Tissue Diagnostics Market
Tissue Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
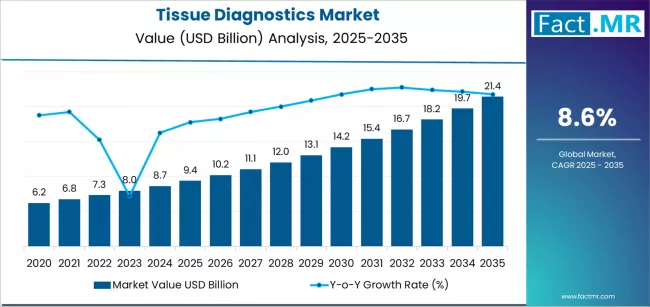
Tissue diagnostics market is projected to grow from USD 9.4 billion in 2025 to USD 21.4 billion by 2035, at a CAGR of 8.6%. Immunohistochemistry will dominate with a 25.8% market share, while breast cancer will lead the application segment with a 50.5% share.
Tissue Diagnostics Market Forecast and Outlook 2025 to 2035
The global tissue diagnostics market is projected to reach USD 21.4 billion by 2035, marking an absolute increase of USD 12.0 billion over the forecast period. Valued at USD 9.4 billion in 2025, the market is set to expand at a CAGR of 8.6% through 2035, resulting in a total market growth of nearly 2.3 times.
Increasing global cancer incidence, expanding oncology screening programs, and the growing shift toward precision medicine continue to drive steady adoption of advanced tissue-based diagnostic systems across both mature and developing healthcare markets. These trends support the rising demand for immunohistochemistry technologies, digital pathology systems, and companion diagnostic solutions designed to guide therapy decisions with greater accuracy.
Quick Stats for Tissue Diagnostics Market
- Tissue Diagnostics Market Value (2025): USD 9.4 billion
- Tissue Diagnostics Market Forecast Value (2035): USD 21.4 billion
- Tissue Diagnostics Market Forecast CAGR: 8.6%
- Leading Technology in Tissue Diagnostics Market: Immunohistochemistry
- Key Growth Regions in Tissue Diagnostics Market: Asia Pacific, North America, and Europe
- Top Players in Tissue Diagnostics Market: F. Hoffmann-La Roche Ltd., Abbott Laboratories, Thermo Fisher Scientific Inc., Siemens Healthineers, Danaher Corporation, bioMérieux SA, QIAGEN, BD, Merck KGaA, GE Healthcare
A key market trend is the rapid digital transformation within pathology laboratories. Hospitals and diagnostic networks are increasingly integrating digital pathology scanners, cloud-based image management platforms, and AI-enabled image analysis tools into routine workflows. These technologies allow high-resolution slide evaluation, remote expert consultation, and improved consistency in diagnostic interpretation.
As cancer caseloads rise and pathologist shortages intensify, automated diagnostic assistance and standardized digital workflows are becoming essential components of modern clinical infrastructure. The expansion of AI-powered algorithms for tumor grading, biomarker quantification, and pattern recognition is further strengthening adoption across primary care hospitals, cancer institutes, and large reference laboratories.
Another major trend shaping market expansion is the shift toward biomarker-driven oncology care. Pharmaceutical advancements in targeted therapies and immunotherapies are increasing demand for companion diagnostic tests capable of detecting specific molecular or protein-level indicators required for treatment eligibility. Tissue diagnostics play a central role in identifying actionable biomarkers for cancers such as breast, lung, colorectal, and prostate.
As clinical trials become more biomarker-intensive, partnerships between diagnostic manufacturers, pharmaceutical companies, and research institutions are accelerating assay development and advancing next-generation diagnostic platforms that support personalized treatment selection. This evolution aligns with broader healthcare strategies prioritizing early detection, faster diagnosis, and improved treatment outcomes.
Global investment in diagnostic infrastructure is also widening access to tissue-based technologies, particularly in Asia Pacific, the Middle East, and Latin America. Rising healthcare expenditure, hospital expansion programs, and modernization of laboratory capabilities are enabling broader use of automated staining systems, molecular pathology tools, and digital archiving solutions.
Government initiatives promoting cancer screening and national diagnostic network upgrades further reinforce future demand. Training programs and skill-development initiatives are also gaining momentum as healthcare providers work to improve diagnostic capacity, standardize laboratory quality, and reduce reliance on centralized specialty laboratories.
Despite strong growth drivers, certain structural barriers continue to influence adoption. Variability in reimbursement frameworks across countries affects affordability and test accessibility, especially for advanced immunohistochemistry or digital pathology solutions. Regulatory complexity associated with in vitro diagnostic approvals, laboratory accreditation, and quality assurance requirements can lengthen market entry timelines.
Smaller hospitals and laboratories in resource-limited settings also face capital constraints, making it challenging to invest in digital pathology equipment, automated staining platforms, and continuous reagent supply chains. Addressing these constraints will be essential for ensuring consistent and equitable market expansion across global healthcare ecosystems.
Tissue Diagnostics Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2030, the tissue diagnostics market is projected to expand from USD 9.4 billion to USD 14.2 billion, resulting in a value increase of USD 4.8 billion, which represents 40.0% of the total forecast growth for the decade. This phase of development will be shaped by rising demand for companion diagnostics and biomarker testing, product innovation in digital pathology platforms and AI-enabled image analysis solutions, as well as expanding integration with electronic health records and laboratory information systems. Companies are establishing competitive positions through investment in antibody development and reagent manufacturing capabilities, automated staining platform technologies, and strategic market expansion across hospital pathology departments, reference laboratory networks, and pharmaceutical research applications.
From 2030 to 2035, the market is forecast to grow from USD 14.2 billion to USD 21.4 billion, adding another USD 7.2 billion, which constitutes 60.0% of the overall ten-year expansion. This period is expected to be characterized by the expansion of specialized tissue diagnostic applications, including next-generation sequencing integration with tissue-based testing and artificial intelligence-powered diagnostic algorithms tailored for specific cancer types, strategic collaborations between diagnostic companies and pharmaceutical organizations, and an enhanced focus on standardized testing protocols and quality assurance certifications. The growing emphasis on precision medicine and evidence-based treatment selection will drive demand for clinically validated tissue diagnostic solutions across diverse oncology applications.
Tissue Diagnostics Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 9.4 billion |
| Market Forecast Value (2035) | USD 21.4 billion |
| Forecast CAGR (2025-2035) | 8.6% |
Why is the Tissue Diagnostics Market Experiencing Robust Growth?
The tissue diagnostics market grows by enabling hospital laboratories, diagnostic centers, and pharmaceutical organizations to access advanced pathology technologies that support accurate disease diagnosis while meeting clinical demand for precision oncology solutions. Healthcare providers face mounting pressure to deliver timely and accurate cancer diagnoses with proven biomarker identification capabilities, with immunohistochemistry platforms typically providing 90-95% diagnostic accuracy rates comparable to molecular testing alternatives, making these technologies essential for competitive clinical positioning in oncology care pathways. The diagnostic industry's need for versatile testing platforms and workflow integration creates demand for diverse tissue diagnostic systems that can provide comprehensive biomarker analysis, maintain consistent performance across different tissue types, and ensure regulatory compliance without compromising diagnostic quality or operational efficiency standards.
Government initiatives promoting cancer screening programs and early detection strategies drive adoption in hospital pathology services, reference laboratory applications, and pharmaceutical clinical trial partnerships, where tissue diagnostic testing has a direct impact on patient treatment outcomes and therapeutic development success. The healthcare industry's growing focus on personalized medicine and targeted therapy selection further expands market opportunities, with clinical research demonstrating measurable improvements in treatment response rates, patient survival outcomes, and healthcare cost effectiveness through biomarker-guided treatment decisions. However, supply chain complexity during specialized reagent sourcing and the technical requirements for digital pathology implementation and laboratory workflow automation may limit accessibility among smaller diagnostic facilities and developing regions with limited infrastructure for advanced tissue diagnostic platforms and quality management systems.
Segmental Analysis
The market is segmented by technology, application, modality, end-use, and region. By technology, the market is divided into immunohistochemistry, digital pathology & workflow, in situ hybridization, molecular pathology, anatomic pathology, and others. Based on application, the market is categorized into breast cancer, NSCLC, prostate cancer, and other cancers. By modality, the market is segmented into clinical, pharma/CRO/research, and reference labs/other. Based on end-use, the market is divided into hospitals, diagnostic centers, and pharmaceutical organizations & CROs. Regionally, the market is divided into Europe, North America, Asia Pacific, Latin America, and Middle East & Africa.
What Makes Immunohistochemistry the Dominant Technology Segment in the Tissue Diagnostics Market?
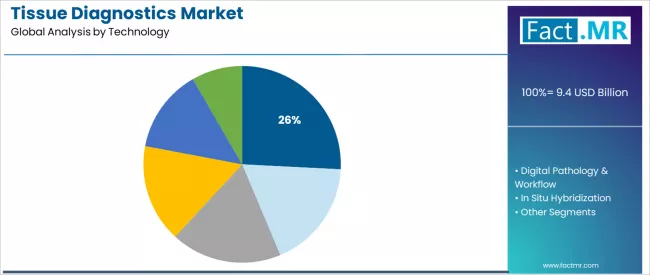
The immunohistochemistry segment represents the dominant force in the tissue diagnostics market, capturing approximately 25.8% of total market share in 2025. This established technology category encompasses solutions featuring antibody-based protein detection capabilities from tissue specimens, including IHC consumables such as antibodies and reagents that enable accurate biomarker identification and consistent diagnostic standards across all pathology laboratory applications.
The immunohistochemistry segment's market leadership stems from its superior diagnostic utility, with platforms capable of meeting diverse testing requirements while maintaining high specificity and operational reliability across all tissue processing environments. Within the immunohistochemistry segment, IHC consumables including antibodies and reagents account for approximately 14.3% share, driven by essential role in routine diagnostic workflows and expanding companion diagnostics applications.
The digital pathology & workflow segment maintains a substantial 22.4% market share, serving laboratories that require advanced imaging technologies with enhanced productivity features for whole slide imaging and remote consultation applications. These technologies offer comprehensive digital solutions for pathology operations while providing sufficient integration capabilities to meet clinical workflow demands and telepathology requirements.
The in situ hybridization segment accounts for approximately 18.9% of the market, serving specialized molecular diagnostics and gene expression analysis applications. Molecular pathology represents 16.0% market share, while anatomic pathology accounts for 16.9%, serving traditional histology and cytology examination requirements.
Key advantages driving the immunohistochemistry segment include:
- Advanced antibody development infrastructure with validated diagnostic markers that reduce testing complexity and ensure consistent biomarker detection
- High specificity capabilities allowing accurate protein expression analysis across different tissue types without significant background interference
- Proven clinical utility, delivering reliable diagnostic information while maintaining cost effectiveness against molecular testing alternatives
- Broad regulatory acceptance enabling straightforward test approvals and reimbursement coverage across multiple healthcare systems
How does the Breast Cancer Segment Lead Application Market Share?

Breast cancer dominates the application segment with approximately 50.5% market share in 2025, reflecting the critical role of tissue diagnostics in supporting global breast oncology patient management and treatment selection worldwide.
The breast cancer segment's market leadership is reinforced by established biomarker testing protocols, comprehensive guideline recommendations, and rising requirements for HER2, ER, and PR testing in surgical pathology specimens, oncology treatment planning, and companion diagnostics across developed and emerging markets.
Within the breast cancer segment, clinical diagnostic use for breast oncology accounts for approximately 31.7% share, driven by routine testing requirements and expanding targeted therapy utilization.
The NSCLC segment represents the second-largest application category, capturing 14.8% market share through growing demand for PD-L1 testing, ALK testing, and EGFR mutation analysis.
This segment benefits from expanding immunotherapy adoption and precision medicine initiatives that meet specific biomarker requirements, treatment eligibility criteria, and therapeutic monitoring protocols in competitive oncology markets. Prostate cancer accounts for 10.6% market share, while other cancers collectively represent 24.1%, serving diverse oncology diagnostic applications.
Key market dynamics supporting application growth include:
- Breast cancer testing expansion driven by guideline-mandated biomarker assessment and companion diagnostics requirements in global markets
- NSCLC testing modernization trends require comprehensive molecular profiling and immunotherapy selection biomarkers
- Integration of tissue-based testing with liquid biopsy approaches enabling complementary diagnostic strategies
- Growing emphasis on multi-cancer screening driving demand for comprehensive, pan-cancer diagnostic solutions
What Drives the Clinical Modality's Market Position?

Clinical modality dominates the segment with approximately 37.8% market share in 2025, reflecting the essential role of routine pathology services and hospital-based laboratories in supporting patient diagnosis and treatment decisions.
The clinical modality segment's market leadership is driven by established diagnostic workflows, high testing volumes, and critical requirements for timely cancer diagnosis across tertiary care hospitals, community hospitals, and academic medical centers. Within the clinical modality segment, clinical histopathology services in hospitals account for approximately 22.9% share, serving routine surgical pathology and biopsy evaluation requirements.
The pharma/CRO/research segment captures 34.2% market share, driven by companion diagnostics development, clinical trial support services, and translational research applications. Reference labs and other modalities represent 28.0% market share, serving specialized testing and consultation services.
Key modality dynamics include:
- Clinical laboratory growth driven by increasing cancer incidence and expanding screening programs requiring comprehensive diagnostic services
- Pharmaceutical research applications expanding through companion diagnostics mandates and biomarker-driven drug development
- Integration of centralized testing models enabling specialized expertise and standardized quality protocols
- Growing emphasis on quality assurance driving demand for accredited, high-complexity testing facilities
How Do Hospitals Lead the End-use Segment?
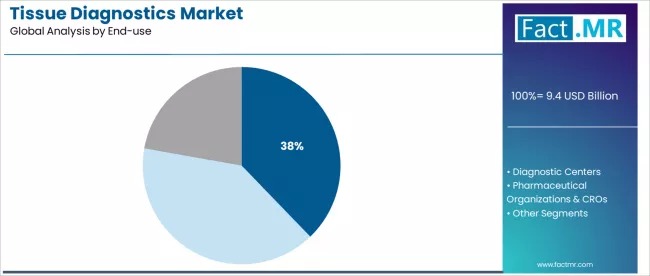
Hospitals dominate the end-use segment with approximately 37.8% market share in 2025, representing the primary setting for tissue diagnostic testing and pathology services worldwide.
The hospital segment's market leadership reflects integrated pathology departments, comprehensive surgical pathology capabilities, and direct patient care responsibilities across cancer diagnosis and treatment workflows. Within the hospital segment, tertiary care hospitals with integrated pathology workflows account for approximately 20.7% share, driven by advanced diagnostic capabilities and specialized oncology services.
Diagnostic centers capture 28.6% market share, serving outpatient testing and specialized pathology services, while pharmaceutical organizations and CROs represent 33.6% market share through drug development and clinical trial applications.
Key end-use dynamics include:
- Hospital laboratory modernization driving adoption of automated staining platforms and digital pathology systems
- Diagnostic center expansion enabling accessible outpatient services and specialized testing capabilities
- Pharmaceutical organization growth through companion diagnostics requirements and biomarker research programs
- Integration of multidisciplinary tumor boards creating comprehensive cancer care ecosystems
What are the Drivers, Restraints, and Key Trends of the Tissue Diagnostics Market?
The market is driven by three concrete demand factors tied to cancer burden and precision medicine adoption. First, increasing global cancer incidence rates create growing demand for tissue diagnostic testing, with worldwide cancer diagnoses expanding by 2-3% annually across major disease categories, requiring comprehensive pathology service infrastructure. Second, pharmaceutical industry mandates for companion diagnostics and biomarker testing drive increased adoption of tissue diagnostic platforms, with regulatory agencies increasingly requiring biomarker evidence for targeted therapy approvals by 2030. Third, technological advancements in digital pathology and artificial intelligence enable more efficient and accurate diagnostic workflows that reduce pathologist workload while improving diagnostic consistency and clinical decision support capabilities.
Market restraints include complex reimbursement landscapes that can deter healthcare providers from adopting advanced diagnostic technologies, particularly in markets where payment policies for specialized testing remain inadequate. Regulatory complexity and clinical validation requirements pose another significant challenge, as tissue diagnostic assays demand extensive analytical and clinical performance studies, potentially causing increased development costs and market entry delays. Pathologist workforce shortages across different regions create additional market challenges for service capacity expansion, demanding ongoing investment in telepathology infrastructure and AI-assisted diagnostic tools.
Key trends indicate accelerated adoption in Asia Pacific markets, particularly India and China, where expanding diagnostic infrastructure and rising cancer awareness drive comprehensive tissue diagnostics utilization. Technology integration trends toward AI-powered image analysis with specific diagnostic algorithms, workflow automation assessments, and quality control applications enable proactive diagnostic approaches that optimize accuracy and minimize interpretation variability. However, the market thesis could face disruption if significant advances in liquid biopsy technologies or alternative diagnostic modalities reduce reliance on traditional tissue-based diagnostic testing.
Analysis of the Tissue Diagnostics Market by Key Countries
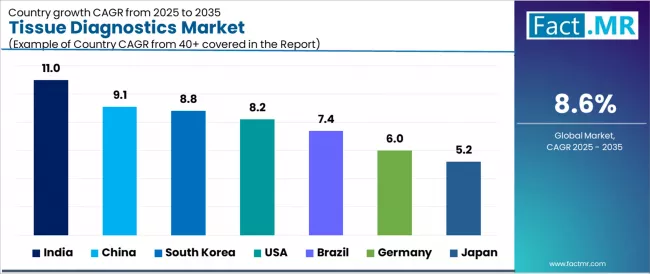
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 11.0% |
| China | 9.1% |
| South Korea | 8.8% |
| USA | 8.2% |
| Brazil | 7.4% |
| Germany | 6.0% |
| Japan | 5.2% |
The tissue diagnostics market is expanding robustly, with India leading at an 11.0% CAGR through 2035, driven by rapid expansion of diagnostic capacity, government cancer screening initiatives, and accelerating adoption of digital pathology in tier-1 and tier-2 cities. China follows at 9.1%, supported by large oncology burden, major investments in pathology automation, and domestic scaling of IHC and ISH reagent manufacturing. South Korea records 8.8%, reflecting high uptake of advanced diagnostics including digital pathology and AI, along with strong hospital purchasing power for precision oncology tools.
USA posts 8.2%, anchored by continued replacement cycle in hospitals and reference labs, growing companion diagnostics demand, and robust reimbursement for advanced testing. Brazil grows at 7.4%, with expanding private healthcare diagnostics, growth of regional reference labs, and increased procurement of digital scanners and reagents.
Germany advances at 6.0%, representing stable, mature market with steady upgrades to digital workflows and sustained R&D spending, while Japan grows at 5.2%, focusing on highly mature clinical diagnostics market with targeted, niche investment in next-generation pathology rather than broad rapid expansion.
India Leads Global Market Expansion
India demonstrates the strongest growth potential in the tissue diagnostics market with a CAGR of 11.0% through 2035. The country's leadership position stems from rapid expansion of diagnostic capacity, government cancer screening initiatives, and accelerating adoption of digital pathology technologies enabling mainstream tissue diagnostics utilization. Growth is concentrated in major metropolitan centers, including Mumbai, Delhi, Bangalore, and Chennai, where tertiary care hospitals and expanding diagnostic chains are implementing comprehensive pathology services for cancer diagnosis and treatment monitoring. Distribution channels through hospital laboratory partnerships, diagnostic center networks, and reference laboratory collaborations expand deployment across oncology programs and surgical pathology services. The country's growing healthcare infrastructure provides policy support for diagnostic service development, including National Cancer Grid initiatives and Ayushman Bharat health coverage programs.
Key market factors:
- Demand concentrated in urban centers and tier-1 hospitals with comprehensive oncology programs and pathology departments
- Diagnostic infrastructure growth through private hospital chains, standalone laboratories, and government medical colleges
- Comprehensive healthcare ecosystem, including established pathology training programs and growing subspecialty expertise
- Technology integration featuring digital pathology platforms, automated IHC stainers, and telepathology connectivity solutions
China Emerges as High-Growth Market
In Beijing, Shanghai, Guangzhou, and Shenzhen, the adoption of tissue diagnostic technologies is accelerating across hospital pathology departments and reference laboratory segments, driven by oncology burden growth and pathology automation investments. The market demonstrates strong growth momentum with a CAGR of 9.1% through 2035, linked to large base oncology burden, major investments in pathology automation, and domestic scaling of IHC and ISH reagent manufacturing. Chinese healthcare providers are implementing advanced tissue diagnostic platforms and molecular pathology technologies to enhance diagnostic capabilities while meeting growing demand in expanding cancer care programs and precision medicine initiatives. The country's Healthy China 2030 strategy creates persistent demand for diagnostic infrastructure, while increasing emphasis on early cancer detection drives adoption of comprehensive screening and diagnostic programs.
Key development areas:
- Hospital pathology departments and regional medical centers leading tissue diagnostic technology adoption with comprehensive modernization programs
- Domestic manufacturing providing integrated supply chains with 75% self-sufficiency in reagent production capabilities
- Technology partnerships between international diagnostic companies and Chinese healthcare institutions are expanding market reach
- Integration of AI-powered diagnostic tools and comprehensive digital pathology workflows
Brazil Shows Strong Regional Leadership
Brazil's market expansion is driven by diverse healthcare demand, including private hospital diagnostics in São Paulo and Rio de Janeiro, and expanding reference laboratory networks across multiple regions. The country demonstrates promising growth potential with a CAGR of 7.4% through 2035, supported by growing private healthcare diagnostics, expansion of regional reference labs, and increased procurement of digital scanners and reagents. Brazilian healthcare providers face implementation challenges related to public sector budget constraints, requiring private investment growth and support from international diagnostic partners. However, growing cancer awareness and healthcare access expansion create compelling business cases for tissue diagnostics adoption, particularly in metropolitan areas where diagnostic quality has a direct impact on treatment outcomes and patient satisfaction.
Market characteristics:
- Private healthcare and diagnostic chains showing fastest growth with 12-15% annual increase in advanced pathology testing volumes
- Regional expansion trends focused on southeastern states and major metropolitan centers
- Future projections indicate the need for pathologist training programs and laboratory accreditation development
- Growing emphasis on reference laboratory consolidation and specialized testing centralization
Germany Demonstrates Technology Innovation
The German market leads in premium tissue diagnostics adoption based on integration with comprehensive quality management systems and evidence-based clinical pathways for enhanced diagnostic performance. The country shows steady potential with a CAGR of 6.0% through 2035, driven by demand for low-VOC coatings and advanced pigment technologies, representing stable, mature market with steady upgrades to digital workflows and sustained R&D spending. German pathology practices are adopting digital pathology platforms and AI-assisted diagnostic tools for efficiency optimization and quality improvement, particularly in university medical centers and large pathology group practices demanding comprehensive diagnostic excellence. Technology deployment channels through established equipment distributors and direct manufacturer relationships expand coverage across hospital networks and specialized pathology institutes.
Leading market segments:
- University hospitals and comprehensive cancer centers implementing advanced digital pathology with AI integration
- Reference laboratory partnerships with hospital networks, achieving 98% quality accreditation compliance rates
- Strategic collaborations between diagnostic companies and pathology professional societies are expanding best practice adoption
- Focus on standardization initiatives and external quality assessment programs
USA Emphasizes Market Maturity
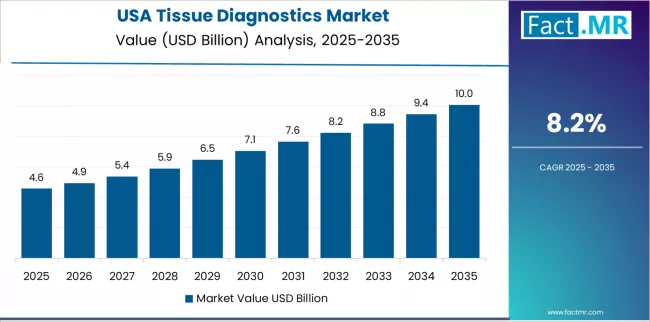
In major healthcare markets including academic medical centers, integrated health systems, and national reference laboratories, pathology departments are implementing comprehensive tissue diagnostic platforms to modernize existing workflows and expand molecular testing capabilities, with documented implementation showing successful integration through laboratory information system connectivity. The market shows steady growth potential with a CAGR of 8.2% through 2035, linked to continued replacement cycle in hospitals and reference labs, growing companion diagnostics demand, and robust reimbursement for advanced testing. American healthcare providers benefit from established tissue diagnostic infrastructure and comprehensive molecular pathology services to enhance patient care while maintaining regulatory compliance standards demanded by CAP accreditation and CLIA certification requirements. The country's mature diagnostic ecosystem creates persistent demand for technology innovation and workflow optimization solutions that integrate with existing laboratory operations and electronic medical record systems.
Market development factors:
- Academic medical centers and reference laboratories leading tissue diagnostic innovation across USA
- Reimbursement policies providing coverage support for companion diagnostics and specialized biomarker testing
- Strategic partnerships between pharmaceutical companies and diagnostic providers are expanding companion diagnostics development
- Emphasis on laboratory consolidation and standardized testing protocols across integrated health systems
South Korea Shows Advanced Technology Adoption
South Korea's tissue diagnostics market demonstrates sophisticated technology integration focused on digital pathology and artificial intelligence adoption, with documented implementation of whole slide imaging achieving comprehensive deployment across tertiary care hospitals. The country maintains strong growth momentum with a CAGR of 8.8% through 2035, driven by high uptake of advanced diagnostics including digital pathology and AI, along with strong hospital purchasing power for precision oncology tools. Major healthcare markets, including Seoul, Busan, and Incheon metropolitan areas, showcase advanced deployment of tissue diagnostic technologies where digital pathology platforms integrate seamlessly with hospital information systems and comprehensive tumor board workflows.
Key market characteristics:
- Tertiary hospitals and comprehensive cancer centers driving advanced diagnostic technology adoption with emphasis on AI integration
- Government healthcare programs enabling comprehensive cancer screening and diagnostic service coverage
- Technology collaboration between Korean hospitals and international diagnostic companies is expanding capabilities
- Emphasis on precision medicine initiatives and molecular tumor board implementation
Japan Emphasizes Quality and Specialization
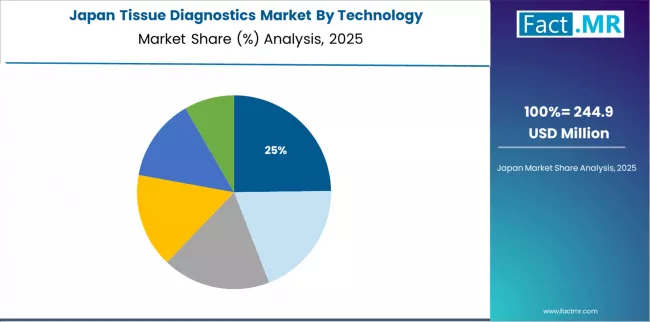
Japan's tissue diagnostics market demonstrates mature and quality-focused landscape, characterized by sophisticated integration of advanced diagnostic technologies with existing pathology workflows across academic institutions, regional medical centers, and specialized pathology laboratories. The country shows stable growth momentum with a CAGR of 5.2% through 2035, representing highly mature clinical diagnostics market with targeted, niche investment in next-generation pathology rather than broad rapid expansion.
Japan's emphasis on diagnostic quality and subspecialization creates requirements for premium tissue diagnostic platforms that support comprehensive performance standards and regulatory compliance requirements in clinical operations. The market benefits from strong relationships between international equipment providers and domestic distribution partners, creating comprehensive service ecosystems that prioritize reliability and technical support. Healthcare markets in major regions showcase advanced tissue diagnostic implementations where digital pathology systems achieve 92% adoption rates in university hospitals through integrated quality programs.
Key market characteristics:
- University hospitals and cancer centers driving specialized diagnostic requirements with emphasis on quality excellence
- Quality assurance programs enabling 96% external quality assessment compliance with comprehensive proficiency testing
- Technology collaboration between Japanese healthcare institutions and international diagnostic providers is expanding capabilities
- Emphasis on pathology subspecialization and molecular pathology integration methodologies
Europe Market Split by Country
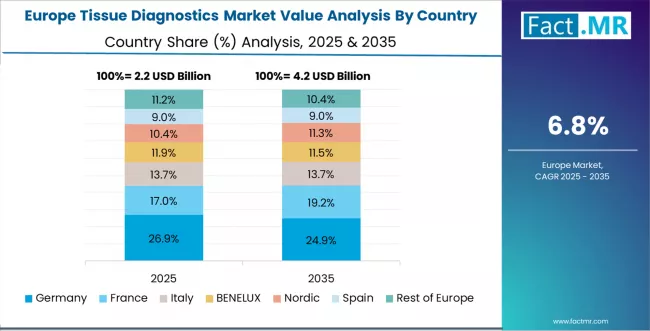
The tissue diagnostics market in Europe is projected to grow from USD 2.8 billion in 2025 to USD 6.1 billion by 2035, registering a CAGR of 8.1% over the forecast period. Germany is expected to maintain its leadership position with a 31.2% market share in 2025, declining slightly to 30.8% by 2035, supported by its advanced healthcare infrastructure, comprehensive pathology networks, and strong academic medical centers across major metropolitan regions.
The UK follows with a 23.5% share in 2025, projected to reach 23.8% by 2035, driven by NHS diagnostic modernization programs and expanding molecular pathology services. France holds a 19.8% share in 2025, expected to reach 20.1% by 2035 through comprehensive cancer plan implementation and pathology laboratory consolidation.
Italy commands a 12.4% share, while Spain accounts for 8.6% in 2025. The rest of Europe region is anticipated to maintain steady position, with collective share moving from 4.5% to 4.9% by 2035, attributed to increasing tissue diagnostics adoption in Nordic countries and emerging Eastern European healthcare systems implementing cancer care modernization programs.
Competitive Landscape of the Tissue Diagnostics Market
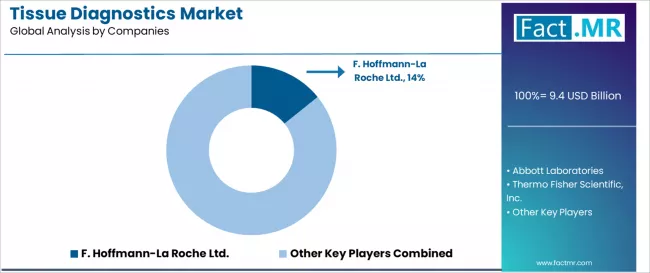
The tissue diagnostics market features approximately 20-25 meaningful players with moderate concentration, where the top three companies control roughly 35-45% of global market share through established product portfolios and extensive laboratory and pharmaceutical industry relationships. Competition centers on diagnostic accuracy, reagent quality, and comprehensive workflow solutions rather than price competition alone.
Market leaders include F. Hoffmann-La Roche Ltd., Abbott Laboratories, and Thermo Fisher Scientific Inc., which maintain competitive advantages through comprehensive tissue diagnostic solution portfolios, global distribution networks, and deep expertise in the immunohistochemistry, molecular pathology, and digital pathology sectors, creating high customer loyalty among pathology laboratories.
These companies leverage established regulatory approvals and ongoing product innovation services to defend market positions while expanding into companion diagnostics and digital pathology applications. F. Hoffmann-La Roche Ltd. commands approximately 14.2% market share through strategic ventana platform excellence and comprehensive companion diagnostics partnerships.
Challengers encompass Siemens Healthineers and Danaher Corporation, which compete through diversified diagnostic portfolios and strong regional presence in key healthcare markets. Diagnostic specialists, including bioMérieux SA, QIAGEN, and BD, focus on specific technology platforms or application areas, offering differentiated capabilities in automated workflows, molecular solutions, and specimen processing systems.
Regional players and emerging diagnostic companies create competitive pressure through innovative testing methodologies and specialized biomarker solutions, particularly in high-growth markets including China and India, where local presence provides advantages in regulatory navigation and customer service responsiveness. Market dynamics favor companies that combine advanced diagnostic technologies with comprehensive clinical evidence generation programs that address the complete customer journey from test adoption through clinical validation and laboratory implementation. Strategic collaborations between diagnostic manufacturers and pharmaceutical companies accelerate companion diagnostics development, while laboratory automation partnerships enable workflow optimization and efficiency improvements across pathology operations.
Global Tissue Diagnostics Market - Stakeholder Contribution Framework
Tissue diagnostic products represent a critical cancer diagnosis technology that enables hospital laboratories, diagnostic centers, and pharmaceutical organizations to deliver accurate pathology services without substantial workflow disruption, typically providing 90-95% diagnostic accuracy rates comparable to molecular testing alternatives while enabling comprehensive biomarker assessment. With the market projected to grow from USD 9.4 billion in 2025 to USD 21.4 billion by 2035 at an 8.6% CAGR, these technologies offer compelling advantages - superior diagnostic precision, enhanced workflow efficiency, and comprehensive clinical utility - making them essential for hospital pathology services (growing segment), reference laboratory operations (expanding adoption), and diverse pharmaceutical applications seeking proven companion diagnostics solutions. Scaling market penetration and diagnostic capabilities requires coordinated action across healthcare policy, laboratory standards, diagnostic manufacturers, pathology professional organizations, and research institutions.
How Governments Could Spur Diagnostic Infrastructure and Adoption?
- Healthcare Infrastructure Programs: Include tissue diagnostics capabilities in national cancer control initiatives, providing targeted funding for pathology laboratory modernization in regional medical centers and supporting local diagnostic service development through capacity building grants and technical assistance programs.
- Reimbursement Policy & Coverage Support: Implement comprehensive coverage policies for essential biomarker testing, provide adequate reimbursement rates for complex pathology services including digital pathology and molecular testing, and establish favorable coding systems that recognize advanced diagnostic value.
- Regulatory Framework Development: Create streamlined diagnostic approval processes across clinical and research applications, establish clear quality standards for tissue diagnostic laboratories, and develop international harmonization protocols that facilitate diagnostic innovation and cross-border clinical trial support.
- Workforce Development & Training: Fund pathology residency programs, laboratory technologist training initiatives, and continuing medical education for subspecialty pathology. Invest in telepathology infrastructure that bridges rural healthcare facilities with expert consultation services and academic pathology centers.
How Professional Societies Could Support Market Development?
- Quality Standards & Accreditation: Define standardized performance metrics for tissue diagnostic testing across hospital, reference laboratory, and pharmaceutical applications, establish universal antibody validation and quality control protocols, and create accreditation programs for laboratory excellence that healthcare providers can benchmark.
- Clinical Guidelines & Best Practices: Lead evidence-based recommendations that demonstrate tissue diagnostics clinical utility, emphasizing proven diagnostic accuracy, treatment selection impact, and patient outcome improvements compared to empiric therapeutic approaches.
- Technology Assessment Standards: Develop consensus guidelines for digital pathology implementation, comprehensive AI algorithm validation requirements, and diagnostic algorithm platforms, ensuring consistent quality across different laboratory environments and testing scenarios.
How Manufacturers and Technology Providers Could Strengthen the Ecosystem?
- Advanced Platform Development: Develop next-generation automated staining systems with enhanced reproducibility capabilities, improved antibody specificity profiles, and integrated quality control features that enhance diagnostic reliability while improving laboratory productivity.
- Clinical Evidence Programs: Provide comprehensive validation studies that integrate analytical performance data, clinical utility assessments, health economic modeling, and real-world evidence generation, enabling laboratories to maximize test adoption and clinical integration success rates.
- Laboratory Partnership Networks: Offer flexible collaboration programs for hospital laboratories and reference facilities, including workflow optimization consultation services, quality assurance support programs, and training pathways that keep tissue diagnostic applications current with clinical guidelines and regulatory requirements.
Key Players in the Tissue Diagnostics Market
- F. Hoffmann-La Roche Ltd.
- Abbott Laboratories
- Thermo Fisher Scientific, Inc.
- Siemens Healthineers
- Danaher Corporation
- bioMérieux SA
- QIAGEN
- BD
- Merck KGaA
- GE Healthcare
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 9.4 Billion |
| Technology | Immunohistochemistry, Digital Pathology & Workflow, In Situ Hybridization, Molecular Pathology, Anatomic Pathology, Others |
| Application | Breast Cancer, NSCLC, Prostate Cancer, Other Cancers |
| Modality | Clinical, Pharma/CRO/Research, Reference Labs/Other |
| End-use | Hospitals, Diagnostic Centers, Pharmaceutical Organizations & CROs |
| Regions Covered | Europe, North America, Asia Pacific, Latin America, Middle East & Africa |
| Country Covered | India, China, South Korea, USA, Brazil, Germany, Japan, and 40+ countries |
| Key Companies Profiled | F. Hoffmann-La Roche Ltd., Abbott Laboratories, Thermo Fisher Scientific Inc., Siemens Healthineers, Danaher Corporation, bioMérieux SA, QIAGEN, BD, Merck KGaA, GE Healthcare |
| Additional Attributes | Dollar sales by technology, application, modality, and end-use categories, regional adoption trends across Europe, North America, and Asia Pacific, competitive landscape with diagnostic equipment providers and reagent manufacturers, clinical validation requirements and regulatory pathways, integration with laboratory information systems and digital pathology platforms. |
Tissue Diagnostics Market by Segments
-
Technology :
- Immunohistochemistry
- Digital Pathology & Workflow
- In Situ Hybridization
- Molecular Pathology
- Anatomic Pathology
- Others
-
Application :
- Breast Cancer
- NSCLC
- Prostate Cancer
- Other Cancers
-
Modality :
- Clinical
- Pharma/CRO/Research
- Reference Labs/Other
-
End-use :
- Hospitals
- Diagnostic Centers
- Pharmaceutical Organizations & CROs
-
Region :
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Rest of Middle East & Africa
- Europe
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Technology
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Technology, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Technology, 2025 to 2035
- Immunohistochemistry
- Digital Pathology & Workflow
- In Situ Hybridization
- Molecular Pathology
- Anatomic Pathology
- Others
- Y to o to Y Growth Trend Analysis By Technology, 2020 to 2024
- Absolute $ Opportunity Analysis By Technology, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- Breast Cancer
- NSCLC
- Prostate Cancer
- Other Cancers
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Modality
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Modality, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Modality, 2025 to 2035
- Clinical
- Pharma/CRO/Research
- Reference Labs/Other
- Y to o to Y Growth Trend Analysis By Modality, 2020 to 2024
- Absolute $ Opportunity Analysis By Modality, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End-use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End-use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End-use, 2025 to 2035
- Hospitals
- Diagnostic Centers
- Pharmaceutical Organizations & CROs
- Y to o to Y Growth Trend Analysis By End-use, 2020 to 2024
- Absolute $ Opportunity Analysis By End-use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Technology
- By Application
- By Modality
- By End-use
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- By Modality
- By End-use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Technology
- By Application
- By Modality
- By End-use
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- By Modality
- By End-use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Technology
- By Application
- By Modality
- By End-use
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- By Modality
- By End-use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Technology
- By Application
- By Modality
- By End-use
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- By Modality
- By End-use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Technology
- By Application
- By Modality
- By End-use
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- By Modality
- By End-use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Technology
- By Application
- By Modality
- By End-use
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- By Modality
- By End-use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Technology
- By Application
- By Modality
- By End-use
- By Country
- Market Attractiveness Analysis
- By Country
- By Technology
- By Application
- By Modality
- By End-use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Technology
- By Application
- By Modality
- By End-use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Technology
- By Application
- By Modality
- By End-use
- Competition Analysis
- Competition Deep Dive
- F. Hoffmann-La Roche Ltd.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Abbott Laboratories
- Thermo Fisher Scientific, Inc.
- Siemens Healthineers
- Danaher Corporation
- bioMérieux SA
- QIAGEN
- BD
- Merck KGaA
- GE Healthcare
- F. Hoffmann-La Roche Ltd.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 5: Global Market Value (USD Million) Forecast by End-use, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 9: North America Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 10: North America Market Value (USD Million) Forecast by End-use, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 13: Latin America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 14: Latin America Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 15: Latin America Market Value (USD Million) Forecast by End-use, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: Western Europe Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 18: Western Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 19: Western Europe Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 20: Western Europe Market Value (USD Million) Forecast by End-use, 2020 to 2035
- Table 21: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: Eastern Europe Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 23: Eastern Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 24: Eastern Europe Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 25: Eastern Europe Market Value (USD Million) Forecast by End-use, 2020 to 2035
- Table 26: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 27: East Asia Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 28: East Asia Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 29: East Asia Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 30: East Asia Market Value (USD Million) Forecast by End-use, 2020 to 2035
- Table 31: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 32: South Asia and Pacific Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 33: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 34: South Asia and Pacific Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 35: South Asia and Pacific Market Value (USD Million) Forecast by End-use, 2020 to 2035
- Table 36: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 37: Middle East & Africa Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 38: Middle East & Africa Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 39: Middle East & Africa Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 40: Middle East & Africa Market Value (USD Million) Forecast by End-use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Technology
- Figure 6: Global Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Application
- Figure 9: Global Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Modality
- Figure 12: Global Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by End-use
- Figure 15: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 16: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 17: Global Market Attractiveness Analysis by Region
- Figure 18: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 23: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 24: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 25: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 26: North America Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Technology
- Figure 29: North America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Application
- Figure 32: North America Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 33: North America Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 34: North America Market Attractiveness Analysis by Modality
- Figure 35: North America Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 36: North America Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 37: North America Market Attractiveness Analysis by End-use
- Figure 38: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 39: Latin America Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Technology
- Figure 42: Latin America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 43: Latin America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 44: Latin America Market Attractiveness Analysis by Application
- Figure 45: Latin America Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 46: Latin America Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 47: Latin America Market Attractiveness Analysis by Modality
- Figure 48: Latin America Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 49: Latin America Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 50: Latin America Market Attractiveness Analysis by End-use
- Figure 51: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 52: Western Europe Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 53: Western Europe Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 54: Western Europe Market Attractiveness Analysis by Technology
- Figure 55: Western Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 56: Western Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 57: Western Europe Market Attractiveness Analysis by Application
- Figure 58: Western Europe Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 59: Western Europe Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 60: Western Europe Market Attractiveness Analysis by Modality
- Figure 61: Western Europe Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 62: Western Europe Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 63: Western Europe Market Attractiveness Analysis by End-use
- Figure 64: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 65: Eastern Europe Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 66: Eastern Europe Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 67: Eastern Europe Market Attractiveness Analysis by Technology
- Figure 68: Eastern Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 69: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 70: Eastern Europe Market Attractiveness Analysis by Application
- Figure 71: Eastern Europe Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 72: Eastern Europe Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 73: Eastern Europe Market Attractiveness Analysis by Modality
- Figure 74: Eastern Europe Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 75: Eastern Europe Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 76: Eastern Europe Market Attractiveness Analysis by End-use
- Figure 77: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 78: East Asia Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 79: East Asia Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 80: East Asia Market Attractiveness Analysis by Technology
- Figure 81: East Asia Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 82: East Asia Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 83: East Asia Market Attractiveness Analysis by Application
- Figure 84: East Asia Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 85: East Asia Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 86: East Asia Market Attractiveness Analysis by Modality
- Figure 87: East Asia Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 88: East Asia Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 89: East Asia Market Attractiveness Analysis by End-use
- Figure 90: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 91: South Asia and Pacific Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 92: South Asia and Pacific Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 93: South Asia and Pacific Market Attractiveness Analysis by Technology
- Figure 94: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 95: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 96: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 97: South Asia and Pacific Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 98: South Asia and Pacific Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 99: South Asia and Pacific Market Attractiveness Analysis by Modality
- Figure 100: South Asia and Pacific Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 101: South Asia and Pacific Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 102: South Asia and Pacific Market Attractiveness Analysis by End-use
- Figure 103: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 104: Middle East & Africa Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 105: Middle East & Africa Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 106: Middle East & Africa Market Attractiveness Analysis by Technology
- Figure 107: Middle East & Africa Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 108: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 109: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 110: Middle East & Africa Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 111: Middle East & Africa Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 112: Middle East & Africa Market Attractiveness Analysis by Modality
- Figure 113: Middle East & Africa Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 114: Middle East & Africa Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 115: Middle East & Africa Market Attractiveness Analysis by End-use
- Figure 116: Global Market - Tier Structure Analysis
- Figure 117: Global Market - Company Share Analysis
- FAQs -
How big is the tissue diagnostics market in 2025?
The global tissue diagnostics market is estimated to be valued at USD 9.4 billion in 2025.
What will be the size of tissue diagnostics market in 2035?
The market size for the tissue diagnostics market is projected to reach USD 21.4 billion by 2035.
How much will be the tissue diagnostics market growth between 2025 and 2035?
The tissue diagnostics market is expected to grow at a 8.6% CAGR between 2025 and 2035.
What are the key product types in the tissue diagnostics market?
The key product types in tissue diagnostics market are immunohistochemistry, digital pathology & workflow, in situ hybridization, molecular pathology, anatomic pathology and others.
Which application segment to contribute significant share in the tissue diagnostics market in 2025?
In terms of application, breast cancer segment to command 50.5% share in the tissue diagnostics market in 2025.