Bioconjugation Market
Bioconjugation Market Size and Share Forecast Outlook 2025 to 2035
Bioconjugation market is projected to grow from USD 6.1 billion in 2025 to USD 14.7 billion by 2035, at a CAGR of 9.2%. Consumables will dominate with a 45.5% market share, while antibodies will lead the biomolecule type segment with a 52.4% share.
Bioconjugation Market Forecast and Outlook 2025 to 2035
The global bioconjugation market is projected to reach USD 14.7 billion by 2035, recording an absolute increase of USD 8.6 billion over the forecast period. The market is valued at USD 6.1 billion in 2025 and is set to rise at a CAGR of 9.2% during the assessment period.
Quick Stats for Bioconjugation Market
- Bioconjugation Market Value (2025): USD 6.1 billion
- Bioconjugation Market Forecast Value (2035): USD 14.7 billion
- Bioconjugation Market Forecast CAGR: 9.2%
- Leading Product & Service in Bioconjugation Market: Consumables
- Key Growth Regions in Bioconjugation Market: North America, Europe, and Asia Pacific
- Top Players in Bioconjugation Market: Thermo Fisher Scientific, Danaher, Lonza Group, Merck KGaA (MilliporeSigma), Sartorius AG, Abbvie, Inc., Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., Catalent, Inc., BD
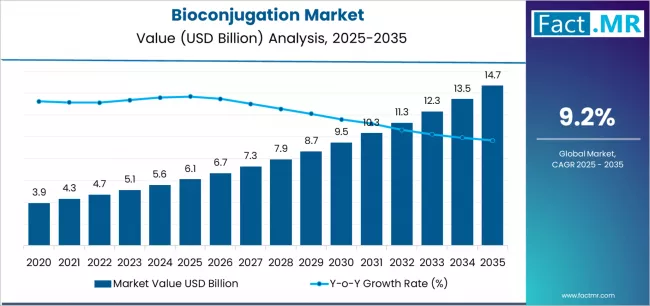
The overall market size is expected to grow by approximately 2.4 times during the same period, supported by increasing development of antibody-drug conjugates and rising demand for targeted therapeutic delivery systems worldwide, driving investments in advanced conjugation technologies and precision medicine innovations globally.
The pharmaceutical and biotechnology sectors face mounting pressure to improve drug efficacy while meeting evolving requirements for site-specific conjugation and stable linker chemistry, with modern bioconjugation techniques providing documented payload delivery optimization and therapeutic index enhancement capabilities compared to traditional drug development approaches.
Rising biologics commercialization and expanding contract manufacturing infrastructure development across emerging economies create substantial opportunities for reagent manufacturers and service providers. However, technical complexity in conjugation chemistry optimization and regulatory challenges for novel conjugate products may pose obstacles to market expansion.
The consumables segment dominates market activity with approximately 45.5% share, driven by the extensive life sciences research base requiring specialized reagents and kits with proven conjugation performance across pharmaceutical development and diagnostic applications worldwide. Researchers and drug developers increasingly recognize the practical benefits of ready-to-use conjugation reagents, with typical product offerings providing reliable crosslinking chemistry and reproducible conjugate formation at competitive pricing through established scientific supply networks.
The services segment demonstrates the fastest growth potential with a CAGR of 11.7%, supported by rising outsourcing trends and biopharmaceutical company preference for specialized custom synthesis and conjugation services in antibody-drug conjugate manufacturing. Antibodies emerge as the critical biomolecule category with 52.4% share, reflecting pharmaceutical emphasis on antibody-based therapeutics and immunoconjugate development. Therapeutics applications represent the dominant end-use segment with 57.8% share, driven by antibody-drug conjugate commercialization requirements and targeted oncology therapy development across diverse cancer indications.
Regional dynamics show North America maintaining market leadership supported by robust pharmaceutical R&D infrastructure and extensive antibody-drug conjugate pipeline activity across the USA and Canada. Europe demonstrates strong innovation momentum driven by contract development and manufacturing expansion and advanced biologics expertise, while Asia Pacific emphasizes biosimilar development and emerging bioconjugation service capacity.
The USA leads country-level activity through comprehensive pharmaceutical innovation ecosystem and regulatory pathway establishment for conjugated therapeutics. The competitive landscape features moderate concentration with Thermo Fisher Scientific holding a 9.2% market share, while established players including Danaher, Lonza Group, and Merck KGaA compete through comprehensive product portfolios and integrated service capabilities across diverse bioconjugation applications.
Bioconjugation Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the bioconjugation market is projected to expand from USD 6.1 billion to USD 8.7 billion, resulting in a value increase of USD 2.6 billion, which represents 30.2% of the total forecast growth for the period. This phase of development will be shaped by rising adoption of site-specific conjugation technologies and enzyme-mediated coupling methods in antibody-drug conjugate development, regulatory approvals for next-generation ADC products with improved therapeutic windows, as well as expanding integration with click chemistry platforms and bioorthogonal reaction systems. Companies are establishing competitive positions through investment in specialized reagent development, custom conjugation service capabilities, and strategic partnerships across pharmaceutical manufacturing, contract research, and academic collaboration networks.
From 2029 to 2035, the market is forecast to grow from USD 8.7 billion to USD 14.7 billion, adding another USD 6.0 billion, which constitutes 69.8% of the overall expansion. This period is expected to be characterized by the expansion of oligonucleotide conjugation applications for RNA therapeutics and gene editing delivery systems, strategic collaborations between bioconjugation technology providers and pharmaceutical companies for proprietary conjugate platform development, and an enhanced focus on GMP-compliant manufacturing services and scalable conjugation process development. The growing emphasis on diagnostics applications including companion diagnostics development and rising adoption of bioorthogonal chemistry approaches will drive demand for comprehensive bioconjugation solutions across diverse research and commercial applications.
Bioconjugation Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 6.1 billion |
| Market Forecast Value (2035) | USD 14.7 billion |
| Forecast CAGR (2025-2035) | 9.2% |
Why is the Bioconjugation Market Growing?
The bioconjugation market grows by enabling pharmaceutical researchers and biotechnology companies to create targeted therapeutic constructs while accessing advanced crosslinking chemistries without substantial process development complexity requirements.
Drug developers and protein engineers face mounting pressure to improve therapeutic targeting and reduce systemic toxicity while managing diverse payload attachment needs across antibody scaffolds and biomolecular platforms, with modern bioconjugation technologies typically providing superior site-specificity and linker stability benefits compared to traditional random conjugation methodologies, making technology adoption essential for competitive drug development positioning.
The biologics industry's need for precise molecular engineering and payload-specific coupling capabilities creates demand for comprehensive bioconjugation solutions that can provide reproducible conjugate formation, maintain protein functionality, and ensure stable therapeutic performance without compromising drug-like properties or manufacturability standards.
Pharmaceutical industry initiatives emphasizing antibody-drug conjugate development and targeted therapy innovation drive adoption in drug discovery laboratories, biopharmaceutical manufacturing facilities, and contract service organizations, where conjugation efficiency has a direct impact on therapeutic efficacy and commercial viability.
The success of approved antibody-drug conjugates in oncology has created sustained interest in conjugation platform technologies, supporting ongoing demand for specialized reagents and services across all therapeutic development stages.
Rising investments in biologics manufacturing capacity and biosimilar development enable greater access to conjugation technologies with proven scalability and regulatory acceptability characteristics. However, intellectual property constraints around proprietary conjugation platforms and technical challenges in achieving homogeneous conjugate formation may limit accessibility of advanced site-specific technologies among organizations with constrained licensing budgets for sophisticated conjugation methodologies.
Segmental Analysis
The market is segmented by product & services, biomolecule type, technique, application, end use, and region. By product & services, the market is divided into consumables, instruments, and services. Based on biomolecule type, the market is categorized into antibodies, proteins, peptides, oligonucleotides, and other biomolecules. By technique, the market includes chemical conjugation, enzyme-mediated conjugation, click chemistry, photoreactive crosslinking, and other techniques.
By application, the market encompasses therapeutics, diagnostics, and research & development. By end use, the market includes pharmaceutical & biotechnology companies, CROs & CMOs, academic & research institutes, and hospitals, clinical & diagnostic laboratories. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
By Product & Services, Which Segment Accounts for the Dominant Market Share?
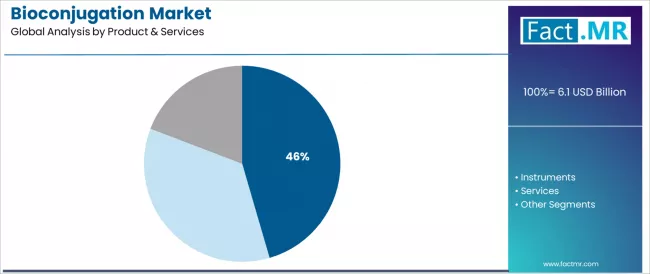
The consumables segment represents the dominant force in the bioconjugation market, capturing approximately 45.5% of total market share. This established product category encompasses reagents and kits featuring proven crosslinking chemistry and biocompatible linker systems, including advanced coupling reagents and specialized conjugation buffers that enable reliable bioconjugate formation and consistent experimental reproducibility across all research and development applications.
The consumables segment's market leadership stems from recurring purchase patterns in research workflows, with products capable of addressing diverse conjugation requirements while maintaining straightforward protocols and broad compatibility across all biomolecular coupling scenarios.
Within the consumables segment, reagents and kits dominate usage patterns, driven by researcher preference for ready-to-use formulations where product standardization complements routine conjugation workflows in pharmaceutical and academic laboratories. This subsegment benefits from established supplier relationships and comprehensive technical documentation supporting method optimization in conjugation applications.
The instruments segment maintains meaningful market presence, serving laboratories requiring automated conjugation workstations and analytical instrumentation for conjugate characterization including mass spectrometry systems and chromatography equipment for purity assessment.
The services segment demonstrates the fastest growing category with a CAGR of 11.7%, driven by pharmaceutical company preference for outsourcing complex conjugation development to specialized contract service providers. These solutions offer custom synthesis capabilities and GMP manufacturing support while providing sufficient expertise to meet stringent regulatory and quality requirements. The services segment demonstrates exceptional growth potential, driven by expanding antibody-drug conjugate pipeline activity and increasing outsourcing of specialized bioconjugation processes beyond internal capabilities.
Within the services segment, custom synthesis and conjugation services command dominant adoption, driven by biopharmaceutical company needs for specialized conjugate manufacturing that requires sophisticated chemistry expertise and GMP-compliant production infrastructure unavailable in typical drug development organizations.
Key technological advantages driving the consumables segment include:
- Comprehensive reagent portfolios with validated crosslinking chemistries that enable diverse conjugation strategies and ensure reproducible results across experimental conditions
- Established manufacturing processes allowing reliable supply across different product formats without extensive batch-to-batch variability concerns
- Enhanced kit convenience features enabling streamlined conjugation workflows while maintaining protocol flexibility and troubleshooting support
- Superior cost-effectiveness providing optimal price-performance balance for various research budgets and high-throughput screening requirements
By Biomolecule Type, Which Segment Accounts for the Largest Market Share?
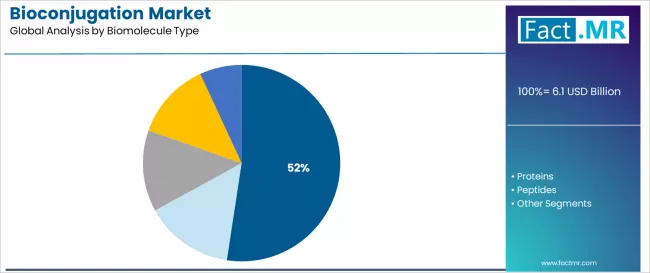
Antibodies dominate the bioconjugation market landscape with approximately 52.4% market share, reflecting the central role of antibody-based therapeutics in driving bioconjugation technology development across pharmaceutical research and commercial manufacturing worldwide. The antibodies segment's market leadership is reinforced by extensive antibody-drug conjugate pipeline activity, established therapeutic validation, and versatile scaffold properties combined with proven targeting capabilities in oncology and immunology applications.
Within the biomolecule landscape, antibodies maintain dominance through their combination of specific antigen recognition, favorable pharmacokinetic properties, and well-characterized conjugation sites that enable therapeutic payload delivery. This biomolecule class benefits from decades of antibody engineering advancement and comprehensive manufacturing infrastructure supporting scalable conjugate production.
The proteins segment represents an important biomolecule category, serving researchers requiring conjugation of enzymes, growth factors, and other functional proteins for diagnostic reagent development, biosensor fabrication, and targeted protein delivery applications. Peptides maintain meaningful presence through therapeutic peptide conjugation and cell-penetrating peptide delivery system development.
The oligonucleotides segment demonstrates the fastest growing category with a CAGR of 12.0%, showing exceptional expansion through specialized requirements for RNA therapeutic delivery, antisense oligonucleotide targeting, and nucleic acid-based diagnostic probe development. This segment benefits from RNA therapeutics advancement and technical innovation enabling efficient conjugation to lipophilic moieties and targeting ligands.
The other biomolecules segment encompasses polysaccharides, lipids, small molecule drugs, and synthetic polymers requiring bioconjugation for various biomedical applications including vaccine development and drug delivery system construction.
Key market dynamics supporting biomolecule segmentation include:
- Antibody conjugate expansion driven by antibody-drug conjugate commercial success and continuing pipeline development across oncology and other therapeutic areas
- Oligonucleotide conjugation growth acceleration trends require enabling delivery technologies for RNA therapeutics and gene editing applications
- Integration of multi-specific antibody formats enabling complex conjugate architectures through complementary binding specificities and enhanced functional capabilities
- Growing emphasis on bioorthogonal conjugation methods driving site-specific antibody modification without interference with native protein function
By Application, Which Segment Demonstrates Strong Market Presence?
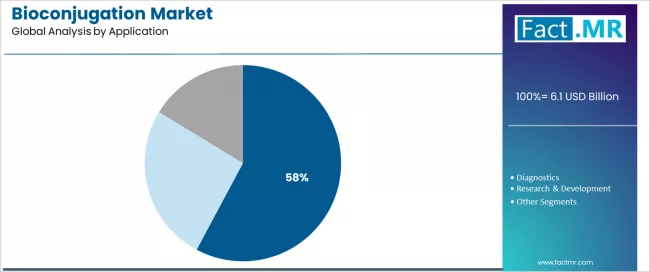
The therapeutics segment represents the leading application category in the bioconjugation market with approximately 57.8% market share, reflecting the extensive drug development activity focused on conjugated therapeutic modalities. The therapeutics segment demonstrates consistent demand driven by antibody-drug conjugate commercialization, peptide-drug conjugate development, and targeted therapy innovation across oncology and other disease areas.
Within therapeutics applications, antibody-drug conjugates (ADCs) demonstrate dominant market activity, driven by multiple approved products achieving commercial success and robust pipeline expansion addressing diverse cancer types. ADC development emphasizes optimized conjugation technologies enabling defined drug-antibody ratios and stable linker chemistry supporting favorable therapeutic windows.
The diagnostics segment emerges as the fastest growing application category with a CAGR of 12.3%, driven by immunoassay development requirements, companion diagnostic conjugate preparation, and molecular imaging probe synthesis for disease detection and monitoring applications. Diagnostics applications require fluorescent dye conjugation, enzyme labeling for detection systems, and radionuclide attachment for imaging modalities.
The research & development segment maintains substantial market share, serving academic researchers and pharmaceutical discovery teams requiring bioconjugation for target validation studies, assay development, and proof-of-concept therapeutic exploration. R&D applications encompass diverse conjugation needs including affinity probe preparation, biosensor construction, and biomaterial functionalization.
Key application dynamics include:
- Therapeutics development driving sustained bioconjugation demand with emphasis on ADC pipeline advancement and next-generation conjugate format exploration
- Diagnostics applications accelerating adoption of conjugated detection reagents in point-of-care devices and companion diagnostic development programs
- Research & development priorities emphasizing tool development for chemical biology investigation and fundamental protein engineering research
- Emerging applications including vaccine conjugate development and cell therapy modification expanding bioconjugation technology utilization beyond traditional markets
By End Use, Which Segment Accounts for Dominant Market Presence?
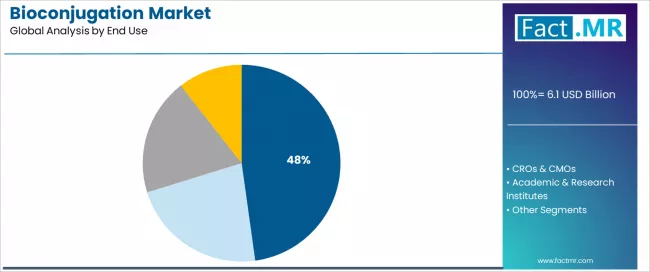
Pharmaceutical & biotechnology companies represent the leading end-use segment in the bioconjugation market with approximately 47.8% market share, reflecting the extensive internal R&D capabilities and therapeutic development programs in biopharmaceutical organizations. The pharmaceutical & biotechnology companies segment demonstrates consistent demand driven by integrated drug discovery operations, process development activities, and clinical manufacturing requirements across large pharmaceutical corporations and specialized biotech firms.
Within pharmaceutical end users, large biopharmaceutical companies demonstrate substantial bioconjugation technology adoption, driven by comprehensive ADC development programs and internal conjugation expertise supporting proprietary platform development. These organizations maintain dedicated bioconjugation laboratories with specialized instrumentation and experienced scientific staff.
The CROs & CMOs segment emerges as the fastest growing end-use category with a CAGR of 12.1%, driven by increasing outsourcing of specialized conjugation services and contract manufacturing of conjugated therapeutics. CROs & CMOs provide comprehensive capabilities from early-stage conjugation development through GMP manufacturing supporting clinical trial material production and commercial supply.
Academic & research institutes maintain meaningful market share, serving university laboratories and government research organizations requiring bioconjugation for fundamental biology research, method development, and proof-of-concept therapeutic exploration. Hospitals, clinical & diagnostic laboratories account for adoption driven by in-house diagnostic assay preparation and specialized clinical testing requiring conjugated detection reagents.
Key end-use dynamics include:
- Pharmaceutical & biotechnology companies driving core market demand with emphasis on internal capability development and strategic technology partnerships
- CROs & CMOs segment requirements accelerating through outsourcing trends and specialized service provider capacity expansion for complex conjugation processes
- Academic & research institutes prioritizing method innovation and fundamental conjugation chemistry advancement supporting future therapeutic applications
- Hospitals, clinical & diagnostic laboratories emphasizing quality-controlled conjugate preparation for specialized diagnostic testing and research applications
What are the Drivers, Restraints, and Key Trends of the Bioconjugation Market?
The market is driven by three concrete demand factors tied to therapeutic outcomes. First, expanding antibody-drug conjugate pipeline development and commercial success create increasing demand for advanced bioconjugation technologies, with site-specific conjugation representing a critical enabler for therapeutic index optimization worldwide, requiring comprehensive platform availability. Second, pharmaceutical industry investment in targeted therapy innovation and precision medicine approaches drives adoption of bioconjugation solutions, with many organizations implementing conjugate development programs and establishing internal conjugation capabilities by 2030. Third, technological advancements in bioorthogonal chemistry and enzyme-mediated coupling enable more precise biomolecular engineering that improves therapeutic performance while reducing manufacturing complexity and conjugate heterogeneity concerns.
Market restraints include intellectual property complexity and platform licensing requirements that can challenge pharmaceutical developers in accessing proprietary conjugation technologies, particularly for emerging biotech companies where upfront licensing fees and royalty obligations represent significant financial commitments. Technical challenges in achieving homogeneous conjugate formation and consistent drug-to-antibody ratio control pose another significant hurdle, as conjugation chemistry depends on biomolecule properties and reaction optimization, potentially affecting manufacturing reproducibility and regulatory approval timelines. Analytical characterization complexity for heterogeneous conjugate mixtures creates additional challenges for quality control, demanding sophisticated analytical capabilities and method validation initiatives.
Key trends indicate accelerated site-specific conjugation adoption in antibody-drug conjugate development, particularly in North America and Europe, where pharmaceutical companies demonstrate preference for defined conjugation technologies enabling improved therapeutic consistency. Oligonucleotide conjugation advancement trends toward lipid nanoparticle alternatives and cell-targeting ligand attachment with bioorthogonal linker systems enable RNA therapeutic delivery optimization that addresses cellular uptake limitations. However, the market thesis could face disruption if significant advances in non-conjugate targeted delivery systems or major breakthroughs in biologics half-life extension eliminate conjugation requirements for therapeutic enhancement.
Analysis of the Bioconjugation Market by Key Countries
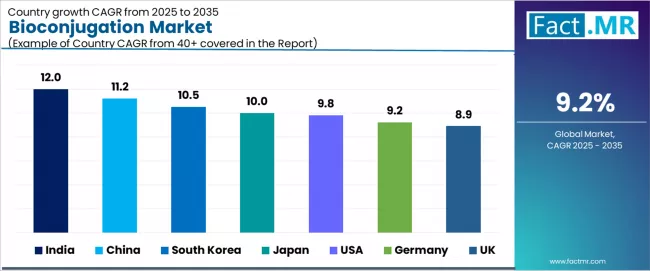
| Country | CAGR (2025-2035) |
|---|---|
| India | 12.0% |
| China | 11.2% |
| South Korea | 10.5% |
| Japan | 10.0% |
| USA | 9.8% |
| Germany | 9.2% |
| UK | 8.9% |
The global bioconjugation market is expanding steadily, with India leading at a 12.0% CAGR through 2035, driven by growing pharmaceutical R&D infrastructure, rising antibody-drug conjugate adoption, and expanding contract manufacturing capabilities supporting global biopharmaceutical outsourcing. China follows at 11.2%, supported by expanding biotechnology industry, government healthcare investments, and comprehensive biologics manufacturing capacity development. South Korea records 10.5%, reflecting rapid adoption of bioconjugation technologies and government initiatives promoting precision medicine and biologics innovation.
Japan advances at 10.0%, leveraging advanced pharmaceutical infrastructure, ADC demand growth, and government support for innovative drug development. USA grows at 9.8%, anchored by strong R&D investments and well-established biotechnology sector. Germany posts 9.2%, focusing on leading pharmaceutical sector strength and precision medicine focus, while UK demonstrates 8.9% growth through academic-industry collaborations and strong biotechnology investments.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the bioconjugation market with a CAGR of 12.0% through 2035. The country's leadership position stems from rapidly expanding pharmaceutical R&D capabilities, growing contract research and manufacturing infrastructure, and increasing adoption of antibody-drug conjugate technologies by domestic and international pharmaceutical companies.
Growth is concentrated in major pharmaceutical and biotechnology hubs, including Hyderabad, Bangalore, Pune, and Ahmedabad, where contract service organizations are establishing specialized bioconjugation capabilities and pharmaceutical companies are developing biosimilar ADC programs to serve emerging domestic oncology markets.
Distribution channels through specialized life science distributors and direct manufacturer partnerships expand reagent accessibility across contract organizations and pharmaceutical research facilities. The country's growing emphasis on biotechnology innovation, skilled scientific workforce availability, and cost-competitive service delivery provides strong momentum for bioconjugation market expansion, including comprehensive adoption across contract development, manufacturing organizations, and emerging domestic biotech companies.
Key market factors:
- Contract manufacturing organization expansion concentrated in pharmaceutical clusters with rising bioconjugation service capabilities and GMP infrastructure development
- Pharmaceutical R&D growth through domestic companies and multinational research centers enabling increased bioconjugation reagent consumption
- Comprehensive skill development ecosystem, including specialized training programs in bioconjugation chemistry and conjugate analytical characterization
- Technology partnerships featuring collaborations between Indian CDMOs and international pharmaceutical companies for conjugation service delivery
Why is China Emerging as a High-Growth Market?
In major biopharmaceutical centers including Shanghai, Beijing, Suzhou, and Guangzhou, the adoption of advanced bioconjugation solutions is accelerating across pharmaceutical companies and contract service organizations, driven by government healthcare investment policies and expanding biotechnology industry infrastructure. The market demonstrates strong growth momentum with a CAGR of 11.2% through 2035, linked to comprehensive biologics manufacturing expansion and increasing focus on innovative antibody-drug conjugate development beyond biosimilar programs. Chinese pharmaceutical companies are implementing sophisticated bioconjugation platforms and establishing internal expertise to enhance competitive positioning while meeting growing domestic demand for targeted oncology therapeutics. The country's substantial biotechnology investment climate creates ongoing demand for conjugation reagents and services, while increasing emphasis on innovative drug development drives adoption of site-specific conjugation technologies and advanced linker-payload systems.
Key development areas:
- Pharmaceutical companies and biotech firms leading bioconjugation adoption with emphasis on ADC pipeline development and manufacturing capability establishment
- Government policy support through healthcare modernization initiatives and innovative drug development incentive programs
- Technology infrastructure expansion enabling advanced analytical characterization and quality-controlled conjugation manufacturing
- Growing integration between domestic reagent suppliers and international technology platform providers expanding local market access
What Drives South Korea Market Innovation?
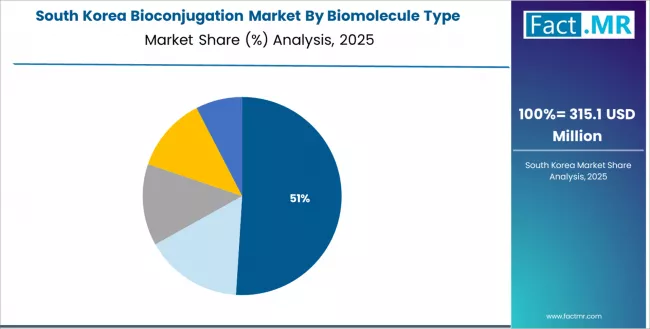
South Korea market expansion is driven by rapid technology adoption patterns, government-backed biotechnology initiatives, and established pharmaceutical manufacturing capabilities. The country demonstrates strong growth potential with a CAGR of 10.5% through 2035, supported by aggressive innovation strategies from pharmaceutical companies and comprehensive government support for precision medicine development. Korean biopharmaceutical organizations face opportunities in biosimilar ADC development and proprietary conjugation platform innovation, requiring advanced bioconjugation technologies to provide competitive differentiation and manufacturing efficiency. However, established biologics manufacturing expertise and strong analytical capabilities create stable demand for bioconjugation solutions, particularly in contract manufacturing settings where Korean CDMOs serve international pharmaceutical clients requiring high-quality conjugation services.
Market characteristics:
- Pharmaceutical and biotechnology companies showing robust bioconjugation adoption with substantial investment in ADC development programs
- Government initiatives supporting biotechnology sector growth through funding programs and regulatory pathway facilitation
- Contract manufacturing organizations expanding specialized conjugation capabilities for domestic and international pharmaceutical clients
- Growing emphasis on proprietary conjugation platform development and licensing strategies creating intellectual property value
How Does Japan Demonstrate Advanced Capabilities?
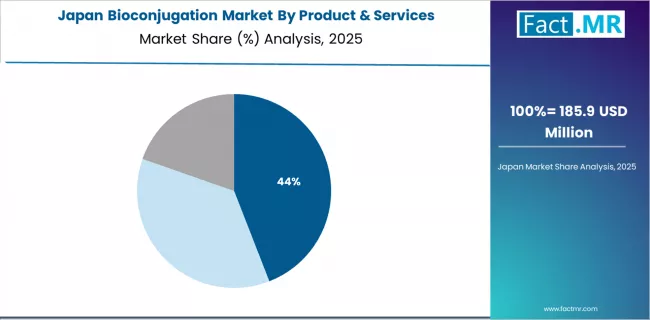
The Japan market leads in precision bioconjugation manufacturing based on integration with advanced pharmaceutical infrastructure and rigorous quality control systems for therapeutic conjugate development. The country shows strong potential with a CAGR of 10.0% through 2035, driven by antibody-drug conjugate clinical demand and government support for innovative drug development in major pharmaceutical regions, including Tokyo, Osaka, Kyoto, and Nagoya. Japanese pharmaceutical companies are adopting advanced conjugation technologies and implementing comprehensive analytical characterization protocols for quality assurance, particularly in proprietary ADC programs demonstrating competitive differentiation through superior conjugation chemistry and manufacturing control. Distribution channels through established scientific distributors and direct supplier relationships expand reagent availability across pharmaceutical research facilities and contract manufacturing organizations.
Leading market segments:
- Pharmaceutical companies in major research centers implementing proprietary conjugation platforms with rigorous process control and quality standards
- Government support through regulatory science advancement and innovative drug development incentive programs
- Strategic emphasis on antibody engineering excellence enabling sophisticated conjugation approaches and optimized therapeutic properties
- Focus on analytical instrumentation and characterization methodology supporting comprehensive conjugate quality assessment
What Positions USA for Continued Leadership?
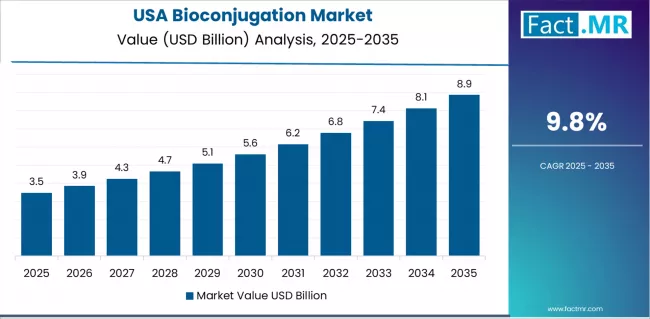
In major biotechnology hubs including Boston/Cambridge, San Francisco Bay Area, San Diego, and Research Triangle Park, pharmaceutical companies are implementing cutting-edge bioconjugation technologies featuring next-generation site-specific platforms and novel linker-payload combinations, with extensive pipeline activity demonstrating commercial validation through multiple approved antibody-drug conjugates and robust development programs. The market shows steady growth potential with a CAGR of 9.8% through 2035, linked to sustained R&D investment, established biotechnology sector infrastructure, and comprehensive clinical development ecosystem in major innovation regions. American pharmaceutical and biotechnology companies are developing proprietary conjugation platforms and advancing novel conjugate therapeutic modalities to maintain competitive advantages while addressing diverse oncology and non-oncology therapeutic opportunities. The country's mature bioconjugation market creates opportunities for technology differentiation that emphasizes innovation in emerging application areas and next-generation conjugation methodologies.
Market development factors:
- Pharmaceutical and biotech companies leading innovation with emphasis on site-specific conjugation platforms and novel payload development
- Well-established supply chain infrastructure providing comprehensive reagent access and specialized service availability
- Strategic venture capital and corporate investment supporting emerging conjugation technology companies and platform development
- Emphasis on regulatory pathway clarity through FDA guidance supporting conjugated therapeutic development and approval processes
How Does Germany Leverage Pharmaceutical Excellence?
The Germany market demonstrates strong bioconjugation capabilities based on leading pharmaceutical and biotechnology sector strength and precision chemistry expertise supporting contract manufacturing excellence. The country shows solid potential with a CAGR of 9.2% through 2035, driven by contract development and manufacturing organization infrastructure and precision medicine focus in major industrial regions, including Bavaria, Baden-Württemberg, North Rhine-Westphalia, and Hesse. German contract manufacturers and pharmaceutical companies are implementing validated bioconjugation processes and comprehensive quality systems for therapeutic conjugate production, particularly in GMP manufacturing settings serving European and global pharmaceutical clients demanding rigorous quality standards. Supply infrastructure through established chemical suppliers and specialized bioconjugation service providers expands technology access across pharmaceutical development organizations and academic research institutions.
Leading market segments:
- Contract development and manufacturing organizations providing specialized conjugation services with emphasis on quality and regulatory compliance
- Chemical and life science companies including Merck KGaA (MilliporeSigma) and Sartorius AG offering comprehensive bioconjugation reagent portfolios
- Academic-industrial partnerships enabling method development and technology validation supporting commercial applications
- Focus on process analytical technology and quality-by-design approaches ensuring reproducible conjugation manufacturing
What Characterizes UK's Research-Driven Market?
In major research centers including Cambridge, Oxford, London, and Edinburgh, academic institutions are implementing innovative bioconjugation methodologies featuring bioorthogonal chemistry development and novel conjugation platform technologies, with established research programs demonstrating fundamental advancement through chemical biology expertise and translational research initiatives. The market shows steady growth potential with a CAGR of 8.9% through 2035, linked to academic-industry collaboration strength, biotechnology investment growth, and emerging therapeutic conjugate development in university spinout companies and established pharmaceutical organizations. The researchers and biotechnology companies are developing novel conjugation approaches and translating academic innovations into commercial applications to create competitive advantages while contributing to global bioconjugation technology advancement. The country's research excellence infrastructure creates opportunities for method innovation that differentiates through scientific leadership in conjugation chemistry fundamentals and emerging application development.
Market development factors:
- Academic research institutions leading bioconjugation method development with emphasis on chemical biology innovation and bioorthogonal chemistry
- Biotechnology investment supporting spinout company formation and technology commercialization from university research programs
- Strategic collaboration frameworks between universities and pharmaceutical companies enabling technology translation and validation
- Emphasis on early-stage conjugation technology development creating pipeline of innovations for future therapeutic applications
Competitive Landscape of the Bioconjugation Market
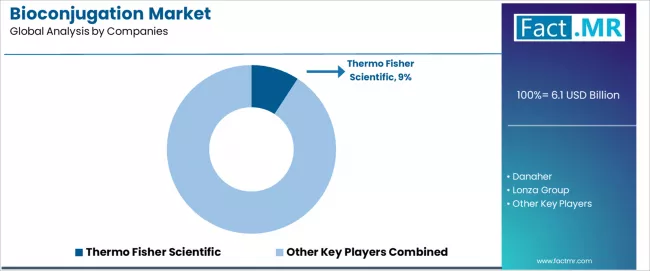
The bioconjugation market features approximately 15-20 meaningful players with moderate concentration, where the top three companies control roughly 20-25% of global market share through established product portfolios and comprehensive service offerings. Competition centers on technology innovation, application expertise, and integrated solution capabilities rather than price competition alone.
Market leaders include Thermo Fisher Scientific, Danaher, and Lonza Group, which maintain competitive advantages through comprehensive bioconjugation product catalogs, proven service capabilities, and deep expertise in the life sciences sector, creating strong customer relationships among pharmaceutical and research organizations. These companies leverage established distribution networks and ongoing technology development initiatives to defend market positions while expanding into adjacent categories including cell and gene therapy manufacturing services.
Challengers encompass Merck KGaA (MilliporeSigma) and Sartorius AG, which compete through specialized reagent portfolios and European market strength with integrated bioprocessing capabilities. Life science companies, including Abbvie, Inc., Agilent Technologies, Inc., and Bio-Rad Laboratories, Inc., focus on specific conjugation applications or complementary analytical instrumentation, offering differentiated capabilities in therapeutic development, chromatography systems, and protein analysis platforms.
Contract service specialists including Catalent, Inc. and established life science suppliers like BD create competitive dynamics through specialized GMP conjugation manufacturing and comprehensive laboratory product portfolios, particularly in high-growth pharmaceutical development markets where outsourcing trends and quality requirements provide advantages for specialized service providers with regulatory expertise. Market dynamics favor companies that combine proven reagent performance with comprehensive technical support capabilities that address the complete value chain from conjugation chemistry selection through analytical characterization and manufacturing scale-up. Strategic emphasis on site-specific conjugation platforms, bioorthogonal chemistry development, and integrated drug-linker-payload systems enables differentiation in increasingly sophisticated bioconjugation applications across therapeutic development and diagnostic manufacturing segments.
Global Bioconjugation Market - Stakeholder Contribution Framework
Bioconjugation solutions represent critical enabling technologies that allow pharmaceutical researchers and biotechnology companies to create targeted therapeutic constructs and functionalized biomolecules without prohibitive chemistry expertise requirements, typically providing superior precision and reproducibility compared to empirical conjugation approaches while ensuring consistent product quality and regulatory compliance capabilities. With the market projected to grow from USD 6.1 billion in 2025 to USD 14.7 billion by 2035 at a 9.2% CAGR, these solutions offer compelling advantages - targeted payload delivery, reproducible conjugate formation, and diverse application versatility - making them essential for therapeutics applications (57.8% share), antibody bioconjugation (52.4% biomolecule share), and pharmaceutical & biotechnology end users (47.8% share) seeking reliable molecular engineering solutions. Scaling technology adoption and innovation advancement requires coordinated action across research funding, reagent standardization, technology providers, pharmaceutical developers, and analytical method development initiatives.
How Could Governments Spur Innovation and Access?
- Research & Development Funding: Include bioconjugation technology in national biotechnology research programs, providing targeted support for academic method development and supporting technology transfer through commercialization grants.
- Regulatory Guidance Development: Establish clear analytical characterization expectations for conjugated biologics, provide regulatory pathway clarity for novel conjugate formats, and develop harmonized standards facilitating international development programs.
- Intellectual Property Framework: Create balanced patent policies encouraging platform innovation while preventing excessive licensing barriers, support freedom-to-operate analysis resources, and establish reasonable licensing frameworks for publicly-funded conjugation technologies.
- Infrastructure Investment: Fund core facility development at research institutions with specialized conjugation equipment and analytical capabilities. Support training programs developing expertise in conjugation chemistry and conjugate characterization methodologies.
- Therapeutic Development Incentives: Provide tax credits for antibody-drug conjugate development programs, support orphan drug conjugate development through expedited review pathways, and establish funding mechanisms for academic-industrial conjugation technology partnerships.
How Could Industry Associations Support Market Development?
- Technology Standards & Best Practices: Define standardized terminology for conjugation methodologies, establish analytical characterization guidelines for conjugate assessment, and create quality benchmarks supporting reproducible conjugation outcomes.
- Education & Training Programs: Lead technical workshops demonstrating conjugation chemistry fundamentals, emphasizing reaction optimization strategies, analytical method development, and regulatory considerations for conjugated products.
- Analytical Method Harmonization: Develop consensus approaches for drug-to-antibody ratio determination, establish standard protocols for conjugate stability assessment, and create reference materials supporting analytical method validation.
- Technology Assessment: Provide objective evaluation frameworks comparing conjugation platforms, publish application-specific technology recommendations, and facilitate information exchange about emerging conjugation methodologies.
How Could Technology Providers Strengthen the Ecosystem?
- Platform Innovation: Develop next-generation bioorthogonal chemistries with enhanced reaction kinetics, site-specific conjugation technologies enabling homogeneous products, and scalable GMP-compatible conjugation processes supporting commercial manufacturing.
- Integrated Solutions: Provide comprehensive workflows combining reagents with analytical characterization support, develop automated conjugation workstations reducing manual handling, and offer validated methods accelerating development timelines.
- Technical Support Services: Deliver expert consultation on conjugation strategy selection, provide troubleshooting assistance for challenging conjugation scenarios, and offer analytical characterization services validating conjugate quality.
- Collaborative Development: Build partnerships with pharmaceutical companies for proprietary platform development, establish academic collaborations advancing conjugation methodology, and create consortia addressing common technical challenges.
How Could Pharmaceutical Developers Navigate Technology Selection?
- Strategic Technology Assessment: Evaluate conjugation platforms across consumables (45.5% product dominance), services (fastest growth 11.7% CAGR), and emerging click chemistry techniques (11.9% CAGR) considering therapeutic requirements and development timelines.
- Application-Specific Optimization: Implement appropriate conjugation strategies for antibodies (52.4% biomolecule share), emerging oligonucleotide conjugates (12.0% CAGR), and diverse payload types matching conjugation chemistry to biomolecule properties.
- Manufacturing Scalability Planning: Design conjugation processes with commercial manufacturing considerations, establish analytical methods supporting regulatory filings, and validate conjugation procedures ensuring batch-to-batch consistency.
- Outsourcing Strategy Development: Partner with CROs & CMOs (12.1% CAGR) for specialized conjugation development, leverage contract manufacturing for GMP production, and maintain internal expertise for platform evaluation and oversight.
How Could Investors and Industry Partners Unlock Value?
- Technology Platform Investment: Provide capital for established companies like Thermo Fisher Scientific, Danaher, and Lonza Group expanding conjugation portfolios and service capabilities, particularly in site-specific conjugation and bioorthogonal chemistry domains.
- Innovation Financing: Back emerging companies developing novel conjugation methodologies, next-generation linker-payload systems, and enabling technologies for oligonucleotide and cell therapy conjugation applications.
- Service Capacity Expansion: Finance GMP conjugation manufacturing facilities at CDMOs, support analytical characterization laboratory development, and enable automation technology adoption improving throughput and reproducibility.
- Strategic Partnership Formation: Support collaborative ventures between reagent suppliers and pharmaceutical companies, facilitate technology licensing agreements enabling platform access, and structure value-sharing arrangements aligning stakeholder incentives.
Key Players in the Bioconjugation Market
- Thermo Fisher Scientific
- Danaher
- Lonza Group
- Merck KGaA (MilliporeSigma)
- Sartorius AG
- Abbvie, Inc.
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Catalent, Inc.
- BD
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 6.1 Billion |
| Product & Services | Consumables, Instruments, Services |
| Biomolecule Type | Antibodies, Proteins, Peptides, Oligonucleotides, Other Biomolecules |
| Technique | Chemical Conjugation, Enzyme-Mediated Conjugation, Click Chemistry, Photoreactive Crosslinking, Other Techniques |
| Application | Therapeutics, Diagnostics, Research & Development |
| End Use | Pharmaceutical & Biotechnology Companies, CROs & CMOs, Academic & Research Institutes, Hospitals, Clinical & Diagnostic Laboratories |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Country Covered | USA, Germany, UK, France, China, Japan, India, and 40+ countries |
| Key Companies Profiled | Thermo Fisher Scientific, Danaher, Lonza Group, Merck KGaA (MilliporeSigma), Sartorius AG, Abbvie, Inc., Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., Catalent, Inc., BD |
| Additional Attributes | Dollar sales by product & services and biomolecule type categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with life science reagent manufacturers and contract service organizations, technology specifications and conjugation methodology frameworks, integration with antibody-drug conjugate development programs and RNA therapeutic delivery systems, innovations in site-specific conjugation technologies and bioorthogonal chemistry approaches, and development of specialized applications with GMP manufacturing capabilities and analytical characterization services. |
Bioconjugation Market by Segments
-
Product & Services :
- Consumables
- Instruments
- Services
-
Biomolecule Type :
- Antibodies
- Proteins
- Peptides
- Oligonucleotides
- Other Biomolecules
-
Technique :
- Chemical Conjugation
- Enzyme-Mediated Conjugation
- Click Chemistry
- Photoreactive Crosslinking
- Other Techniques
-
Application :
- Therapeutics
- Diagnostics
- Research & Development
-
End Use :
- Pharmaceutical & Biotechnology Companies
- CROs & CMOs
- Academic & Research Institutes
- Hospitals, Clinical & Diagnostic Laboratories
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Latin America
- Brazil
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Union
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product & Services
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product & Services, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product & Services, 2025 to 2035
- Consumables
- Instruments
- Services
- Y to o to Y Growth Trend Analysis By Product & Services, 2020 to 2024
- Absolute $ Opportunity Analysis By Product & Services, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Biomolecule Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Biomolecule Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Biomolecule Type, 2025 to 2035
- Antibodies
- Proteins
- Peptides
- Oligonucleotides
- Other Biomolecules
- Y to o to Y Growth Trend Analysis By Biomolecule Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Biomolecule Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- Therapeutics
- Diagnostics
- Research & Development
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Pharmaceutical & Biotechnology Companies
- CROs & CMOs
- Academic & Research Institutes
- Hospitals, Clinical & Diagnostic Laboratories
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product & Services
- By Biomolecule Type
- By Application
- By End Use
- Competition Analysis
- Competition Deep Dive
- Thermo Fisher Scientific
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Danaher
- Lonza Group
- Merck KGaA (MilliporeSigma)
- Sartorius AG
- Abbvie, Inc.
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Catalent, Inc.
- BD
- Thermo Fisher Scientific
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Product & Services, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Biomolecule Type, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 5: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Product & Services, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Biomolecule Type, 2020 to 2035
- Table 9: North America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 10: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Product & Services, 2020 to 2035
- Table 13: Latin America Market Value (USD Million) Forecast by Biomolecule Type, 2020 to 2035
- Table 14: Latin America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 15: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: Western Europe Market Value (USD Million) Forecast by Product & Services, 2020 to 2035
- Table 18: Western Europe Market Value (USD Million) Forecast by Biomolecule Type, 2020 to 2035
- Table 19: Western Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 20: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 21: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: Eastern Europe Market Value (USD Million) Forecast by Product & Services, 2020 to 2035
- Table 23: Eastern Europe Market Value (USD Million) Forecast by Biomolecule Type, 2020 to 2035
- Table 24: Eastern Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 25: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 26: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 27: East Asia Market Value (USD Million) Forecast by Product & Services, 2020 to 2035
- Table 28: East Asia Market Value (USD Million) Forecast by Biomolecule Type, 2020 to 2035
- Table 29: East Asia Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 30: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 31: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 32: South Asia and Pacific Market Value (USD Million) Forecast by Product & Services, 2020 to 2035
- Table 33: South Asia and Pacific Market Value (USD Million) Forecast by Biomolecule Type, 2020 to 2035
- Table 34: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 35: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 36: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 37: Middle East & Africa Market Value (USD Million) Forecast by Product & Services, 2020 to 2035
- Table 38: Middle East & Africa Market Value (USD Million) Forecast by Biomolecule Type, 2020 to 2035
- Table 39: Middle East & Africa Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 40: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Product & Services, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Product & Services, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Product & Services
- Figure 6: Global Market Value Share and BPS Analysis by Biomolecule Type, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Biomolecule Type, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Biomolecule Type
- Figure 9: Global Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Application
- Figure 12: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by End Use
- Figure 15: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 16: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 17: Global Market Attractiveness Analysis by Region
- Figure 18: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 23: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 24: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 25: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 26: North America Market Value Share and BPS Analysis by Product & Services, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Product & Services, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Product & Services
- Figure 29: North America Market Value Share and BPS Analysis by Biomolecule Type, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Biomolecule Type, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Biomolecule Type
- Figure 32: North America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 33: North America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 34: North America Market Attractiveness Analysis by Application
- Figure 35: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 36: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 37: North America Market Attractiveness Analysis by End Use
- Figure 38: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 39: Latin America Market Value Share and BPS Analysis by Product & Services, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Product & Services, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Product & Services
- Figure 42: Latin America Market Value Share and BPS Analysis by Biomolecule Type, 2025 and 2035
- Figure 43: Latin America Market Y to o to Y Growth Comparison by Biomolecule Type, 2025 to 2035
- Figure 44: Latin America Market Attractiveness Analysis by Biomolecule Type
- Figure 45: Latin America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 46: Latin America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 47: Latin America Market Attractiveness Analysis by Application
- Figure 48: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 49: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 50: Latin America Market Attractiveness Analysis by End Use
- Figure 51: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 52: Western Europe Market Value Share and BPS Analysis by Product & Services, 2025 and 2035
- Figure 53: Western Europe Market Y to o to Y Growth Comparison by Product & Services, 2025 to 2035
- Figure 54: Western Europe Market Attractiveness Analysis by Product & Services
- Figure 55: Western Europe Market Value Share and BPS Analysis by Biomolecule Type, 2025 and 2035
- Figure 56: Western Europe Market Y to o to Y Growth Comparison by Biomolecule Type, 2025 to 2035
- Figure 57: Western Europe Market Attractiveness Analysis by Biomolecule Type
- Figure 58: Western Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 59: Western Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 60: Western Europe Market Attractiveness Analysis by Application
- Figure 61: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 62: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 63: Western Europe Market Attractiveness Analysis by End Use
- Figure 64: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 65: Eastern Europe Market Value Share and BPS Analysis by Product & Services, 2025 and 2035
- Figure 66: Eastern Europe Market Y to o to Y Growth Comparison by Product & Services, 2025 to 2035
- Figure 67: Eastern Europe Market Attractiveness Analysis by Product & Services
- Figure 68: Eastern Europe Market Value Share and BPS Analysis by Biomolecule Type, 2025 and 2035
- Figure 69: Eastern Europe Market Y to o to Y Growth Comparison by Biomolecule Type, 2025 to 2035
- Figure 70: Eastern Europe Market Attractiveness Analysis by Biomolecule Type
- Figure 71: Eastern Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 72: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 73: Eastern Europe Market Attractiveness Analysis by Application
- Figure 74: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 75: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 76: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 77: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 78: East Asia Market Value Share and BPS Analysis by Product & Services, 2025 and 2035
- Figure 79: East Asia Market Y to o to Y Growth Comparison by Product & Services, 2025 to 2035
- Figure 80: East Asia Market Attractiveness Analysis by Product & Services
- Figure 81: East Asia Market Value Share and BPS Analysis by Biomolecule Type, 2025 and 2035
- Figure 82: East Asia Market Y to o to Y Growth Comparison by Biomolecule Type, 2025 to 2035
- Figure 83: East Asia Market Attractiveness Analysis by Biomolecule Type
- Figure 84: East Asia Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 85: East Asia Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 86: East Asia Market Attractiveness Analysis by Application
- Figure 87: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 88: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 89: East Asia Market Attractiveness Analysis by End Use
- Figure 90: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 91: South Asia and Pacific Market Value Share and BPS Analysis by Product & Services, 2025 and 2035
- Figure 92: South Asia and Pacific Market Y to o to Y Growth Comparison by Product & Services, 2025 to 2035
- Figure 93: South Asia and Pacific Market Attractiveness Analysis by Product & Services
- Figure 94: South Asia and Pacific Market Value Share and BPS Analysis by Biomolecule Type, 2025 and 2035
- Figure 95: South Asia and Pacific Market Y to o to Y Growth Comparison by Biomolecule Type, 2025 to 2035
- Figure 96: South Asia and Pacific Market Attractiveness Analysis by Biomolecule Type
- Figure 97: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 98: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 99: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 100: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 101: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 102: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 103: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 104: Middle East & Africa Market Value Share and BPS Analysis by Product & Services, 2025 and 2035
- Figure 105: Middle East & Africa Market Y to o to Y Growth Comparison by Product & Services, 2025 to 2035
- Figure 106: Middle East & Africa Market Attractiveness Analysis by Product & Services
- Figure 107: Middle East & Africa Market Value Share and BPS Analysis by Biomolecule Type, 2025 and 2035
- Figure 108: Middle East & Africa Market Y to o to Y Growth Comparison by Biomolecule Type, 2025 to 2035
- Figure 109: Middle East & Africa Market Attractiveness Analysis by Biomolecule Type
- Figure 110: Middle East & Africa Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 111: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 112: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 113: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 114: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 115: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 116: Global Market - Tier Structure Analysis
- Figure 117: Global Market - Company Share Analysis
- FAQs -
How big is the bioconjugation market in 2025?
The global bioconjugation market is estimated to be valued at USD 6.1 billion in 2025.
What will be the size of bioconjugation market in 2035?
The market size for the bioconjugation market is projected to reach USD 14.7 billion by 2035.
How much will be the bioconjugation market growth between 2025 and 2035?
The bioconjugation market is expected to grow at a 9.2% CAGR between 2025 and 2035.
What are the key product types in the bioconjugation market?
The key product types in bioconjugation market are consumables, instruments and services.
Which biomolecule type segment to contribute significant share in the bioconjugation market in 2025?
In terms of biomolecule type, antibodies segment to command 52.4% share in the bioconjugation market in 2025.