Lysosomal Storage Disease Treatment Market
Lysosomal Storage Disease Treatment Market Size and Share Forecast Outlook 2025 to 2035
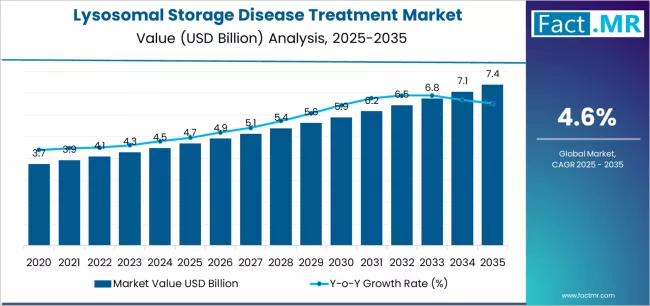
Lysosomal storage disease treatment market is projected to grow from USD 4.7 billion in 2025 to USD 7.4 billion by 2035, at a CAGR of 4.6%. Enzyme Replacement Therapy will dominate with a 84.0% market share, while gaucher disease will lead the disease type segment with a 29.3% share.
Lysosomal Storage Disease Treatment Market Forecast and Outlook 2025 to 2035
The global lysosomal storage disease treatment market is projected to grow from USD 4.7 billion in 2025 to approximately USD 7.4 billion by 2035, recording a substantial absolute increase of USD 2.7 billion over the forecast period. This translates into a total growth of 58.1%, with the market forecast to expand at a compound annual growth rate (CAGR) of 4.6% between 2025 and 2035.
The market is expected to grow by over 1.6X during this period, supported by expanding patient diagnosis rates, advancing therapeutic development programs, and growing emphasis on rare disease treatment accessibility and specialized enzyme replacement therapy optimization across global healthcare operations.
Quick Stats on Lysosomal Storage Disease Treatment Market
- Lysosomal Storage Disease Treatment Market Value (2025): USD 4.7 billion
- Lysosomal Storage Disease Treatment Market Forecast Value (2035): USD 7.4 billion
- Lysosomal Storage Disease Treatment Market Forecast CAGR (2025 to 2035): 4.6%
- Leading Treatment Type in Lysosomal Storage Disease Treatment Market: Enzyme Replacement Therapy (84.0%)
- Leading Disease Type in Lysosomal Storage Disease Treatment Market: Fabry Disease (29.3%)
- Key Growth Regions in Lysosomal Storage Disease Treatment Market: North America, Europe, and Asia Pacific
- Key Players in Lysosomal Storage Disease Treatment Market: Sanofi (Genzyme), Pfizer Inc, Takeda Pharmaceutical Company Limited (Shire Plc), BioMarin, Johnson & Johnson (Actelion Pharmaceuticals Ltd), Amicus Therapeutics Inc, Alexion Pharmaceuticals Inc, Sigilon Therapeutics Inc, Orphazyme A/S, ISU Abxis
The lysosomal storage disease treatment market is positioned for substantial expansion, driven by increasing recognition of rare genetic disorder management importance, growing patient advocacy initiatives with enhanced diagnostic capabilities, and rising adoption of advanced enzyme replacement and substrate reduction technologies across specialized rare disease treatment centers globally.
The market demonstrates robust fundamentals supported by expanding orphan drug development networks, healthcare professionals' focus on early intervention protocols, and rising recognition of lysosomal storage disease therapies as critical healthcare components in achieving enhanced patient quality of life outcomes, metabolic function preservation capabilities, and disease progression management effectiveness within modern rare disease healthcare architectures across diverse patient populations.
Market growth is underpinned by therapeutic innovations in enzyme replacement procedures, particularly advanced recombinant enzyme formulations and substrate reduction system integration, which offer enhanced treatment efficacy, improved patient outcomes, and superior compatibility with comprehensive rare disease management protocols prevalent in contemporary specialized medical practices.
Healthcare providers increasingly prioritize lysosomal storage disease treatments that deliver optimal balance between clinical effectiveness, long-term therapeutic reliability, and cost-effectiveness while adhering to increasingly stringent patient safety standards and orphan drug regulatory requirements across global pharmaceutical markets.
The convergence of rare disease treatment expansion in developed healthcare regions, specialized metabolic clinic development in emerging markets, and orphan drug incentive program advancement creates multifaceted growth opportunities for lysosomal storage disease treatment providers and specialized pharmaceutical operators.
The lysosomal storage disease treatment landscape is experiencing transformative changes as clinicians adopt sophisticated diagnostic protocols including advanced genetic testing, comprehensive metabolic screening panels, and enzyme activity measurement systems that enable early disease detection and precise treatment planning.
These diagnostic advancements are complemented by evolving therapeutic capabilities encompassing next-generation enzyme replacement formulations for Gaucher disease management, advanced substrate reduction approaches for multiple lysosomal disorders, and innovative gene therapy techniques under clinical investigation that significantly improve treatment success rates and patient metabolic function outcomes.
The integration of patient registry platforms and specialized rare disease clinical networks further expands access to lysosomal storage disease treatment expertise, particularly benefiting underdiagnosed patient populations and underserved healthcare regions where specialist availability remains limited.
Between 2025 and 2030, the lysosomal storage disease treatment market is projected to expand from USD 4.7 billion to USD 5.9 billion, demonstrating strong foundational growth driven by global rare disease awareness expansion, increasing prevalence of diagnosed lysosomal storage disorders, and initial deployment of advanced enzyme replacement and substrate reduction technologies across specialized metabolic treatment platforms. This growth phase establishes market infrastructure, validates specialized therapeutic protocols, and creates comprehensive care delivery networks supporting global rare disease healthcare operations.
From 2030 to 2035, the market is forecast to reach USD 7.4 billion, driven by mature enzyme replacement therapy penetration, next-generation substrate reduction procedures requiring sophisticated clinical expertise, and comprehensive integration of personalized treatment systems demanding enhanced patient monitoring capabilities. The growing adoption of newborn screening programs, specialized rare disease diagnostic initiatives, and orphan drug reimbursement coverage expansion will drive demand for comprehensive lysosomal storage disease treatments with enhanced therapeutic outcomes and seamless rare disease healthcare network integration functionality.
Lysosomal Storage Disease Treatment Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value (2025E) | USD 4.7 billion |
| Forecast Value (2035F) | USD 7.4 billion |
| Forecast CAGR (2025 to 2035) | 4.6% |
Why is the Lysosomal Storage Disease Treatment Market Growing?
Market expansion is being supported by the exponential increase in rare disease awareness and the corresponding need for sophisticated orphan drug therapies in lysosomal storage disorder management applications across global healthcare operations. Healthcare practitioners are increasingly focused on advanced enzyme replacement therapies that can improve patient outcomes, enhance quality of life, and optimize metabolic function management protocols while meeting stringent regulatory requirements.
The proven efficacy of specialized lysosomal storage disease treatments in various patient applications makes them an essential component of comprehensive rare disease healthcare strategies and genetic disorder management programs. The growing emphasis on orphan drug development and specialized metabolic clinic integration is driving demand for advanced lysosomal storage disease treatments that meet stringent performance specifications and operational requirements for rare disease applications.
Healthcare providers' preference for reliable, high-performance therapeutic protocols that can ensure consistent clinical outcomes is creating opportunities for innovative enzyme formulations and customized treatment solutions. The rising influence of patient advocacy organizations and rare disease regulatory frameworks is also contributing to increased adoption of premium-grade lysosomal storage disease therapies across different patient categories and healthcare systems requiring advanced therapeutic technology.
Opportunity Pathways - Lysosomal Storage Disease Treatment Market
The lysosomal storage disease treatment market represents a transformative growth opportunity, expanding from USD 4.7 billion in 2025 to USD 7.4 billion by 2035 at a 4.6% CAGR. As healthcare practitioners prioritize rare disease management optimization, specialized metabolic disorder treatment, and clinical excellence in complex genetic disorder environments, lysosomal storage disease therapies have evolved from a niche specialty area to an essential healthcare component enabling precise enzyme deficiency correction, comprehensive metabolic function preservation strategies, and multi-symptom management operations across diverse genetic disorder platforms and orphan disease applications.
The convergence of rare disease awareness acceleration, increasing orphan drug development penetration, advanced diagnostic technology integration, and stringent patient safety mandates creates momentum in demand. High-precision enzyme replacement therapies offering superior clinical outcomes, cost-effective substrate reduction solutions balancing functionality with economics, and specialized treatments for multiple lysosomal disorder applications will capture market premiums, while geographic expansion into high-growth Asian healthcare markets and emerging rare disease ecosystems will drive volume leadership. Healthcare provider emphasis on clinical innovation and treatment reliability provides structural support.
- Pathway A - Enzyme Replacement Therapy Dominance: Leading with 84.0% market share, enzyme replacement therapy applications drive primary demand through complex metabolic disorder workflows requiring comprehensive enzyme supplementation for cellular function optimization. Advanced therapeutic protocols enabling improved disease management, reduced symptom progression, and enhanced patient quality of life outcomes command premium pricing from healthcare providers requiring stringent performance specifications and regulatory compliance. Expected revenue pool: USD 3.9-6.2 billion.
- Pathway B - Fabry Disease Treatment Leadership: Dominating with 29.3% market share through optimal balance of clinical necessity and therapeutic intervention requirements, Fabry disease applications serve the largest single disease category while meeting diverse patient care demands. This disease type addresses both acute symptom management and chronic disease control expectations, making it the preferred category for healthcare practitioners and rare disease treatment operations seeking comprehensive clinical capabilities. Opportunity: USD 1.4-2.2 billion.
- Pathway C - North American Market Leadership: USA (5.4% CAGR) leads global growth through orphan drug development infrastructure, comprehensive rare disease diagnostic capabilities, and specialized metabolic clinic advancement. Strategic partnerships with patient advocacy organizations, treatment accessibility initiatives, and clinical network optimization enable the expansion of lysosomal storage disease therapies in major healthcare and research hubs. Geographic expansion upside: USD 1.8-3.1 billion.
- Pathway D - European Market Consolidation: Germany (4.9% CAGR) and UK (4.5% CAGR) maintain strong positions serving critical rare disease treatment applications requiring specialized therapies for diverse patient populations. Optimized therapeutic protocols supporting multiple lysosomal disorders, healthcare system integration requirements, and proven clinical effectiveness maintain significant volumes from healthcare practitioners and specialized metabolic facilities. Revenue potential: USD 1.2-2.0 billion.
- Pathway E - Advanced Formulation Technologies & Therapeutic Innovation: Companies investing in next-generation enzyme replacement formulations, innovative substrate reduction approaches, and gene therapy development programs gain competitive advantages through consistent clinical delivery and treatment success. Advanced capabilities enabling customized patient dosing specifications and rapid therapeutic protocol development capture premium healthcare partnerships. Technology premium: USD 0.9-1.5 billion.
- Pathway F - Patient Access Programs & Reimbursement Optimization: Specialized rare disease reimbursement strategies, patient assistance program integration, and reliable treatment access systems create competitive differentiation in healthcare markets requiring consistent lysosomal storage disease therapy availability. Companies offering guaranteed patient support services, comprehensive disease management programs, and orphan drug reimbursement navigation gain preferred provider status with quality-focused healthcare operators. Access program value: USD 0.7-1.2 billion.
- Pathway G - Emerging Applications & Clinical Pipeline Development: Beyond traditional enzyme replacement applications, lysosomal storage disease treatments in novel substrate reduction indications, gene therapy approaches, and combination treatment protocols represent growth opportunities. Companies developing new therapeutic mechanisms, supporting clinical trial initiatives, and expanding into adjacent rare disease markets capture incremental demand while diversifying revenue streams. Emerging opportunity: USD 0.5-0.9 billion.
Segmental Analysis
The market is segmented by treatment type, disease type, and region. By treatment type, the market is divided into enzyme replacement therapy (including velaglucerase alfa, alglucosidase alfa, idursulfase, imiglucerase, and others), substrate reduction therapy, and others.
Based on disease type, the market is categorized into Gaucher disease, mucopolysaccharidoses, Pompe disease, Fabry disease, and other diseases. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
Which Treatment Type is the Preferred Option for Lysosomal Storage Disease?
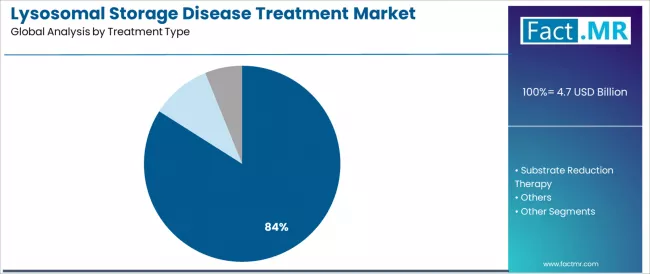
Enzyme replacement therapy is projected to account for 84.0% of the lysosomal storage disease treatment market in 2025, reaffirming its position as the category's dominant treatment type specification. Healthcare providers increasingly recognize the optimal balance of therapeutic effectiveness and metabolic function restoration offered by enzyme replacement therapies for lysosomal storage disorder applications, particularly in Gaucher disease management and Pompe disease treatment environments.
This treatment category addresses both acute symptom relief requirements and chronic disease management demands while providing reliable therapeutic outcomes across diverse patient populations. This segment forms the foundation of most rare disease treatment protocols for lysosomal storage disorder management and specialized metabolic care, as it represents the most clinically validated and commercially established treatment category in the lysosomal storage disease industry.
Clinical validation standards and extensive real-world application continue to strengthen confidence in enzyme replacement therapies among healthcare practitioners and rare disease specialists. With increasing recognition of enzyme deficiency correction impact on patient outcomes and disease progression management requirements, enzyme replacement therapy aligns with both current clinical practices and rare disease treatment evolution goals, making it the central growth driver of comprehensive lysosomal storage disease healthcare strategies across multiple patient platforms.
Within enzyme replacement therapy applications, imiglucerase (Cerezyme) represents a significant portion of Gaucher disease treatment protocols, reflecting established clinical efficacy and healthcare provider familiarity.
Alglucosidase alfa (Myozyme/Lumizyme) accounts for substantial Pompe disease treatment applications, driven by proven therapeutic effectiveness in addressing enzyme deficiency. Idursulfase (Elaprase) serves mucopolysaccharidosis type II patient populations, while velaglucerase alfa and other enzyme replacement formulations address specific lysosomal storage disorder subtypes requiring targeted therapeutic interventions.
What drives Fabry Disease Indication Segment Prominence?
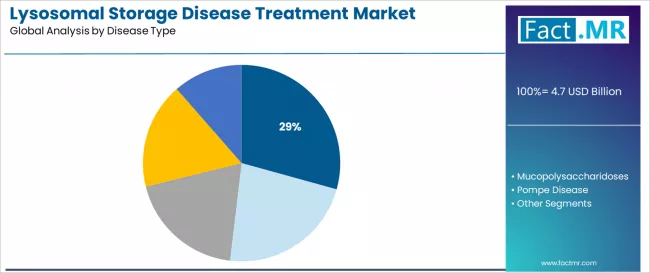
The Fabry disease segment is projected to account for 29.3% of the lysosomal storage disease treatment market in 2025, establishing its position as the leading disease type category. Healthcare practitioners increasingly recognize that Fabry disease, an X-linked lysosomal storage disorder affecting multiple organ systems, represents the most clinically significant and treatment-responsive category requiring specialized enzyme replacement intervention due to disease prevalence and multi-organ manifestation severity.
This disease type addresses both cardiac complication management and renal function preservation requirements while delivering critical therapeutic outcomes across varied patient populations. The segment is supported by the continuing nature of Fabry disease prevalence in both pediatric and adult populations, driven by genetic inheritance patterns, progressive organ damage manifestations, and multi-system complications requiring immediate therapeutic intervention.
Healthcare practitioners are increasingly focusing on advanced enzyme replacement protocols that enhance treatment effectiveness and organ function preservation while maintaining clinical safety standards. As rare disease diagnostic expertise expands and genetic screening capabilities grow, Fabry disease management services will continue to serve a crucial role in ensuring patient quality of life and organ function preservation within the global lysosomal storage disease treatment market.
Gaucher disease represents another substantial portion of indication types, reflecting significant historical market development and established treatment protocols across multiple disease subtypes. Mucopolysaccharidoses account for diverse lysosomal storage disorder applications, driven by multiple disease variants requiring specialized enzyme replacement approaches.
Pompe disease represents critical glycogen storage disorder treatment requirements addressing cardiac and skeletal muscle dysfunction, while other lysosomal storage diseases encompass specialized conditions including Niemann-Pick disease, metachromatic leukodystrophy, and additional rare enzyme deficiency disorders requiring targeted therapeutic services.
What are the Drivers, Restraints, and Key Trends of the Lysosomal Storage Disease Treatment Market?
The lysosomal storage disease treatment market is advancing rapidly due to increasing recognition of rare genetic disorder management importance and growing demand for specialized orphan drug solutions across the healthcare sector.
The market faces challenges, including treatment cost pressures in healthcare systems with limited rare disease funding, restricted availability of specialized metabolic centers in developing regions, and concerns about long-term therapy accessibility in emerging healthcare markets. Innovation in enzyme replacement technologies and advanced genetic screening algorithms continues to influence therapeutic development and market expansion patterns.
Proliferation of Advanced Diagnostic Technologies and Genetic Screening Systems
The accelerating adoption of sophisticated diagnostic platforms is enabling the development of more precise lysosomal storage disease identification applications and treatment protocols that can meet stringent clinical and safety requirements.
Healthcare practitioners demand comprehensive diagnostic integration for rare disease management, including high-resolution genetic testing capabilities and multi-modal enzyme activity measurement formulations that are particularly important for achieving accurate disease identification requirements in complex patient applications.
Advanced diagnostic technologies provide access to early disease detection that can optimize treatment initiation strategies and enhance clinical outcomes while maintaining cost-effectiveness for diverse healthcare system environments.
Integration of Patient Registry Platforms and Specialized Clinical Networks
Modern healthcare organizations are incorporating advanced technologies such as digital patient registry systems, rare disease clinical network capabilities, and specialized metabolic center interfaces to enhance lysosomal storage disease treatment utility and patient care accessibility.
These systems improve specialist collaboration opportunities, enable seamless primary care-metabolic center transitions, and provide better integration between general practitioners and rare disease specialists throughout the diagnostic and treatment experience.
Advanced patient management capabilities also enable customized treatment protocols and early identification of disease progression or therapeutic response patterns, supporting proactive intervention management and improved patient quality of life outcomes.
Analysis of the Lysosomal Storage Disease Treatment Market by Key Countries
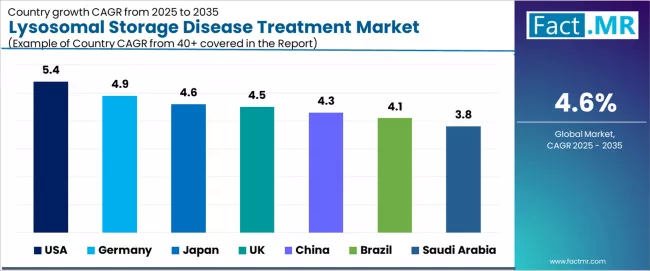
| Country | CAGR (2025 to 2035) |
|---|---|
| USA | 5.4% |
| Germany | 4.9% |
| Japan | 4.6% |
| UK | 4.5% |
| China | 4.3% |
| Brazil | 4.1% |
| Saudi Arabia | 3.8% |
The lysosomal storage disease treatment market is experiencing substantial growth globally, with the USA leading at a 5.4% CAGR through 2035, driven by comprehensive orphan drug development infrastructure, advanced rare disease diagnostic capabilities, and specialized metabolic clinic networks across major healthcare and research centers. Germany follows at 4.9%, supported by robust healthcare system integration, established rare disease treatment protocols, and comprehensive patient access programs. Japan records 4.6% growth, benefiting from advanced healthcare standards and specialized rare disease management capabilities.
The UK demonstrates 4.5% growth, emphasizing orphan drug development excellence and National Health Service rare disease framework implementation. China shows 4.3% growth with expanding rare disease awareness and healthcare infrastructure modernization. Brazil records 4.1% growth, representing growing rare disease recognition and healthcare access improvement, while Saudi Arabia shows 3.8% growth, representing healthcare system development and specialized treatment availability enhancement.
How does USA Demonstrate Market Leadership with Advanced Orphan Drug Infrastructure?
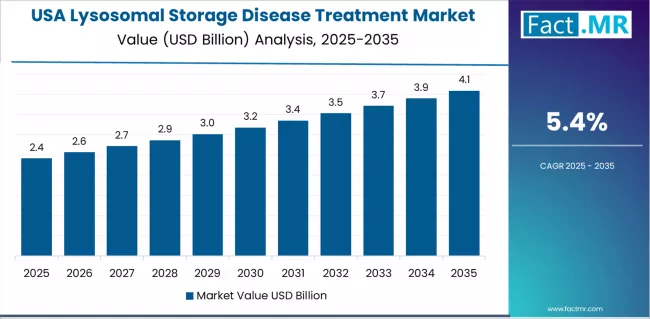
The lysosomal storage disease treatment market in the USA is projected to exhibit exceptional growth with a CAGR of 5.4% through 2035, driven by the world's most advanced orphan drug development ecosystem and comprehensive rare disease diagnostic infrastructure creating significant opportunities for lysosomal storage disease therapy deployment across both pediatric and adult patient populations.
The country's sophisticated rare disease research capabilities and extensive specialized metabolic clinic networks are creating substantial demand for enzyme replacement therapy services across Gaucher disease, Fabry disease, and multiple lysosomal storage disorder patient segments throughout the USA's diverse healthcare regions. The government's strategic emphasis on orphan drug development through regulatory incentives and rare disease research funding is driving substantial investments in therapeutic innovation capabilities and clinical trial infrastructure.
This policy support, combined with the country's extensive patient advocacy networks and comprehensive healthcare insurance coverage, creates a favorable environment for lysosomal storage disease treatment market development. American healthcare practitioners are increasingly focusing on personalized treatment approaches to optimize patient outcomes, with advanced enzyme replacement therapies representing a key component in this rare disease management capability building.
- Government initiatives supporting orphan drug development and rare disease patient access programs are driving demand for specialized lysosomal storage disease treatments across pediatric and adult patient segments
- Healthcare infrastructure expansion and specialized metabolic center networks are supporting appropriate utilization of enzyme replacement therapies among practitioners and rare disease facilities nationwide
- Patient advocacy organizations and rare disease foundations are increasingly promoting early diagnosis importance in lysosomal storage disorder management, creating new treatment demand capabilities
- Rising healthcare investment and growing awareness of enzyme replacement therapy benefits are accelerating lysosomal storage disease treatment adoption across patient categories
What makes Germany Demonstrate European Market Leadership with Healthcare System Integration?
The lysosomal storage disease treatment market in Germany is expanding at a CAGR of 4.9%, supported by comprehensive healthcare system integration, established rare disease treatment protocols, and advancing specialized metabolic clinic infrastructure across the country's developed healthcare corridors.
The country's robust orphan drug reimbursement framework and increasing sophistication of rare disease diagnostic platforms are driving demand for specialized enzyme replacement therapy solutions in both metabolic disorder and multi-system disease applications. International pharmaceutical providers and domestic healthcare systems are establishing treatment capacity to serve the growing demand for quality lysosomal storage disease therapies while supporting the country's position as a leading European rare disease treatment market.
Germany's healthcare sector continues to benefit from favorable regulatory policies, established specialized care infrastructure, and comprehensive patient access programs. The country's focus on expanding rare disease expertise capabilities is driving investments in critical metabolic services including enzyme replacement therapy and advanced diagnostic technologies. This development is particularly important for lysosomal storage disease treatment applications, as healthcare practitioners seek reliable therapeutic sources for orphan drug delivery to enhance clinical capabilities and maintain European rare disease treatment standards.
- Rising awareness about rare disease management and improving domestic specialized care capabilities are creating opportunities for advanced enzyme replacement therapy solutions
- Growing patient diagnosis rates and healthcare system modernization are supporting increased deployment of specialized lysosomal storage disease treatments across patient categories
- Expanding rare disease education capacity and emerging clinical excellence standards are driving innovative applications of enzyme replacement therapies in pediatric and adult patient applications
- Specialized metabolic clinic advancement is enabling delivery of sophisticated treatment protocols, supporting market growth and ensuring comprehensive patient care delivery
Why does Japan maintain Advanced Healthcare Standards Focus?
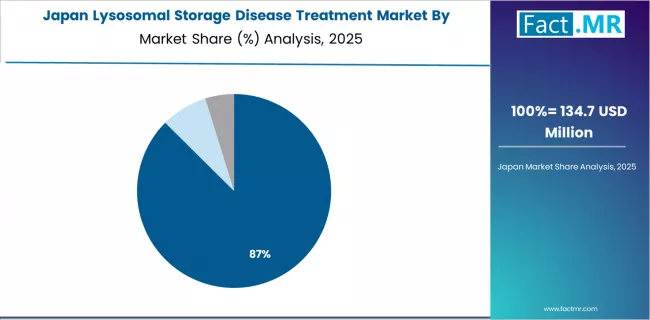
The lysosomal storage disease treatment market in Japan is projected to exhibit solid growth with a CAGR of 4.6% through 2035, driven by advanced healthcare quality standards and established rare disease management protocols. The country's position as a leading developed healthcare market and sophisticated clinical practice environment are creating significant opportunities for enzyme replacement therapy integration across both pediatric metabolic disorder and adult lysosomal storage disease applications.
Japanese healthcare practitioners are leveraging clinical excellence expertise to serve the growing demand for specialized lysosomal storage disease treatments while supporting the country's position as a major Asian rare disease treatment economy. The market benefits from strong healthcare infrastructure supporting comprehensive patient care, enabling optimized therapeutic deployment and seamless rare disease management integration.
This development is particularly important for lysosomal storage disease treatment applications, as practitioners seek sophisticated clinical solutions that maximize patient outcomes while ensuring regulatory compliance in specialized metabolic care and orphan drug treatment environments.
Strategic Market Considerations:
- Specialized rare disease centers and comprehensive healthcare systems leading growth with focus on clinical precision and patient outcome optimization applications
- Advanced healthcare quality requirements are driving sophisticated therapeutic portfolios from standard enzyme replacement protocols to cutting-edge treatment intervention platforms
- Clinical excellence and rare disease expertise supporting competitive positioning in Asian rare disease treatment markets
- Healthcare regulations and patient safety standards beginning to influence therapeutic specifications and orphan drug adoption timelines
How does the UK Demonstrate Orphan Drug Development Excellence?
The UK's advanced rare disease market demonstrates sophisticated lysosomal storage disease treatment deployment with documented effectiveness in National Health Service frameworks and specialized metabolic clinic platforms through integration with cutting-edge diagnostic technologies and treatment protocols. The country leverages orphan drug development leadership and comprehensive rare disease framework infrastructure to maintain a 4.5% CAGR through 2035.
Specialized metabolic centers, including major teaching hospitals in metropolitan regions and university medical facilities, showcase advanced enzyme replacement therapy implementations where sophisticated treatment protocols integrate with comprehensive patient registry programs and ongoing clinical research initiatives to optimize therapeutic effectiveness and patient outcomes.
The healthcare practitioners prioritize evidence-based medicine and long-term patient outcomes in rare disease management delivery, creating demand for premium lysosomal storage disease treatments with advanced capabilities, including sophisticated enzyme replacement formulations and integration with comprehensive National Health Service rare disease frameworks. The market benefits from established specialized care infrastructure and commitment to orphan drug access that drives continuous therapeutic advancement.
Strategic Market Considerations:
- Pediatric and adult patient segments leading growth with focus on clinical evidence and comprehensive treatment protocol applications
- NHS rare disease framework requirements are driving sophisticated therapeutic portfolios from conventional enzyme replacement procedures to advanced substrate reduction platforms
- Orphan drug development excellence and clinical research leadership supporting continued innovation in lysosomal storage disease treatment delivery
- Professional standards and clinical guideline adherence ensuring consistent treatment quality and patient outcome optimization
What Drives China Market Growth with Rare Disease Awareness Expansion?
China's expanding lysosomal storage disease treatment market demonstrates accelerating therapy adoption with a 4.3% CAGR through 2035, driven by growing rare disease awareness, healthcare infrastructure modernization, and comprehensive diagnostic capability development across major healthcare regions. The country's increasing focus on orphan drug access and expanding specialized metabolic clinic networks is creating substantial demand for enzyme replacement therapy solutions across diverse patient populations.
Chinese healthcare practitioners and specialized hospitals are increasingly prioritizing advanced lysosomal storage disease treatments that incorporate proven therapeutic protocols for optimal patient outcomes and clinical care enhancement. Market dynamics focus on accessible lysosomal storage disease treatments that balance clinical effectiveness with cost considerations important to Chinese healthcare system requirements and patient access priorities. Growing domestic rare disease expertise creates opportunities for integrated patient care programs and therapeutic protocol deployment.
Strategic Market Considerations:
- Pediatric patient populations demonstrating focused growth with emphasis on early diagnosis and comprehensive treatment applications
- Expanding healthcare access requirements driving diverse therapeutic specifications with proven efficacy characteristics and patient outcome optimization
- Rare disease awareness supported by patient advocacy initiatives and healthcare policy development capabilities
- Healthcare practitioner preferences emphasizing established treatment protocols and comprehensive clinical validation in lysosomal storage disease applications
Why Does Brazil Maintain Latin American Healthcare Leadership?
The lysosomal storage disease treatment market in Brazil is projected to exhibit growth with a CAGR of 4.1% through 2035, driven by expanding healthcare access initiatives and growing rare disease recognition. The country's position as the largest Latin American healthcare market and increasing orphan drug availability are creating opportunities for enzyme replacement therapy integration across both public health system and private healthcare applications.
Brazilian healthcare practitioners are working to serve the growing demand for specialized lysosomal storage disease treatments while supporting the country's position as a regional rare disease treatment center.
The market benefits from healthcare policy reforms supporting rare disease management, enabling improved therapeutic access and comprehensive patient care integration. This development is particularly important for lysosomal storage disease treatment applications, as healthcare systems seek effective clinical solutions that address patient needs while managing healthcare expenditure in public health and specialized care environments.
Strategic Market Considerations:
- Public healthcare system and private care sectors driving growth with focus on treatment accessibility and patient outcome applications
- Healthcare economics and orphan drug pricing policies influencing therapeutic adoption from basic enzyme replacement protocols to specialized treatment platforms
- Rare disease advocacy and healthcare access initiatives supporting market development in Latin American healthcare systems
- Healthcare regulations and reimbursement frameworks beginning to shape therapeutic specifications and patient access timelines
What Drives Saudi Arabia Market Development with Healthcare System Advancement?
Saudi Arabia's developing lysosomal storage disease treatment market demonstrates emerging therapy adoption with a 3.8% CAGR through 2035, driven by healthcare system modernization, specialized care infrastructure development, and comprehensive medical capability advancement across major healthcare regions.
The country's strategic healthcare transformation initiatives and expanding rare disease treatment availability is creating opportunities for enzyme replacement therapy deployment across diverse patient care platforms. Saudi healthcare practitioners and specialized facilities are working to establish advanced lysosomal storage disease treatment capabilities that support optimal patient outcomes and regional healthcare leadership.
Market dynamics focus on establishing lysosomal storage disease treatment infrastructure that addresses clinical effectiveness requirements while building specialized care capabilities important to Saudi healthcare development goals and regional medical leadership aspirations. Emerging rare disease management expertise creates opportunities for therapeutic protocol implementation and clinical network development.
Strategic Market Considerations:
- Healthcare system modernization and specialized care development driving initial growth with focus on treatment availability and clinical capability applications
- Healthcare transformation priorities influencing therapeutic portfolios from foundational enzyme replacement services to advancing specialized treatment platforms
- Medical expertise development supported by international healthcare partnerships and clinical training program advancement
- Healthcare investment and infrastructure development establishing foundations for expanded rare disease treatment capabilities and patient access enhancement
Europe Market Split by Country
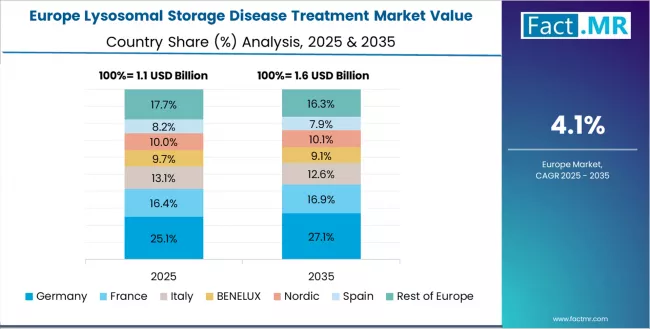
The lysosomal storage disease treatment market in Europe is projected to grow from USD 1.3 billion in 2025 to USD 2.1 billion by 2035, registering a CAGR of 4.9% over the forecast period. Germany is expected to maintain its leadership position with a 32.1% market share in 2025, rising to 33.5% by 2035, supported by its comprehensive healthcare system integration, robust orphan drug reimbursement frameworks, and advanced rare disease treatment infrastructure throughout major metabolic centers and specialized hospital networks.
The UK follows with a 26.4% share in 2025, projected to reach 25.8% by 2035, driven by National Health Service rare disease framework implementation, orphan drug development excellence, and expanding specialized metabolic clinic sophistication serving both domestic and European patient populations. France holds a 19.7% share in 2025, expected to increase to 20.3% by 2035, supported by comprehensive healthcare infrastructure and specialized rare disease management capabilities.
Italy commands a 11.2% share in 2025, projected to reach 10.9% by 2035, while Spain accounts for 7.8% in 2025, expected to reach 7.2% by 2035. The rest of Europe region, including Nordic countries with advanced healthcare systems, Eastern European emerging markets, and smaller Western European specialized care centers, is anticipated to hold 2.8% in 2025, declining slightly to 2.3% by 2035, attributed to market consolidation toward larger core markets with established rare disease treatment infrastructure and lysosomal storage disease therapy expertise.
Competitive Landscape of the Lysosomal Storage Disease Treatment Market
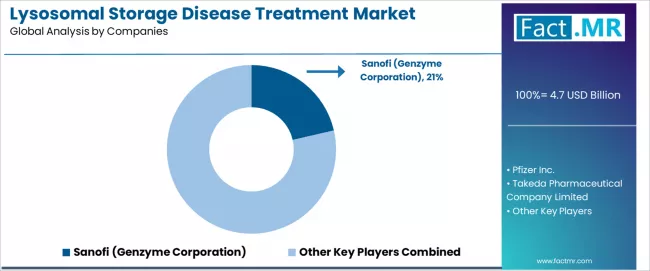
The lysosomal storage disease treatment market is characterized by intense competition among established pharmaceutical companies, specialized orphan drug developers, and biotechnology organizations focused on delivering high-quality, reliable, and accessible rare disease therapeutic solutions.
Companies are investing in clinical trial programs, advanced enzyme formulation development, strategic patient access partnerships, and comprehensive medical education initiatives to deliver effective, efficient, and reliable lysosomal storage disease treatments that meet stringent regulatory standards and patient care expectations. Therapeutic innovation, orphan drug designation optimization, and patient outcome enhancement strategies are central to strengthening product portfolios and market presence.
Sanofi (Genzyme) leads the market with a 21.4% market share, offering comprehensive lysosomal storage disease treatment solutions with a focus on enzyme replacement therapy expertise and advanced therapeutic capabilities for multiple disease indications. Pfizer Inc provides specialized rare disease platforms with emphasis on established therapeutic products and comprehensive patient support program capabilities across global pharmaceutical markets.
Takeda Pharmaceutical Company Limited focuses on orphan drug portfolio excellence and comprehensive rare disease treatment solutions serving international patient communities. BioMarin delivers innovative enzyme replacement therapy development with strong clinical trial capabilities and specialized metabolic disorder focus.
Johnson & Johnson (Actelion Pharmaceuticals Ltd) operates with a focus on bringing advanced rare disease therapeutics to specialized patient populations and orphan drug applications. Amicus Therapeutics Inc provides dedicated lysosomal storage disease treatment platforms emphasizing substrate reduction therapy innovation and comprehensive patient support capabilities.
Alexion Pharmaceuticals Inc specializes in complement-mediated rare disease therapeutics and emerging lysosomal storage disorder treatment applications with emphasis on clinical excellence. Sigilon Therapeutics Inc delivers cell therapy innovation to enhance treatment accessibility and provide next-generation therapeutic solutions. Orphazyme A/S and ISU Abxis focus on specialized heat shock protein therapy approaches and emerging enzyme replacement formulations, emphasizing clinical differentiation and comprehensive treatment protocol development through dedicated rare disease pharmaceutical strategies.
Key Players in the Lysosomal Storage Disease Treatment Market
- Sanofi (Genzyme Corporation)
- Pfizer Inc.
- Takeda Pharmaceutical Company Limited
- BioMarin Pharmaceutical Inc.
- Johnson & Johnson (Actelion Pharmaceuticals Ltd.)
- Amicus Therapeutics, Inc.
- Alexion Pharmaceuticals, Inc.
- Sigilon Therapeutics, Inc.
- Orphazyme A/S
- ISU Abxis Co., Ltd.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 4.7 Billion |
| Treatment Type | Enzyme Replacement Therapy (Velaglucerase Alfa, Alglucosidase Alfa, Idursulfase, Imiglucerase, Others), Substrate Reduction Therapy, Others |
| Disease Type | Gaucher Disease, Mucopolysaccharidoses, Pompe Disease, Fabry Disease, Other Diseases |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | USA, Germany, UK, Japan, China, Brazil, Saudi Arabia and 40+ countries |
| Key Companies Profiled | Sanofi (Genzyme), Pfizer Inc, Takeda Pharmaceutical Company Limited (Shire Plc), BioMarin, Johnson & Johnson (Actelion Pharmaceuticals Ltd), Amicus Therapeutics Inc, Alexion Pharmaceuticals Inc, Sigilon Therapeutics Inc, Orphazyme A/S, ISU Abxis |
| Additional Attributes | Dollar sales by treatment type, disease type, regional demand trends, competitive landscape, healthcare practitioner preferences for specific enzyme replacement therapies, integration with comprehensive rare disease management systems, innovations in therapeutic formulation development, orphan drug regulatory advancement, and patient outcome optimization capabilities |
Lysosomal Storage Disease Treatment Market by Segments
-
Treatment Type :
- Enzyme Replacement Therapy
- Velaglucerase Alfa
- Alglucosidase Alfa (Myozyme/Lumizyme)
- Idursulfase (Elaprase)
- Imiglucerase (Cerezyme)
- Others
- Substrate Reduction Therapy
- Others
- Enzyme Replacement Therapy
-
Disease Type :
- Gaucher Disease
- Mucopolysaccharidoses
- Pompe Disease
- Fabry Disease
- Other Diseases
-
Region :
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Countries
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- USA Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- USA Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- USA Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Treatment Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Treatment Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Treatment Type, 2025 to 2035
- Enzyme Replacement Therapy
- Substrate Reduction Therapy
- Others
- Y to o to Y Growth Trend Analysis By Treatment Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Treatment Type, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Disease Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Disease Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Disease Type, 2025 to 2035
- Gaucher Disease
- Mucopolysaccharidoses
- Pompe Disease
- Fabry Disease
- Other Diseases
- Y to o to Y Growth Trend Analysis By Disease Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Disease Type, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- USA
- Market Attractiveness Analysis By Region
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- By Treatment Type
- By Disease Type
- Market Attractiveness Analysis
- By Country
- By Treatment Type
- By Disease Type
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Treatment Type
- By Disease Type
- Competition Analysis
- Competition Deep Dive
- Sanofi (Genzyme Corporation)
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Pfizer Inc.
- Takeda Pharmaceutical Company Limited
- BioMarin Pharmaceutical Inc.
- Johnson & Johnson (Actelion Pharmaceuticals Ltd.)
- Amicus Therapeutics, Inc.
- Alexion Pharmaceuticals, Inc.
- Sigilon Therapeutics, Inc.
- Orphazyme A/S
- ISU Abxis Co., Ltd
- Sanofi (Genzyme Corporation)
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: USA Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 3: USA Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
- Table 4: USA Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: USA Market Value (USD Million) Forecast by Treatment Type, 2020 to 2035
- Table 6: USA Market Value (USD Million) Forecast by Disease Type, 2020 to 2035
List Of Figures
- Figure 1: USA Market Pricing Analysis
- Figure 2: USA Market Value (USD Million) Forecast 2020-2035
- Figure 3: USA Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 4: USA Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 5: USA Market Attractiveness Analysis by Treatment Type
- Figure 6: USA Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 7: USA Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 8: USA Market Attractiveness Analysis by Disease Type
- Figure 9: USA Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: USA Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: USA Market Attractiveness Analysis by Region
- Figure 12: USA Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: USA Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 14: USA Market Value Share and BPS Analysis by Treatment Type, 2025 and 2035
- Figure 15: USA Market Y to o to Y Growth Comparison by Treatment Type, 2025 to 2035
- Figure 16: USA Market Attractiveness Analysis by Treatment Type
- Figure 17: USA Market Value Share and BPS Analysis by Disease Type, 2025 and 2035
- Figure 18: USA Market Y to o to Y Growth Comparison by Disease Type, 2025 to 2035
- Figure 19: USA Market Attractiveness Analysis by Disease Type
- Figure 20: USA Market - Tier Structure Analysis
- Figure 21: USA Market - Company Share Analysis
- FAQs -
How big is the lysosomal storage disease treatment market in 2025?
The global lysosomal storage disease treatment market is estimated to be valued at USD 4.7 billion in 2025.
What will be the size of lysosomal storage disease treatment market in 2035?
The market size for the lysosomal storage disease treatment market is projected to reach USD 7.4 billion by 2035.
How much will be the lysosomal storage disease treatment market growth between 2025 and 2035?
The lysosomal storage disease treatment market is expected to grow at a 4.6% CAGR between 2025 and 2035.
What are the key product types in the lysosomal storage disease treatment market?
The key product types in lysosomal storage disease treatment market are enzyme replacement therapy, substrate reduction therapy and others.
Which disease type segment to contribute significant share in the lysosomal storage disease treatment market in 2025?
In terms of disease type, gaucher disease segment to command 29.3% share in the lysosomal storage disease treatment market in 2025.