diagnostic accessibility improves and enzyme replacement therapy availability gains traction in developed markets.
Enzyme replacement therapy remains the dominant treatment modality at 78.4%, reflecting the need for proven clinical efficacy, regulatory a7oval establishment, and demonstrated therapeutic outcomes across rare disease patient populations. Sebelipase alfa at 64.1% within ERT expands as reimbursement coverage improves, orphan drug designation benefits strengthen, and clinical evidence accumulation validates treatment effectiveness in metabolic disorder applications.
North America leads growth, with USA at 4.9% and supportive regulatory frameworks, supported by orphan drug incentive advantages, patient registry development, and rare disease treatment infrastructure expansion. Germany, Japan, and UK demonstrate robust development through advanced healthcare systems, comprehensive genetic testing programs, and specialized rare disease center establishment. Competitive advantage is consolidating around regulatory a7oval speed, orphan drug designation achievement, clinical trial execution capabilities, and patient access program development rather than treatment modality diversification alone.
From 2025-2030, the market will witness the market climbing from USD 1.12 billion to a7oximately USD 1.42 billion, adding USD 0.30 billion in value, which constitutes 45% of the total forecast growth period. This phase will be characterized by the continued dominance of enzyme replacement therapy in established markets, combined with accelerating substrate reduction therapy development in clinical trial environments where novel therapeutic a7oaches and small molecule innovation create favorable research advancement conditions.
Manufacturers will concentrate on sebelipase alfa distribution optimization, rare disease diagnosis enhancement for earlier patient identification, and specialist physician network expansion in underserved territories where lysosomal acid lipase deficiency awareness remains limited among general medical practitioners. Enhanced genetic counseling services and patient registry integration will become standard treatment support priorities rather than optional program differentiators.
From 2030-2035, the market will witness sustained expansion from USD 1.42 billion to USD 1.78 billion, representing an addition of USD 0.36 billion or 55% of the decade's growth. This period will be defined by the broadening acceptance of substrate reduction therapies beyond experimental protocols, integration of gene therapy a7oaches across clinical development pipelines, and strengthened diagnostic networks in previously underserved emerging markets.
Where revenue comes from - Now Vs Next (industry-level view)
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | Wolman disease | 61.3% | Early detection dominance |
| Infantile-onset Wolman | 35.7% | Newborn screening focus | |
| CESD | 4.9% CAGR | Late-onset expansion | |
| Enzyme replacement therapy | 78.4% | Regulatory a7oval strength | |
| Sebelipase alfa | 64.1% | Clinical efficacy leadership | |
| Substrate reduction therapy | 5.2% CAGR | Experimental development | |
| North America markets | Data-driven growth | Orphan drug incentives | |
| Future (3-5 yrs) | Wolman disease platforms | 58-62% | Sustained diagnostic leadership |
| CESD treatment expansion | 6-8% CAGR | Emerging market penetration | |
| Enzyme replacement therapy | 74-78% | Maintained therapeutic standard | |
| Substrate reduction therapy | 8-12% CAGR | Clinical trial advancement | |
| Gene therapy a7oaches | 5-9% | Curative modality development | |
| Emerging market access | 15-19% | Diagnostic infrastructure growth | |
| Patient registry integration | 42-46% | Treatment optimization programs |
Lysosomal Acid Lipase Deficiency Treatment Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 1.12 billion |
| Market Forecast (2035) ↑ | USD 1.78 billion |
| Growth Rate ★ | 4.7% CAGR |
| Leading Indication → | Wolman Disease |
| Primary Treatment Type → | Enzyme Replacement Therapy |
The market demonstrates solid fundamentals with Wolman disease capturing a commanding 61.3% share through proven early detection capabilities and comprehensive neonatal screening integration. Enzyme replacement therapy configurations drive primary treatment demand at 78.4% share, supported by established regulatory a7oval infrastructure and clinical efficacy advantages that maintain therapeutic effectiveness across diverse metabolic disorder patient populations.
Treatment concentration remains anchored in enzyme replacement modalities with sebelipase alfa at 64.1% therapy dominance and robust clinical evidence ecosystems, while substrate reduction a7oaches show accelerated development rates driven by experimental therapeutic innovation and orphan drug designation support mechanisms.
Imperatives for Stakeholders in Lysosomal Acid Lipase Deficiency Treatment Market
Design for efficacy, not just availability
- Offer complete therapeutic solutions: enzyme replacement optimization + substrate reduction innovation + gene therapy development + clinical outcome monitoring + patient support program integration.
- Preconfigured treatment protocols: infantile-onset Wolman specifications, late-onset CESD management a7oaches, enzyme dosing optimization packages, and genetic counseling integration for comprehensive patient satisfaction.
Regulatory readiness for orphan drug pathways
- Real-time clinical trial monitoring systems, orphan drug designation documentation, and supply chain transparency (active pharmaceutical ingredient traceability, manufacturing standards, quality control protocols).
Affordability-by-access a7oach
- Patient assistance program development, specialized rare disease reimbursement navigation, healthcare provider partnership establishment, and transparent treatment cost documentation.
Distribution-focused patient penetration
- Clear specialty pharmacy expansion strategies + established metabolic disorder centers (urban academic institutions, regional referral networks, telemedicine consultation access); patient advocacy collaboration for disease awareness and treatment accessibility development.
Segmental Analysis
The market segments by indication into Wolman disease, cholesteryl ester storage disease, and related manifestations, representing the evolution from acute infantile presentations toward chronic late-onset conditions requiring specialized diagnostic recognition and therapeutic intervention a7oaches.
The treatment type segmentation divides the market into enzyme replacement therapy (78.4%) and substrate reduction therapy (5.2% CAGR), reflecting distinct therapeutic priorities for proven clinical efficacy and regulatory validation versus innovative therapeutic mechanism exploration and novel treatment development.
The regional segmentation reveals North America's regulatory leadership position, followed by Europe and Asia Pacific, demonstrating varied healthcare infrastructure maturity levels and rare disease management framework development stages.
Which Indication Type is the Most Dominant in the Lysosomal Acid Lipase Deficiency Treatment Market?
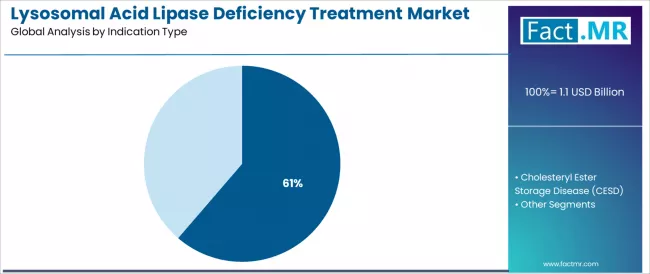
Wolman disease commands the leading position in the lysosomal acid lipase deficiency treatment market with dominant 61.3% market share through proven clinical severity recognition, including critical neonatal presentation characteristics, comprehensive genetic screening integration, and established therapeutic protocol requirements that enable clinicians to achieve timely intervention across varied healthcare infrastructure levels and diagnostic capability applications.
The segment benefits from medical community awareness regarding acute clinical manifestations that provide rapid diagnosis facilitation, manageable treatment protocol requirements, and established enzyme replacement therapy accessibility without requiring experimental therapeutic environments.
Advanced newborn screening technologies enable earlier patient identification, genetic confirmation acceleration, and integration with existing metabolic disorder detection infrastructure, where clinical urgency and therapeutic intervention timing represent critical patient outcome determinants. Infantile-onset presentations hold 35.7% share within the Wolman disease segment, appealing to neonatal intensive care specialists seeking immediate therapeutic intervention and comprehensive metabolic crisis management.
Wolman disease variants differentiate through proven clinical recognition patterns, extensive metabolic specialist awareness, and integration with established rare disease diagnostic frameworks that enhance treatment initiation confidence while maintaining optimal therapeutic timing standards for diverse geographic and healthcare capability applications.
Key market characteristics:
- Advanced genetic screening technology with optimized enzymatic activity measurement and molecular confirmation capabilities
- Superior clinical urgency recognition, enabling rapid diagnosis establishment with characteristic presentation pattern identification
- Healthcare infrastructure compatibility, including metabolic disorder center integration, neonatal intensive care coordination, and rare disease specialist consultation for critical intervention applications
Why does Cholesteryl Ester Storage Disease (CESD) Represent a Growing Late-Onset Segment?
Cholesteryl ester storage disease (CESD) maintains substantial growth momentum with 4.9% CAGR due to its expanding diagnostic recognition and improved therapeutic accessibility.
These clinical manifestations appeal to adult metabolic specialists and primary care physicians recognizing non-specific presentations including hepatomegaly and dyslipidemia, offering treatment opportunity through established enzyme replacement protocols and clinical monitoring frameworks.
Market growth is driven by late-onset CESD awareness advancement, emphasizing diagnostic consideration platforms and therapeutic intervention accessibility through comprehensive patient identification efforts capturing 21.6% within the indication segment.
Which Treatment Type is Preferred for Lysosomal Acid Lipase Deficiency?
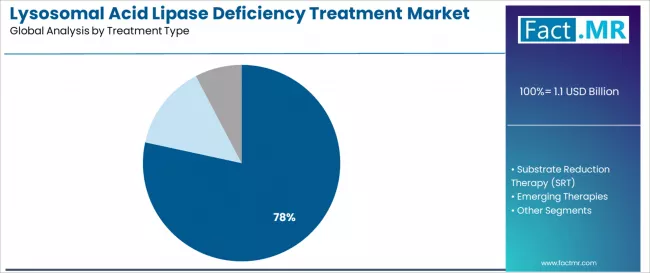
Enzyme replacement therapy (ERT) configurations demonstrate treatment modality leadership in the lysosomal acid lipase deficiency market with a 78.4% share due to widespread regulatory a7oval establishment and sustained focus on proven clinical efficacy, FDA/EMA validation achievement, and demonstrated therapeutic outcomes that maximize patient accessibility while maintaining acceptable safety profile standards.
Healthcare providers prioritize treatment validation credibility, reimbursement coverage availability, and integration with existing rare disease treatment infrastructure that enables coordinated care delivery experiences across diverse healthcare markets. The segment benefits from substantial clinical trial evidence accumulation and real-world effectiveness data maturity that emphasize enzyme replacement therapy selection for therapeutic reliability and outcome predictability applications.
Sebelipase alfa formulations capture 64.1% share within the ERT segment, demonstrating healthcare provider preference for regulatory-validated therapeutic platforms with comprehensive clinical evidence support. Orphan drug designation programs incorporate accelerated a7oval pathways as standard regulatory frameworks for rare disease therapeutic development, while patient advocacy initiatives increase demand for treatment access capabilities that comply with clinical effectiveness standards and minimize disease progression risk exposure.
What drives Substrate Reduction Therapy Development in Experimental Protocols?
Substrate reduction therapy captures 5.2% CAGR through comprehensive therapeutic innovation requirements in novel mechanism exploration, small molecule development advancement, and clinical trial protocol establishment.
These therapeutic a7oaches demand robust preclinical validation capable of demonstrating mechanism feasibility while providing effective pathway modulation characteristics and acceptable toxicity profiles, appealing to research-focused pharmaceutical companies and academic medical centers seeking evidence-based therapeutic alternative development beyond enzyme replacement limitations.
What are the Drivers, Restraints, and Key Trends of the Lysosomal Acid Lipase Deficiency Treatment Market?
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Newborn screening expansion & genetic testing advancement (early detection, comprehensive panels) | ★★★★★ | Large-scale screening implementation enables earlier patient identification for timely intervention; diagnostic technology improvement shifting toward comprehensive metabolic disorder detection drives treatment demand across neonatal care environments. |
| Driver | Orphan drug designation benefits & regulatory incentives (accelerated a7oval, market exclusivity) | ★★★★★ | Drives pharmaceutical investment in rare disease therapeutic development and clinical trial execution; manufacturers receiving regulatory incentives gain competitive advantage in specialized treatment market segments. |
| Driver | Patient advocacy effectiveness & rare disease awareness (community engagement, education programs) | ★★★★☆ | Healthcare providers and patients demand improved diagnostic recognition and treatment accessibility; advocacy advancement expanding addressable patient populations beyond historically underdiagnosed rare disease demographics. |
| Restraint | High treatment costs & reimbursement challenges (payer negotiation, budget impact) | ★★★★★ | Manufacturers face healthcare system resistance and coverage limitation scrutiny; increases market access complexity and affects patient treatment initiation in cost-constrained healthcare environments. |
| Restraint | Limited patient population & diagnostic underrecognition (rare disease prevalence, awareness gaps) | ★★★☆☆ | Therapeutic developers face commercial viability concerns and clinical trial recruitment challenges, limiting market expansion potential and affecting research investment in ultra-rare metabolic disorder categories. |
| Trend | Gene therapy development & curative a7oaches (genetic correction, one-time intervention) | ★★★★★ | Growing research expectation for permanent therapeutic solutions beyond chronic enzyme replacement maintenance; gene therapy advancement becomes core differentiation strategy for future treatment paradigm transformation. |
| Trend | Patient registry expansion & real-world evidence (outcome tracking, treatment optimization) | ★★★★☆ | Rare disease management evolving beyond isolated treatment episodes toward comprehensive longitudinal care coordination; registry integration drives improved clinical decision-making and regulatory a7oval support in specialized patient populations. |
Analysis of the Lysosomal Acid Lipase Deficiency Treatment Market by Key Countries
The lysosomal acid lipase deficiency treatment market demonstrates robust regional growth dynamics with established leaders including USA (4.9% CAGR) and Germany (4.8% CAGR) driving expansion through comprehensive regulatory frameworks and specialized treatment infrastructure.
Strong performers encompass Japan (4.6% CAGR), UK (4.5% CAGR), and China (4.4% CAGR), benefiting from advancing diagnostic capabilities and rare disease policy development. Emerging markets feature India (4.3% CAGR) and Brazil (4.2% CAGR), where genetic testing expansion and public health program integration support consistent growth patterns.
Regional synthesis reveals developed markets leading treatment accessibility through comprehensive orphan drug frameworks and specialized metabolic disorder center networks, while emerging countries demonstrate accelerated growth potential supported by diagnostic infrastructure development and international clinical research collaboration. European markets show solid advancement driven by patient registry integration and systematic rare disease management a7oaches.
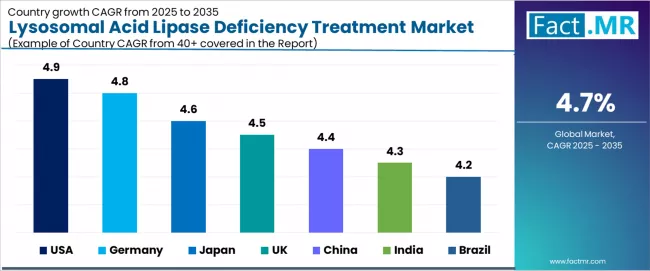
| Region/Country | 2025-2035 Growth | How to win | What to watch out |
|---|---|---|---|
| USA | 4.9% | Focus on orphan drug pathway optimization | Reimbursement complexity; payer restrictions |
| Germany | 4.8% | Lead with patient registry integration | Healthcare budget constraints; regional variation |
| Japan | 4.6% | Offer regulatory-validated therapies | Conservative adoption patterns; demographic challenges |
| UK | 4.5% | Provide NHS framework alignment | Budget impact assessments; commissioning barriers |
| China | 4.4% | Push diagnostic capability development | Market access hurdles; regulatory evolution |
| India | 4.3% | Deliver clinical collaboration platforms | Infrastructure limitations; affordability challenges |
| Brazil | 4.2% | Maintain public program engagement | Economic volatility; healthcare system constraints |
What establishes USA's Market Leadership in Lysosomal Acid Lipase Deficiency Treatment?
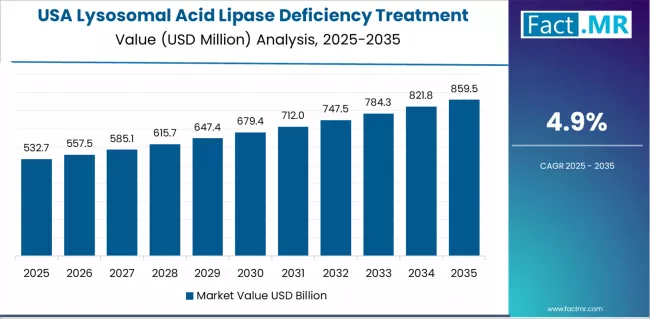
The USA establishes market leadership in the lysosomal acid lipase deficiency treatment sector with 4.9% CAGR due to comprehensive orphan drug regulatory framework and sustained focus on rare disease therapeutic incentivization, specialized treatment center development, and patient advocacy organization strength that maximizes treatment accessibility while maintaining innovation encouragement standards.
Healthcare providers and pharmaceutical manufacturers prioritize FDA a7oval pathway clarity, orphan drug designation benefits, and integration with established rare disease insurance coverage frameworks that enables coordinated treatment commercialization across multiple stakeholder categories. The region benefits from substantial clinical research infrastructure and patient registry development expertise that emphasizes the advancement of validated therapeutic platforms for metabolic disorder intervention and rare disease management applications.
Federal rare disease programs incorporate research funding as standard healthcare priorities for unmet medical need addressing, while growing patient advocacy effectiveness increases demand for treatment development capabilities that meet clinical validation requirements and ensure equitable patient access across diverse geographic regions.
Regional dynamics include:
- Strong growth in specialized metabolic disorder centers with comprehensive enzyme replacement therapy administration capabilities
- Increasing adoption of genetic counseling services for family screening and carrier identification initiatives
- Rising integration with patient assistance programs for treatment cost mitigation and insurance navigation support
How is Germany Advancing in the Lysosomal Acid Lipase Deficiency Treatment Market?
Germany captures a 4.8% CAGR supported by structured rare disease governance frameworks that facilitate consistent diagnostic and treatment coordination across federal and regional healthcare systems. University hospitals maintain established metabolic disorder centers that integrate enzyme replacement therapy into standard clinical pathways while ensuring adherence to national treatment quality benchmarks. The country benefits from active participation in European rare disease networks, which strengthens clinical data exchange and supports regular updates to diagnostic algorithms.
Progress in Germany is also linked to a strong genetic testing ecosystem anchored by accredited molecular laboratories with validated sequencing capabilities. These facilities support early identification of lysosomal acid lipase deficiency through widespread clinician awareness and standardized testing protocols embedded into metabolic disorder evaluation guidelines. National insurance coverage structures maintain predictable reimbursement procedures that enable stable therapeutic access for eligible patients within specialized hospital settings.
Germany additionally demonstrates methodical regulatory alignment with European Medicines Agency requirements, enabling timely integration of a7oved rare disease therapies. Continuous investment in patient registries enhances longitudinal data collection, supporting outcome monitoring and informing clinical practice updates. Collaborative research relationships between academic institutions and international partners contribute to sustained improvement in diagnostic precision, treatment consistency, and real-world evidence generation within the lysosomal acid lipase deficiency treatment environment.
Why is Japan Positioned as an Advanced Metabolic Disorder Market?
Japan holds a 4.6% CAGR with distinctive market maturity indicators driven by structured rare disease designation programs and coordinated academic medical leadership. The country maintains well-developed genetic testing pathways supported by hospital-based molecular diagnostic laboratories, enabling early identification of lysosomal acid lipase deficiency within defined referral networks. Enzyme therapy utilization aligns with national clinical guidance, ensuring consistent treatment delivery under specialized metabolic departments.
Japan’s regulatory environment features harmonized review pathways that facilitate predictable therapy evaluation and post-marketing oversight for rare metabolic disorders. Institutional participation in international clinical research networks encourages knowledge exchange and supports continual updates to diagnostic criteria and therapeutic monitoring practices. Medical centers with research mandates maintain comprehensive patient follow-up systems that reinforce high adherence and reporting standards.
Further growth in Japan is linked to expanding clinician education programs and structured collaborations between government agencies, academic institutes, and pharmaceutical developers. These partnerships help maintain strong diagnostic uptake and reinforce early intervention strategies across major urban healthcare systems. The country’s patient identification efficiency reflects established integration of screening algorithms into tertiary hospital workflows, supporting continued advancement in metabolic disorder treatment capabilities.
What Drives UK's Genetic Testing Leadership?
The UK establishes comprehensive genetic testing integration through progressive rare disease framework implementation and documented effectiveness in newborn screening program expansion across National Health Service infrastructure and specialized metabolic medicine departments. The country leverages established healthcare system coordination and patient registry development expertise to maintain 4.5% growth momentum.
Clinical centers, including London teaching hospitals and regional metabolic units, showcase systematic diagnostic a7oaches where lysosomal acid lipase deficiency identification integrates with comprehensive rare disease strategies and genetic counseling services to optimize patient outcomes and ensure treatment accessibility under evolving NHS specialized commissioning frameworks.
British healthcare providers prioritize systematic diagnostic protocols and evidence-based treatment pathways in clinical decision-making, creating demand for validated enzyme replacement therapies with comprehensive clinical effectiveness documentation, including long-term outcome evidence combined with health economic value assessment and patient quality-of-life considerations. The market benefits from established rare disease patient organizations and clinical research networks that provide treatment optimization opportunities and compliance with UK rare disease framework requirements.
How does China Demonstrate Emerging Rare Disease Framework Development?
China’s 4.4% CAGR reflects systematic efforts to strengthen rare disease recognition through national policy development and expanding specialized hospital networks. Government initiatives support the establishment of rare disease lists and coordinated diagnostic pathways in major metropolitan centers, improving lysosomal acid lipase deficiency identification rates within evolving clinical infrastructure. Modernization of tertiary hospitals continues to enhance testing availability and metabolic disorder management capacity.
Diagnostic progress in China is supported by growing access to genetic sequencing platforms, particularly in large urban hospitals with dedicated molecular diagnostics units. These facilities increasingly adopt standardized workflows that integrate lysosomal acid lipase deficiency assessment into broader metabolic evaluation protocols. International collaboration in clinical research settings deepens clinical exposure to enzyme replacement therapies and supports gradual refinement of treatment practices.
China’s treatment landscape continues to mature as imported rare disease therapies gain controlled access within specialized institutions. Integration of multidisciplinary care, including hepatology and metabolic genetics, strengthens follow-up quality and enables consistent monitoring of long-term treatment responses. Although infrastructure development remains uneven across regions, leading hospitals demonstrate clear progress in aligning with global rare disease management standards.
Why does India Show Growing Clinical Research Collaboration?
India maintains a 4.3% CAGR supported by expanding genetic testing accessibility and increasing adoption of standardized diagnostic workflows for metabolic disorders. Leading academic centers implement structured protocols for lysosomal acid lipase deficiency assessment, supported by clinician training programs focused on rare disease recognition. Growing awareness among hepatology and pediatric metabolic specialists enhances early referral and testing consistency.
Clinical research collaboration plays a central role in India’s market development, with several academic hospitals participating in multinational studies involving rare metabolic disorders. These collaborations strengthen institutional knowledge and contribute to systematic updates in diagnostic and therapeutic decision-making. Improved laboratory infrastructure, particularly in major cities, supports broader availability of sequencing technologies relevant to lysosomal acid lipase deficiency detection.
Government engagement through rare disease policy frameworks creates long-term opportunities for structured patient support systems and improved access mechanisms. As reimbursement pathways evolve, manufacturers explore engagement with healthcare institutions to align treatment availability with growing middle-class healthcare access patterns. Despite variability in regional healthcare capacity, leading hospitals maintain stable progress in developing metabolic disorder management competencies.
What drives Brazil's Public Health Program Integration?
Brazil maintains a 4.2% CAGR driven by selective integration of rare disease treatments within the SUS public healthcare framework and specialized state-level initiatives. Reference centers across major cities provide structured diagnostic pathways for lysosomal acid lipase deficiency, supported by genetic counseling services and trained metabolic disorder teams. Federal policy development promotes gradual expansion of rare disease service capacity under monitored resource allocation mechanisms.
Diagnostic capability improvements in Brazil are linked to institutional investment in molecular laboratories and the establishment of standardized metabolic evaluation workflows. Academic hospitals with specialized research units participate in collaborative studies that enhance understanding of disease progression and treatment outcomes. These facilities maintain structured relationships with patient advocacy groups, enabling community-based support and awareness initiatives that contribute to earlier identification.
Brazil’s a7oach to treatment availability emphasizes regulated incorporation of enzyme replacement therapies within defined clinical protocols, balancing therapeutic need with public sector resource constraints. Hospitals integrate multidisciplinary care models that coordinate hepatology, genetics, and metabolic medicine expertise for comprehensive patient management. Continued policy refinement and institutional collaboration support stable progress in rare disease treatment quality across the national healthcare landscape.
Europe Market Split by Country
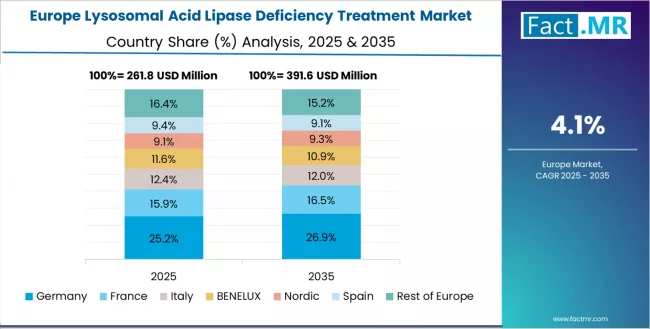
The lysosomal acid lipase deficiency treatment market in Europe is projected to grow from USD 0.34 billion in 2025 to USD 0.54 billion by 2035, registering a CAGR of 4.6% over the forecast period. Germany is expected to maintain its leadership position with a 26.8% market share in 2025, supported by its advanced rare disease infrastructure and comprehensive patient registry systems across specialized metabolic disorder centers.
France follows with a 18.9% share in 2025, driven by comprehensive orphan drug access programs and enzyme replacement therapy integration in university hospital networks. The UK holds a 17.3% share in 2025 through established genetic testing infrastructure and National Health Service rare disease framework implementation.
Italy commands a 14.2% share, while Spain accounts for 11.6% in 2025. The Rest of Europe region maintains 11.2% of the European market, attributed to increasing rare disease awareness in Nordic countries and emerging Eastern European healthcare systems implementing metabolic disorder diagnostic capability enhancement programs.
Competitive Landscape of the Lysosomal Acid Lipase Deficiency Treatment Market
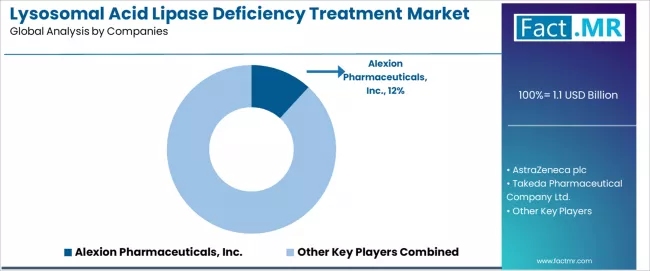
The lysosomal acid lipase deficiency treatment market exhibits a moderately fragmented competitive structure with a7oximately 30-50 active players operating across global pharmaceutical development networks and specialized rare disease portfolios. Alexion Pharmaceuticals Inc. maintains market leadership at an 11.6% share, reflecting strong regulatory positioning across enzyme replacement therapy segments with sophisticated orphan drug development strategies.
This competitive landscape demonstrates the specialization of rare disease therapeutic manufacturing, where established players leverage regulatory expertise, clinical trial execution capabilities, and patient access program development to maintain competitive positions, while emerging gene therapy developers and substrate reduction innovators create differentiation opportunities through novel mechanism exploration and curative a7oach advancement.
Market leadership is maintained through several critical competitive advantages extending beyond manufacturing capabilities and therapeutic formulations. Orphan drug regulatory expertise enables leading players to navigate complex a7oval pathways and access specialized incentive programs including market exclusivity, tax credits, and accelerated review designations across multiple jurisdictions.
Clinical trial execution proficiency and patient recruitment network development represent crucial differentiators in rare disease categories, where limited patient populations, diagnostic challenges, and specialized endpoint selection create substantial barriers for competitors lacking rare disease development experience.
Manufacturing capacity for specialized biologic production, cold chain distribution infrastructure, and regulatory compliance maintenance separate major pharmaceutical companies from smaller biotechnology firms, while comprehensive patient support programs addressing treatment access, reimbursement navigation, and clinical monitoring strengthen market position and support patient retention throughout chronic therapy administration.
The market demonstrates emerging differentiation opportunities in gene therapy categories and substrate reduction a7oaches, where traditional enzyme replacement therapy dominance faces potential disruption from curative modality developers and oral therapeutic innovators offering administration convenience advantages.
Significant competitive advantages persist in established enzyme replacement categories through proven clinical efficacy and regulatory validation. Premium gene therapy platforms with one-time administration models and genetic correction mechanisms command substantial value propositions through curative potential and lifetime treatment burden elimination.
Substrate reduction therapy segments combining oral administration convenience with disease pathway modulation create patient preference positioning that justifies development investment beyond established enzyme replacement competition. Next-generation enzyme formulations emphasizing extended dosing intervals, enhanced tissue penetration, and improved pharmacokinetic profiles generate clinical differentiation and patient experience advantages beyond first-generation therapeutic competition.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Established rare disease pharmaceutical companies | Regulatory expertise; orphan drug development; patient access programs | Clinical trial execution; market access navigation; specialty distribution | Innovation speed; curative modality development; emerging market expansion |
| Gene therapy developers | Novel mechanism platforms; curative a7oach positioning; academic partnerships | Differentiation potential; premium value capture; regulatory breakthrough designation | Manufacturing scale; commercial infrastructure; long-term safety data |
| Substrate reduction innovators | Oral administration convenience; small molecule expertise; pathway modulation | Patient preference advantages; development cost efficiency; manufacturing simplicity | Clinical efficacy validation; mechanism uncertainty; competitive differentiation |
| Academic medical centers | Patient registries; natural history studies; clinical expertise | Disease understanding; diagnostic capability; clinical trial site access | Commercial development; manufacturing capability; global market reach |
| Patient advocacy organizations | Community engagement; awareness campaigns; research funding | Patient identification; treatment demand generation; policy influence | Therapeutic development; clinical trial execution; regulatory navigation |
Key Players in the Lysosomal Acid Lipase Deficiency Treatment Market
- Alexion Pharmaceuticals, Inc.
- AstraZeneca plc
- Takeda Pharmaceutical Company Ltd.
- Amicus Therapeutics, Inc.
- Chiesi Farmaceutici S.p.A
- Orphan Europe SARL
- Sanofi S.A.
- Moderna Therapeutics Inc.
- Ultragenyx Pharmaceutical Inc.
- Regenxbio Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Value (USD Million)s (2025) | USD 1.12 billion |
| Indication Type | Wolman Disease, Cholesteryl Ester Storage Disease (CESD) |
| Treatment Type | Enzyme Replacement Therapy (ERT), Substrate Reduction Therapy (SRT) |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | USA, Germany, Japan, UK, China, India, Brazil, and 15+ additional countries |
| Key Companies Profiled | Alexion Pharmaceuticals Inc., AstraZeneca plc, Takeda Pharmaceutical Company Ltd., Amicus Therapeutics Inc., Chiesi Farmaceutici S.p.A, Orphan Europe SARL, Sanofi S.A., Moderna Therapeutics Inc. |
| Additional Attributes | Dollar sales by indication and treatment type categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with established rare disease pharmaceutical companies and emerging gene therapy developers, healthcare provider preferences for proven enzyme replacement therapy and innovative curative modalities, integration with specialized metabolic disorder centers and patient registry systems, innovations in gene therapy technology and substrate reduction a7oaches, and development of optimized therapeutic platforms with enhanced clinical efficacy profiles and improved patient administration convenience. |
Lysosomal Acid Lipase Deficiency Treatment Market by Segments
-
Indication Type:
- Wolman Disease
- Infantile-Onset Wolman Disease
- Classic Wolman Disease Presentations
- Cholesteryl Ester Storage Disease (CESD)
- Late-Onset CESD
- Adult-Diagnosed CESD
- Wolman Disease
-
Treatment Type :
- Enzyme Replacement Therapy (ERT)
- Sebelipase Alfa
- Next-Generation Enzyme Formulations
- Substrate Reduction Therapy (SRT)
- Experimental Small Molecules
- Pathway Modulation A7oaches
- Emerging Therapies
- Gene Therapy A7oaches
- Novel Mechanism Platforms
- Enzyme Replacement Therapy (ERT)
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- Japan
- China
- India
- South Korea
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Indication Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Indication Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Indication Type, 2025 to 2035
- Wolman Disease
- Cholesteryl Ester Storage Disease (CESD)
- Y to o to Y Growth Trend Analysis By Indication Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Indication Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Treatment Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Treatment Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Treatment Type, 2025 to 2035
- Enzyme Replacement Therapy (ERT)
- Substrate Reduction Therapy (SRT)
- Emerging Therapies
- Y to o to Y Growth Trend Analysis By Treatment Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Treatment Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Indication Type
- By Treatment Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Indication Type
- By Treatment Type
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Indication Type
- By Treatment Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Indication Type
- By Treatment Type
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Indication Type
- By Treatment Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Indication Type
- By Treatment Type
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Indication Type
- By Treatment Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Indication Type
- By Treatment Type
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Indication Type
- By Treatment Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Indication Type
- By Treatment Type
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Indication Type
- By Treatment Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Indication Type
- By Treatment Type
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Indication Type
- By Treatment Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Indication Type
- By Treatment Type
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Indication Type
- By Treatment Type
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Indication Type
- By Treatment Type
- Competition Analysis
- Competition Deep Dive
- Alexion Pharmaceuticals, Inc.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- AstraZeneca plc
- Takeda Pharmaceutical Company Ltd.
- Amicus Therapeutics, Inc.
- Chiesi Farmaceutici S.p.A
- Orphan Europe SARL
- Sanofi S.A.
- Moderna Therapeutics Inc.
- Ultragenyx Pharmaceutical Inc.
- Regenxbio Inc.
- Alexion Pharmaceuticals, Inc.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Indication Type , 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Indication Type , 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Indication Type , 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Indication Type , 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Indication Type , 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Indication Type , 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Indication Type , 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Indication Type , 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Indication Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Indication Type, 2025-2035
- Figure 5: Global Market Attractiveness Analysis by Indication Type
- Figure 6: Global Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Treatment Type, 2025-2035
- Figure 8: Global Market Attractiveness Analysis by Treatment Type
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025-2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025-2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025-2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025-2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025-2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Indication Type, 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Indication Type, 2025-2035
- Figure 22: North America Market Attractiveness Analysis by Indication Type
- Figure 23: North America Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Treatment Type, 2025-2035
- Figure 25: North America Market Attractiveness Analysis by Treatment Type
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Indication Type, 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Indication Type, 2025-2035
- Figure 29: Latin America Market Attractiveness Analysis by Indication Type
- Figure 30: Latin America Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by Treatment Type, 2025-2035
- Figure 32: Latin America Market Attractiveness Analysis by Treatment Type
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Indication Type, 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Indication Type, 2025-2035
- Figure 36: Western Europe Market Attractiveness Analysis by Indication Type
- Figure 37: Western Europe Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by Treatment Type, 2025-2035
- Figure 39: Western Europe Market Attractiveness Analysis by Treatment Type
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Indication Type, 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Indication Type, 2025-2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Indication Type
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by Treatment Type, 2025-2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by Treatment Type
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Indication Type, 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Indication Type, 2025-2035
- Figure 50: East Asia Market Attractiveness Analysis by Indication Type
- Figure 51: East Asia Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by Treatment Type, 2025-2035
- Figure 53: East Asia Market Attractiveness Analysis by Treatment Type
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Indication Type, 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Indication Type, 2025-2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Indication Type
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by Treatment Type, 2025-2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by Treatment Type
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Indication Type, 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Indication Type, 2025-2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Indication Type
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by Treatment Type, 2025-2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by Treatment Type
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the lysosomal acid lipase deficiency treatment market in 2025?
The global lysosomal acid lipase deficiency treatment market is estimated to be valued at USD 1.1 billion in 2025.
What will be the size of lysosomal acid lipase deficiency treatment market in 2035?
The market size for the lysosomal acid lipase deficiency treatment market is projected to reach USD 1.8 billion by 2035.
How much will be the lysosomal acid lipase deficiency treatment market growth between 2025 and 2035?
The lysosomal acid lipase deficiency treatment market is expected to grow at a 4.7% CAGR between 2025 and 2035.
What are the key product types in the lysosomal acid lipase deficiency treatment market?
The key product types in lysosomal acid lipase deficiency treatment market are wolman disease and cholesteryl ester storage disease (cesd).
Which treatment type segment to contribute significant share in the lysosomal acid lipase deficiency treatment market in 2025?
In terms of treatment type, enzyme replacement therapy (ert) segment to command 78.4% share in the lysosomal acid lipase deficiency treatment market in 2025.