Iron Chelators Market
Iron Chelators Market Size and Share Forecast Outlook 2025 to 2035
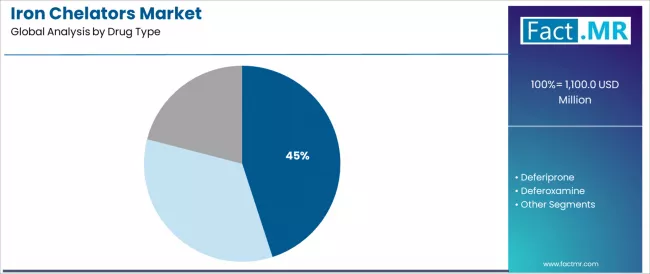
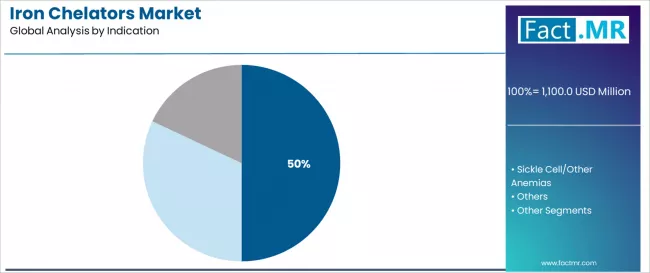
Iron chelators market is projected to grow from USD 1,100.0 million in 2025 to USD 1,700.0 million by 2035, at a CAGR of 4.4%. Deferasirox will dominate with a 45.0% market share, while thalassemia will lead the indication segment with a 50.0% share.
Iron Chelators Market Forecast and Outlook 2025 to 2035
The global iron chelators market is projected to grow from USD 1,100.0 million in 2025 to approximately USD 1,700.0 million by 2035, recording an absolute increase of USD 600.0 million over the forecast period. This translates into a total growth of 54.4%, with the market forecast to expand at a compound annual growth rate (CAGR) of 4.4% between 2025 and 2035.
The overall market size is expected to grow by nearly 1.5X during the same period, supported by increasing global demand for effective iron overload management therapies, growing prevalence of blood disorders requiring iron chelation therapy, and rising awareness of iron toxicity complications driving premium pharmaceutical procurement across various therapeutic applications.
Quick Stats for Iron Chelators Market
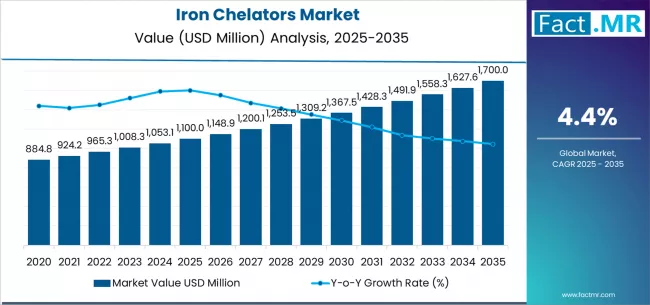
| Iron Chelators Market Value (2025) | USD 1,100.0 million |
|---|---|
| Iron Chelators Market Forecast Value (2035) | USD 1,700.0 million |
| Iron Chelators Market CAGR | 4.4% |
| Leading Segment by Drug Type (2025) | Deferasirox (45.0%) |
| Leading Segment by Indication (2025) | Thalassemia (50.0%) |
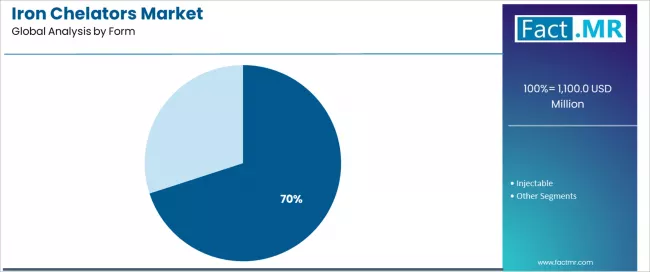
| Leading Segment by Form (2025) | Oral (70.0%) |
| Key Growth Regions | Asia Pacific, North America, and Europe |
| Top Companies by Market Share | Novartis, Cipla, Sun Pharma |
Iron Chelators Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 1,100.0 million |
| Market Forecast Value (2035) | USD 1,700.0 million |
| Forecast CAGR (2025-2035) | 4.4% |
Why is the Iron Chelators Market Growing?
| THERAPEUTIC & CLINICAL TRENDS | PHARMACEUTICAL REQUIREMENTS | REGULATORY & QUALITY STANDARDS |
|---|---|---|
| Growing Prevalence of Blood Disorders Continuous expansion of thalassemia and sickle cell disease incidence across established and emerging markets driving demand for high-efficacy iron chelation therapy solutions. Aging Population Demographics Growing emphasis on iron overload management and advanced chelation therapy technologies creating demand for high-performance iron chelation pharmaceutical ingredients. Premium Therapeutic Performance Superior iron binding properties and gastrointestinal tolerance characteristics making iron chelators essential for performance-focused therapeutic applications. | Advanced Chelation Efficacy Requirements Modern pharmaceutical manufacturing requires high-performance chelation agents delivering precise iron binding control and enhanced bioavailability. Processing Efficiency Demands Pharmaceutical manufacturers investing in premium chelation agents offering consistent performance while maintaining processing efficiency. Quality and Reliability Standards Certified producers with proven track records required for advanced iron chelation pharmaceutical applications. | Drug Approval Standards Regulatory requirements establishing performance benchmarks favoring high-quality iron chelation pharmaceutical materials. rformance Property Standards Quality standards requiring superior iron binding properties and resistance to environmental stresses in processing. Medical Compliance Requirements Diverse therapeutic requirements and quality standards driving need for sophisticated iron chelation pharmaceutical ingredient inputs. |
Iron Chelators Market Segmentation
| Category | Segments Covered |
|---|---|
| By Drug Type | Deferasirox, Deferiprone, Deferoxamine |
| By Indication | Thalassemia, Sickle Cell/Other Anemias, Others |
| By Form | Oral, Injectable |
| By Region | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
Iron Chelators Market Analysis by Drug Type
| Segment | 2025 to 2035 Outlook |
|---|---|
| Deferasirox | Leader in 2025 with 45.0% market share; likely to maintain leadership through 2035. Broadest use (thalassemia, sickle cell disease, transfusion-dependent conditions), mature supply chain, predictable functionality. Momentum: steady-to-strong. Watchouts: raw material price volatility. |
| Deferiprone | Well-established for oral iron chelation therapy applications, but processing complexity limits broader adoption in specialized therapeutic applications. Momentum: steady in traditional chelation therapy; flat to slightly up in premium segments. |
| Deferoxamine | Benefiting from advanced parenteral therapy requirements due to specialized iron binding properties and enhanced performance characteristics. Momentum: rising. Watchouts: manufacturing complexity, technical qualification requirements. |
Iron Chelators Market Analysis by Indication
| Segment | 2025 to 2035 Outlook |
|---|---|
| Thalassemia | At 50.0%, largest indication segment in 2025 with established iron chelation therapy integration. Mature supply chains, standardized treatment protocols. Momentum: steady growth driven by disease prevalence and premium therapy demand. Watchouts: regulatory changes affecting treatment requirements. |
| Sickle Cell/Other Anemias | Strong growth segment driven by expanding diagnosis and treatment awareness. Deferasirox and deferiprone chelators dominate applications. Momentum: strong growth through 2030, supported by therapeutic awareness. Watchouts: competition from alternative iron management technologies. |
| Others | Specialized segment with diverse iron chelation requirements. Growing demand for iron overload management supporting higher-value therapeutic applications. Momentum: moderate growth via therapeutic expansion and medical innovation. Watchouts: regulatory requirements affecting treatment protocols. |
Iron Chelators Market Analysis by Form
| Form | Status & Outlook 2025-2035 |
|---|---|
| Oral | Dominant form in 2025 with 70.0% share for therapy applications. Patient convenience, processing advantages, ease of administration. Momentum: steady growth driven by patient compliance and treatment advantages. Watchouts: bioavailability variations in certain conditions. |
| Injectable | Important for specialized applications requiring precise control. Value-add through superior bioavailability properties, controlled delivery, specialized processing. Momentum: moderate growth as specialty applications demand enhanced properties. Processing complexity may limit broader growth. |
DRIVERS
| DRIVERS | RESTRAINTS | KEY TRENDS |
|---|---|---|
| Global Therapeutic Awareness Growth Continuing expansion of blood disorder awareness and treatment activities across established and emerging markets driving demand for high-efficacy iron chelation therapy solutions. Aging Population Demographics Increasing adoption of advanced iron overload management systems importance in therapeutic efficiency and treatment optimization. Premium Pharmaceutical Demand Growing demand for iron chelators that support both therapeutic benefits and processing efficiency in pharmaceutical manufacturing. | Raw Material Price Volatility Price fluctuations affecting production costs and supply chain predictability for manufacturers. Manufacturing Complexity Complex technical requirements across applications affecting product development and standardization. Technical Qualification Requirements Complex technical requirements across applications affecting product development and standardization. Competition from Alternatives Alternative iron management technologies affecting market selection and development. | Advanced Processing Technologies Integration of advanced iron chelation processing systems, manufacturing innovations, and quality control solutions enabling superior operational efficiency. Performance Enhancement Enhanced iron binding control, improved patient tolerance, and advanced bioavailability capabilities compared to traditional chelation systems. Specialized Formulations Development of specialized iron chelation grades and custom formulations providing enhanced performance benefits and application-specific optimization. Technical Innovation Integration of advanced iron chelation development and intelligent processing management for sophisticated therapeutic solutions. |
Analysis of Iron Chelators Market by Key Countries
| Country | CAGR (2025-2035) |
|---|---|
| India | 5.2% |
| China | 4.8% |
| Brazil | 4.3% |
| USA | 4.2% |
| South Korea | 4.0% |
| Germany | 3.8% |
| Japan | 3.6% |
India Leads Global Market Growth with Therapeutic Excellence
Revenue from iron chelators in India is projected to exhibit strong growth driven by expanding healthcare infrastructure and comprehensive blood disorder management innovation creating opportunities for pharmaceutical suppliers across therapeutic applications, hospital facilities, and specialty medical sectors.
The country's developing pharmaceutical manufacturing tradition and expanding medical awareness capabilities are creating significant demand for both conventional and high-performance iron chelation pharmaceutical ingredients.
Major pharmaceutical companies are establishing comprehensive local processing facilities to support large-scale manufacturing operations and meet growing demand for efficient therapeutic solutions.
- Healthcare industry modernization programs are supporting widespread adoption of advanced iron chelation processing across manufacturing operations, driving demand for high-quality pharmaceutical ingredients
- Therapeutic processing excellence initiatives and specialized drug development are creating opportunities for pharmaceutical suppliers requiring reliable efficacy and cost-effective iron chelation solutions
- Blood disorder treatment growth and pharmaceutical processing development are facilitating adoption of specialty iron chelation ingredients throughout major industrial regions
China Demonstrates Strong Market Potential with Pharmaceutical Processing Growth
Revenue from iron chelators in China is expanding supported by extensive pharmaceutical manufacturing expansion and comprehensive therapeutic processing industry development creating demand for reliable iron chelation ingredients across diverse manufacturing categories and specialty medical segments. The country's dominant pharmaceutical production position and expanding therapeutic manufacturing capabilities are driving demand for iron chelation solutions that provide consistent performance while supporting cost-effective processing requirements. Pharmaceutical processors and manufacturers are investing in local production facilities to support growing manufacturing operations and therapeutic demand.
- Pharmaceutical manufacturing operations expansion and therapeutic processing capability development are creating opportunities for iron chelation ingredients across diverse manufacturing segments requiring reliable efficacy and competitive processing costs
- Manufacturing modernization and processing technology advancement are driving investments in pharmaceutical supply chains supporting performance requirements throughout major industrial regions
- Therapeutic manufacturing growth and specialty drug development programs are enhancing demand for processing-grade iron chelators throughout pharmaceutical production areas
USA Maintains Strong Position with Premium Pharmaceutical Innovation
Demand for iron chelators in USA is projected to grow at 4.2% CAGR supported by the country's expanding pharmaceutical manufacturing base and therapeutic processing technologies requiring advanced iron chelation systems for drug production and medical applications. American pharmaceutical companies are implementing processing systems that support advanced manufacturing techniques, operational efficiency, and comprehensive quality protocols. The market is characterized by focus on operational excellence, therapeutic performance, and compliance with pharmaceutical quality standards.
- Premium pharmaceutical industry investments are prioritizing advanced processing technologies that demonstrate superior efficacy and quality while meeting American manufacturing standards
- Therapeutic processing leadership programs and operational excellence initiatives are driving adoption of precision-engineered iron chelation ingredients that support advanced manufacturing systems and performance optimization
- Research and development programs for efficacy enhancement are facilitating adoption of specialized processing techniques throughout major pharmaceutical centers
Brazil Expands Market with Healthcare Innovation
Revenue from iron chelators in Brazil is growing driven by advanced pharmaceutical manufacturing development programs and increasing therapeutic technology innovation creating opportunities for iron chelation suppliers serving both drug manufacturing operations and healthcare contractors. The country's extensive healthcare base and expanding medical awareness are creating demand for iron chelation ingredients that support diverse performance requirements while maintaining processing performance standards. Pharmaceutical manufacturers and specialty ingredient service providers are developing procurement strategies to support operational efficiency and regulatory compliance.
- Advanced therapeutic development programs and iron chelation manufacturing are facilitating adoption of iron chelator ingredients capable of supporting diverse efficacy requirements and competitive processing standards
- Pharmaceutical ingredient innovation and performance-focused manufacturing development programs are enhancing demand for processing-grade iron chelators that support operational efficiency and therapeutic reliability
- Premium pharmaceutical market expansion and specialty therapeutic development are creating opportunities for advanced iron chelation processing capabilities across Brazilian pharmaceutical manufacturing facilities
Germany Focuses on Premium Pharmaceutical Manufacturing
Demand for iron chelators in Germany is projected to grow driven by premium pharmaceutical manufacturing excellence and specialty iron chelation capabilities supporting advanced therapeutic development and comprehensive drug applications. The country's established pharmaceutical processing tradition and growing performance therapy market segments are creating demand for high-quality iron chelation ingredients that support operational performance and therapeutic standards. Pharmaceutical manufacturers and processing suppliers are maintaining comprehensive development capabilities to support diverse manufacturing requirements.
- Premium pharmaceutical manufacturing and specialty iron chelation programs are supporting demand for processing-grade iron chelators that meet contemporary efficacy and reliability standards
- Drug development and performance-focused manufacturing programs are creating opportunities for specialized iron chelation ingredients that provide comprehensive therapeutic support
- Manufacturing modernization and pharmaceutical quality enhancement programs are facilitating adoption of advanced processing capabilities throughout major pharmaceutical regions
South Korea Focuses on Advanced Pharmaceutical Manufacturing
Demand for iron chelators in South Korea is projected to grow at 4.0% CAGR driven by advanced pharmaceutical manufacturing excellence and specialty iron chelation capabilities supporting therapeutic development and comprehensive medical applications. The country's established pharmaceutical processing tradition and growing performance therapy market segments are creating demand for high-quality iron chelation ingredients that support operational performance and therapeutic standards.
- Advanced pharmaceutical manufacturing and specialty iron chelation programs are supporting demand for processing-grade iron chelators that meet contemporary efficacy and reliability standards
- Therapeutic development and performance-focused manufacturing programs are creating opportunities for specialized iron chelation ingredients that provide comprehensive medical support
- Manufacturing modernization and pharmaceutical quality enhancement programs are facilitating adoption of advanced processing capabilities throughout major pharmaceutical regions
Japan Focuses on Precision Pharmaceutical Manufacturing
Demand for iron chelators in Japan is projected to grow driven by precision pharmaceutical manufacturing excellence and specialty iron chelation capabilities supporting advanced therapeutic development and comprehensive technical applications. The country's established pharmaceutical processing tradition and growing performance therapy market segments are creating demand for high-quality iron chelation ingredients that support operational performance and therapeutic standards.
- Precision pharmaceutical manufacturing and specialty iron chelation programs are supporting demand for processing-grade iron chelators that meet contemporary efficacy and reliability standards
- Therapeutic development and performance-focused manufacturing programs are creating opportunities for specialized iron chelation ingredients that provide comprehensive medical support
- Manufacturing modernization and pharmaceutical quality enhancement programs are facilitating adoption of advanced processing capabilities throughout major pharmaceutical regions
Europe Market Split by Processing Application
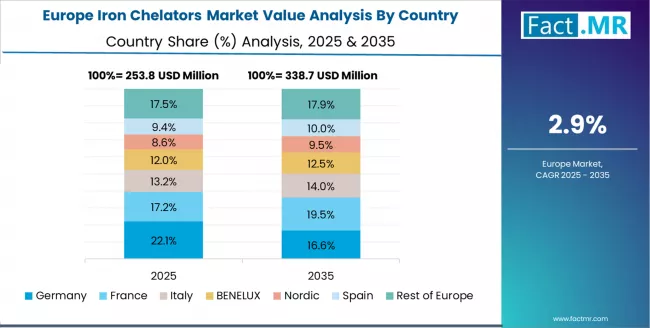
European iron chelators operations are increasingly polarized between Western European precision processing and Eastern European cost-competitive manufacturing. German (USD 250.0 million) and UK facilities (USD 180.0 million) dominate premium therapeutic and pharmaceutical iron chelation processing, leveraging advanced pharmaceutical technologies and strict quality protocols that command price premiums in global markets. German processors maintain leadership in high-performance iron chelation applications, with major pharmaceutical companies driving technical specifications that smaller suppliers must meet to access supply contracts.
France (USD 160.0 million), Italy (USD 130.0 million), and Spain (USD 100.0 million) operations focus on specialized applications and regional market requirements. Rest of Europe (USD 280.0 million) including Eastern European operations in Poland, Hungary, and Czech Republic are capturing volume-oriented processing contracts through labor cost advantages and EU regulatory compliance, particularly in standard iron chelation ingredients for pharmaceutical applications.
The regulatory environment presents both opportunities and constraints. EU pharmaceutical regulations and drug approval directives create barriers for novel iron chelation ingredients but establish quality standards that favor established European processors over imports. Brexit has fragmented UK sourcing from EU suppliers, creating opportunities for direct relationships between processors and British pharmaceutical manufacturers.
Supply chain consolidation accelerates as processors seek economies of scale to absorb rising energy costs and compliance expenses. Vertical integration increases, with major pharmaceutical manufacturers acquiring processing facilities to secure iron chelation supplies and quality control. Smaller processors face pressure to specialize in niche applications or risk displacement by larger, more efficient operations serving mainstream pharmaceutical manufacturing requirements.
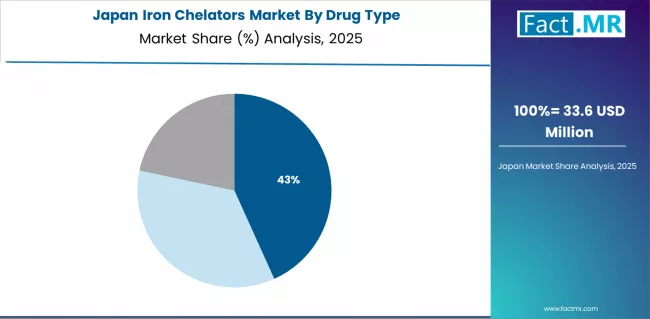
Premium Quality Standards Define Japanese Market Dynamics
Japanese iron chelators operations reflect the country's exacting quality standards and sophisticated therapeutic expectations. Major pharmaceutical manufacturers maintain rigorous supplier qualification processes that often exceed international standards, requiring extensive documentation, batch testing, and facility audits that can take 12-18 months to complete. This creates high barriers for new suppliers but ensures consistent quality that supports premium product positioning.
The Japanese market demonstrates unique application preferences with Thalassemia accounting for 45.0%, Sickle Cell/Other Anemias 35.0%, and Others 20.0%. Companies require specific chelation ratios and purity specifications that differ from Western applications, driving demand for customized processing capabilities.
Japanese market demonstrates unique type preferences with Deferasirox accounting for 50.0%, Deferiprone 30.0%, and Deferoxamine 20.0%. Companies require specific molecular binding and therapeutic specifications that differ from Western applications, driving demand for customized chelation capabilities.
Regulatory oversight emphasizes comprehensive pharmaceutical ingredient management and traceability requirements that surpass most international standards. The drug registration system requires detailed ingredient sourcing information, creating advantages for suppliers with transparent supply chains and comprehensive documentation systems.
Supply chain management focuses on relationship-based partnerships rather than purely transactional procurement. Japanese companies typically maintain long-term supplier relationships spanning decades, with annual contract negotiations emphasizing quality consistency over price competition. This stability supports investment in specialized processing equipment tailored to Japanese specifications.
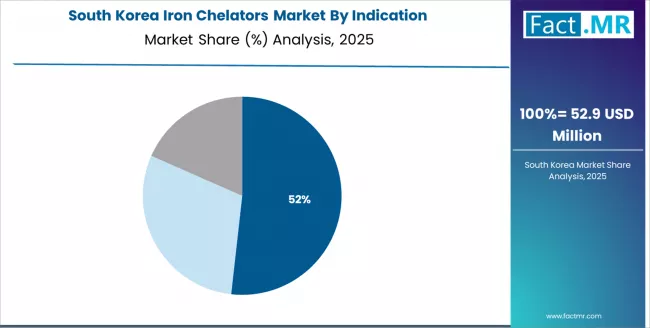
Market Dynamics Drive Innovation in South Korea
South Korean iron chelators operations reflect the country's advanced pharmaceutical manufacturing sector and export-oriented business model. Major pharmaceutical companies drive sophisticated ingredient procurement strategies, establishing direct relationships with global suppliers to secure consistent quality and pricing for their therapeutic and medical operations targeting both domestic and international markets.
The Korean market demonstrates particular strength in application distribution with Thalassemia accounting for 55.0%, Sickle Cell/Other Anemias 30.0%, and Others 15.0%. This health-focused approach creates demand for specific efficacy specifications that differ from Western applications, requiring suppliers to adapt chelation and purification techniques.
Korean market demonstrates unique form preferences with Oral accounting for 75.0% and Injectable 25.0%. Companies require specific delivery mechanisms and therapeutic specifications that differ from Western applications, driving demand for customized processing capabilities.
Regulatory frameworks emphasize pharmaceutical ingredient safety and traceability, with Korean health administration standards often exceeding international requirements. This creates barriers for smaller pharmaceutical suppliers but benefits established processors who can demonstrate compliance capabilities. The regulatory environment particularly favors suppliers with comprehensive certification and documentation systems.
Supply chain efficiency remains critical given Korea's geographic limitations and import dependence. Companies increasingly pursue long-term contracts with suppliers in United States, Germany, and Japan to ensure reliable access to raw materials while managing foreign exchange risks. Technical logistics investments support quality preservation during extended shipping periods.
The market faces pressure from rising labor costs and competition from lower-cost regional manufacturers, driving automation investments and consolidation among smaller processors. However, the premium positioning of Korean pharmaceutical brands internationally continues to support demand for high-quality iron chelation ingredients that meet stringent specifications.
Competitive Landscape of Iron Chelators Market
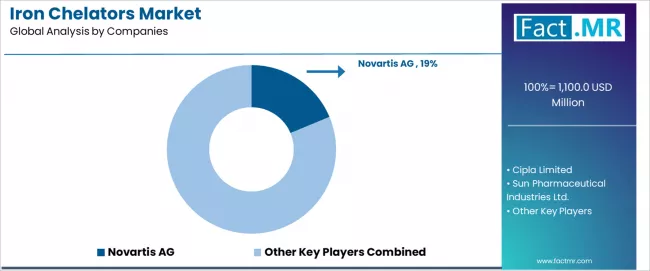
Profit pools are consolidating upstream in scaled iron chelation production systems and downstream in value-added specialty grades for therapeutic applications, blood disorder treatment, and pharmaceutical formulations where certification, traceability, and consistent efficacy command premiums. Value is migrating from raw chemical commodity trading to specification-tight, application-ready iron chelation pharmaceuticals where technical expertise and quality control drive competitive advantage.
Several archetypes set the pace: global pharmaceutical integrators defending share through production scale and technical reliability; multi-grade processors that manage complexity and serve diverse applications; specialty iron chelation developers with formulation expertise and pharmaceutical industry ties; and efficacy-driven suppliers pulling volume in premium therapeutic and medical applications.
Switching costs re-qualification, efficacy testing, performance validation, provide stability for incumbents, while supply shocks and regulatory changes reopen opportunities for diversified suppliers. Consolidation and verticalization continue; digital procurement emerge in commodity grades while premium specifications remain relationship led.
Focus areas: lock pharmaceutical and therapeutic pipelines with application-specific grades and service level agreements; establish multi-grade production capabilities and technical disclosure; develop specialized iron chelation formulations with efficacy claims.
| Stakeholder Type | Primary Advantage | Repeatable Plays |
|---|---|---|
| Global pharmaceutical integrators | Scale, production integration, technical reliability | Long-term contracts, tight specs, co-development with pharmaceuticals/therapeutics |
| Multi-grade processors | Grade diversification, application expertise, supply flexibility | Multi-application serving, technical support, quality assurance across segments |
| Specialty iron chelation developers | Formulation expertise and industry relationships | Custom grades, efficacy science, performance SLAs |
| Efficacy suppliers | Application-focused demand and specialized service | Technical efficacy claims, specialized grades, application activation |
| Pharmaceutical distributors & platforms | Technical support for mid-tier manufacturers | Grade selection, smaller volumes, technical service |
Key Players in the Iron Chelators Market
- Novartis AG
- Cipla Limited
- Sun Pharmaceutical Industries Ltd.
- Apotex Inc.
- Natco Pharma Limited
- Teva Pharmaceutical Industries Ltd.
- Mylan N.V.
- Shionogi & Co. Ltd.
- Novamed Pharmaceuticals
- MacroChem Corporation
- Hikma Pharmaceuticals PLC
- Chiesi Farmaceutici S.p.A.
- Sanofi S.A.
- Lupin Limited
- Takeda Pharmaceutical Company Limited
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 1,100.0 Million |
| Product | Deferasirox, Deferiprone, Deferoxamine |
| Application | Thalassemia, Sickle Cell/Other Anemias, Others |
| Form | Oral, Injectable |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | United States, Germany, China, Japan, India, South Korea, Brazil, and other 40+ countries |
| Key Companies Profiled | Novartis, Cipla, Sun Pharma, Apotex, Natco, Teva, Mylan, Shionogi, Novamed, MacroChem, Hikma, Chiesi, Sanofi, Lupin, Takeda |
| Additional Attributes | Dollar sales by product/application/form, regional demand (NA, EU, APAC), competitive landscape, oral vs. injectable adoption, production/processing integration, and advanced processing innovations driving efficacy enhancement, technical advancement, and efficiency |
Iron Chelators Market Segmentation
-
By Drug Type :
- Deferasirox
- Deferiprone
- Deferoxamine
-
By Indication :
- Thalassemia
- Sickle Cell/Other Anemias
- Others
-
By Form :
- Oral
- Injectable
-
By Region :
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East & Africa
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Drug Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Drug Type , 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Drug Type , 2025 to 2035
- Deferasirox
- Deferiprone
- Deferoxamine
- Y to o to Y Growth Trend Analysis By Drug Type , 2020 to 2024
- Absolute $ Opportunity Analysis By Drug Type , 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Indication
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Indication, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Indication, 2025 to 2035
- Thalassemia
- Sickle Cell/Other Anemias
- Others
- Y to o to Y Growth Trend Analysis By Indication, 2020 to 2024
- Absolute $ Opportunity Analysis By Indication, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Form
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Form, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Form, 2025 to 2035
- Oral
- Injectable
- Y to o to Y Growth Trend Analysis By Form, 2020 to 2024
- Absolute $ Opportunity Analysis By Form, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Drug Type
- By Indication
- By Form
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Type
- By Indication
- By Form
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Drug Type
- By Indication
- By Form
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Type
- By Indication
- By Form
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Drug Type
- By Indication
- By Form
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Type
- By Indication
- By Form
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Drug Type
- By Indication
- By Form
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Type
- By Indication
- By Form
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Drug Type
- By Indication
- By Form
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Type
- By Indication
- By Form
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Drug Type
- By Indication
- By Form
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Type
- By Indication
- By Form
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Drug Type
- By Indication
- By Form
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Type
- By Indication
- By Form
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Type
- By Indication
- By Form
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Drug Type
- By Indication
- By Form
- Competition Analysis
- Competition Deep Dive
- Novartis AG
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Cipla Limited
- Sun Pharmaceutical Industries Ltd.
- Apotex Inc.
- Natco Pharma Limited
- Teva Pharmaceutical Industries Ltd.
- Mylan N.V.
- Shionogi & Co. Ltd.
- Novamed Pharmaceuticals
- MacroChem Corporation
- Hikma Pharmaceuticals PLC
- Chiesi Farmaceutici S.p.A.
- Sanofi S.A.
- Lupin Limited
- Takeda Pharmaceutical Company Limited
- Novartis AG
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Drug Type , 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Form, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Drug Type , 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Form, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Drug Type , 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Form, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Drug Type , 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Form, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Drug Type , 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by Form, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Drug Type , 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by Form, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Drug Type , 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by Form, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Drug Type , 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Indication, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by Form, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Drug Type , 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Drug Type , 2025-2035
- Figure 5: Global Market Attractiveness Analysis by Drug Type
- Figure 6: Global Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Indication, 2025-2035
- Figure 8: Global Market Attractiveness Analysis by Indication
- Figure 9: Global Market Value Share and BPS Analysis by Form, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Form, 2025-2035
- Figure 11: Global Market Attractiveness Analysis by Form
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025-2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025-2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025-2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025-2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025-2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Drug Type , 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Drug Type , 2025-2035
- Figure 25: North America Market Attractiveness Analysis by Drug Type
- Figure 26: North America Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Indication, 2025-2035
- Figure 28: North America Market Attractiveness Analysis by Indication
- Figure 29: North America Market Value Share and BPS Analysis by Form, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Form, 2025-2035
- Figure 31: North America Market Attractiveness Analysis by Form
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Drug Type , 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Drug Type , 2025-2035
- Figure 35: Latin America Market Attractiveness Analysis by Drug Type
- Figure 36: Latin America Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Indication, 2025-2035
- Figure 38: Latin America Market Attractiveness Analysis by Indication
- Figure 39: Latin America Market Value Share and BPS Analysis by Form, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Form, 2025-2035
- Figure 41: Latin America Market Attractiveness Analysis by Form
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Drug Type , 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Drug Type , 2025-2035
- Figure 45: Western Europe Market Attractiveness Analysis by Drug Type
- Figure 46: Western Europe Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Indication, 2025-2035
- Figure 48: Western Europe Market Attractiveness Analysis by Indication
- Figure 49: Western Europe Market Value Share and BPS Analysis by Form, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by Form, 2025-2035
- Figure 51: Western Europe Market Attractiveness Analysis by Form
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Drug Type , 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Drug Type , 2025-2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Drug Type
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Indication, 2025-2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Indication
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by Form, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by Form, 2025-2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by Form
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Drug Type , 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Drug Type , 2025-2035
- Figure 65: East Asia Market Attractiveness Analysis by Drug Type
- Figure 66: East Asia Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Indication, 2025-2035
- Figure 68: East Asia Market Attractiveness Analysis by Indication
- Figure 69: East Asia Market Value Share and BPS Analysis by Form, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by Form, 2025-2035
- Figure 71: East Asia Market Attractiveness Analysis by Form
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Drug Type , 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Drug Type , 2025-2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Drug Type
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Indication, 2025-2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Indication
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by Form, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by Form, 2025-2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by Form
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Drug Type , 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Drug Type , 2025-2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Drug Type
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Indication, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Indication, 2025-2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Indication
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by Form, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by Form, 2025-2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by Form
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the iron chelators market in 2025?
The global iron chelators market is estimated to be valued at USD 1,100.0 million in 2025.
What will be the size of iron chelators market in 2035?
The market size for the iron chelators market is projected to reach USD 1,700.0 million by 2035.
How much will be the iron chelators market growth between 2025 and 2035?
The iron chelators market is expected to grow at a 4.4% CAGR between 2025 and 2035.
What are the key product types in the iron chelators market?
The key product types in iron chelators market are deferasirox, deferiprone and deferoxamine.
Which indication segment to contribute significant share in the iron chelators market in 2025?
In terms of indication, thalassemia segment to command 50.0% share in the iron chelators market in 2025.