Tamper Evident Labels Industry Analysis in the USA
Tamper Evident Labels Industry Analysis in the USA Size and Share Forecast Outlook 2025 to 2035
Tamper evident labels industry analysis in the USA is projected to grow from USD 920.0 million in 2025 to USD 1,498.6 million by 2035, at a CAGR of 5.0%. Void Labels will dominate with a 46.0% market share, while pharmaceuticals will lead the end use segment with a 42.0% share.
Tamper Evident Labels Industry Analysis in the USA 2025 to 2035
The demand for tamper evident labels in the USA is projected to grow from USD 920.0 million in 2025 to approximately USD 1,498.0 million by 2035, the market will rise at a CAGR of 5.0% which recording an absolute increase of USD 578.0 million over the forecast period. The void labels segment is projected to account for 46.0% of USA tamper evident labels demand in 2025. Void labels are widely used in the USA for product security, anti-counterfeiting protection, and packaging authentication where immediate tamper detection, application reliability, and cost-effectiveness remain essential for brand protection and regulatory compliance.
The pharmaceuticals end use segment is expected to represent 42.0% of USA tamper evident labels demand in 2025. Pharmaceutical manufacturers are fundamental to the tamper evident labels industry because they provide the volume demand, precision application requirements, and security performance characteristics required for product safety and regulatory standards.
Quick Stats for USA Tamper Evident Labels Industry
- usa Tamper Evident Labels Sales Value (2025): USD 920.0 million
- usa Tamper Evident Labels Forecast Value (2035): USD 1,498.0 million
- usa Tamper Evident Labels Forecast CAGR: 5.0%
- Leading Product Category in USA Tamper Evident Labels Industry: Void Labels (46.0%)
- Key Growth Regions in USA Tamper Evident Labels Industry: West, Northeast, South, Midwest
- Regional Leadership: Northeast holds the leading position in demand
- Key Players in USA Tamper Evident Labels Industry: Avery Dennison, VeriSeal, CCL Industries, UPM Raflatac, Multi-Color, Fedrigoni, Schreiner Group, Huhtamaki, Securikett, Fort Dearborn
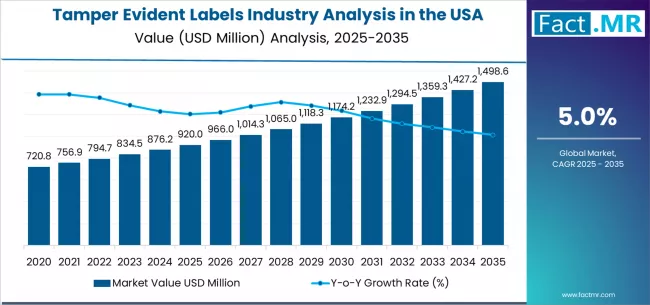
Between 2025 and 2030, demand for tamper evident labels in the USA is projected to expand from USD 920.0 million to USD 1,123.0 million, resulting in a value increase of USD 203.0 million, which represents 35.1% of the total forecast growth for the decade. This phase of growth will be shaped by rising regulatory requirements, increasing brand protection needs, and growing anti-counterfeiting initiatives across USA regions, particularly in areas where pharmaceutical manufacturing and consumer goods production are accelerating tamper evident labels adoption. Increasing integration of advanced authentication technologies in packaging applications and growing adoption of serialization-compliant varieties continue to drive demand. Label manufacturers and packaging companies are expanding their security capabilities to address the growing complexity of modern regulatory requirements and brand protection standards, with USA operations leading investments in application technology and quality assurance systems.
From 2030 to 2035, demand is forecast to grow from USD 1,123.0 million to USD 1,498.0 million, adding another USD 375.0 million, which constitutes 64.9% of the overall ten-year expansion. This period is expected to be characterized by expansion of smart packaging platforms, development of advanced authentication applications, and implementation of comprehensive track-and-trace programs across different industry sectors. The growing adoption of serialization requirements and enhanced security standards, particularly in pharmaceuticals and premium consumer goods, will drive demand for more sophisticated tamper evident label platforms and validated security solutions.
Between 2020 and 2025, tamper evident labels demand in the USA experienced steady expansion, driven by increasing regulatory enforcement in pharmaceutical sectors and growing awareness of tamper evident benefits for product protection and brand integrity. The sector developed as pharmaceutical manufacturers and consumer goods companies, especially in major production centers, recognized the need for proven security solutions and effective authentication systems to achieve compliance objectives while meeting regulatory expectations and cost requirements. Tamper evident label suppliers and packaging providers began emphasizing supply chain optimization and quality validation to maintain competitive advantages and commercial viability.
USA Tamper Evident Labels Industry Key Takeaways
| Metric | Value |
|---|---|
| usa Tamper Evident Labels Sales Value (2025) | USD 920.0 million |
| usa Tamper Evident Labels Forecast Value (2035) | USD 1,498.0 million |
| usa Tamper Evident Labels Forecast CAGR (2025-2035) | 5.0% |
Why is the USA Tamper Evident Labels Industry Growing?
Demand expansion is being supported by the accelerating emphasis on product security enhancement and anti-counterfeiting transformation nationwide, with the USA maintaining its position as a packaging innovation and regulatory compliance leadership region, and the corresponding need for effective security labeling systems for pharmaceutical applications, authentication compliance, and brand protection integration. Modern pharmaceutical manufacturers and consumer goods companies rely on tamper evident label technologies to ensure regulatory compliance, security requirement fulfillment, and optimal pathway achievement toward comprehensive authentication environments.
Advanced security requirements necessitate comprehensive labeling solutions including specialized authentication capabilities, application processing, and detection enhancement infrastructure to address diverse application needs and regulatory specifications.
The growing emphasis on packaging modernization and increasing federal and state-level security regulations, particularly FDA compliance programs across the USA, are driving demand for tamper evident label systems from proven security suppliers with appropriate application expertise and quality management capabilities. Pharmaceutical facilities and consumer goods manufacturers are increasingly investing in tamper evident label sourcing and integrated security solutions to enhance product protection profiles, access authentication optimization trends, and demonstrate compliance leadership in competitive regulatory environments.
Regulatory standards and security compliance requirements are establishing standardized packaging pathways that require tamper evident label systems and authentication assurance, with USA operations often pioneering large-scale implementation of advanced security technologies.
Segmental Analysis
The industry is segmented by type, end use, and region. By type, the industry is divided into void labels, tamper-evident tapes, and shrink bands categories. In terms of end use, the industry is segmented into pharmaceuticals, food & beverage, and cosmetics & others. By region, the industry is divided into West, Northeast, South, and Midwest, with pharmaceuticals representing a key growth and innovation hub for security packaging technologies.
By Type, Void Labels Segment Accounts for 46.00% Share
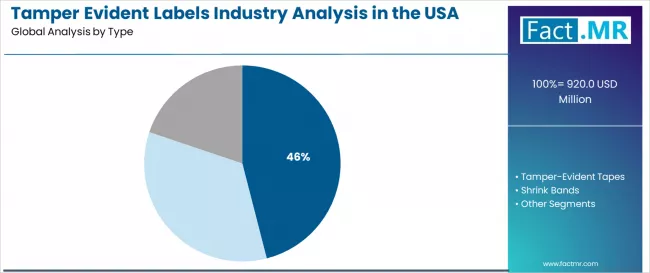
The void labels segment is projected to account for 46.00% of USA tamper evident labels demand in 2025, making it the leading type category across the sector. This dominance reflects the security efficacy requirements and application versatility needs of void label systems for existing pharmaceutical manufacturing facilities and consumer applications where tamper detection performance is optimized through standardized security capabilities and integrated packaging architecture.
In the USA, where substantial pharmaceutical manufacturing infrastructure requires security integration without complete packaging redesign, void label platforms provide practical pathways for authentication enhancement while maintaining production continuity. Continuous innovations are improving adhesive stability, tamper evidence characteristics, and manufacturing integration parameters, enabling pharmaceutical manufacturers to achieve high security standards while maximizing production efficiency.
The segment's strong position is reinforced by the extensive existing pharmaceutical production infrastructure requiring security feature adoption and growing availability of void label suppliers with proven application experience.
- Packaging compatibility and existing production integration make void label platforms the preferred technology for enhancing operating pharmaceutical facilities and consumer product installations.
- Security reliability and manufacturing demonstration track records are enhancing producer confidence and label viability across large-scale adoption initiatives.
By End Use, Pharmaceuticals Segment Accounts for 42.00% Share
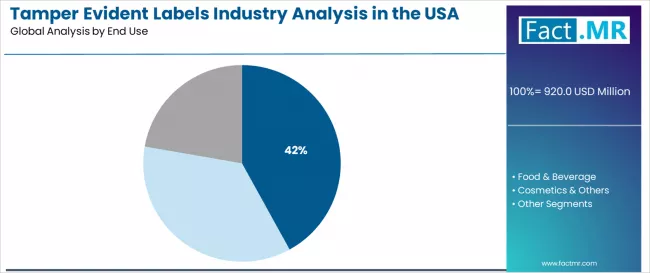
Pharmaceuticals applications are expected to represent 42.00% of USA tamper evident labels demand in 2025, highlighting the critical importance of versatile end use systems requiring comprehensive security solutions. Pharmaceutical manufacturers including major drug companies, over-the-counter producers, and medical product developers generate consistent demand for tamper evident label systems that are technically and economically favorable for regulated applications.
The segment benefits from end use characteristics that often provide superior application versatility compared to alternative applications, reducing packaging complexity and compliance costs. Pharmaceuticals applications also access enhanced USAge optimization through proven performance that improves product safety and regulatory compliance.
In the USA, where mainstream pharmaceutical operations represent substantial portions of security development, authentication excellence requires pharmaceutical integration across diverse packaging environments. In Northeast and Midwest regions, where pharmaceutical innovation concentrations are significant, tamper evident labels demand is elevated by emphasis on maintaining security excellence while achieving authentication integration targets.
- Usage optimization and favorable production economics make this the largest end use segment for tamper evident label technologies.
- Application versatility and regulatory adoption demands drive consistent demand across major drug companies, over-the-counter producers, and medical product developers.
What are the Drivers, Restraints, and Key Trends in the USA Tamper Evident Labels Demand?
usa tamper evident labels demand is advancing steadily due to increasing product security requirements and growing recognition of authentication necessity for regulatory compliance, with Northeast region serving as a key driver of innovation and application development. The sector faces challenges including competition from alternative security technologies, need for specialized application infrastructure development, and ongoing concerns regarding cost efficiency and integration complexity considerations.
Regulatory compliance requirements and anti-counterfeiting initiatives, particularly FDA mandates in Northeast and Midwest regions, continue to influence tamper evident labels selection and deployment timelines.
Expansion of Product Security Requirements and Authentication Standards
The enhancement of regulatory standards, gaining particular significance through pharmaceutical safety regulations and anti-counterfeiting campaigns, is enabling tamper evident labels suppliers to achieve differentiation without prohibitive manufacturing costs, providing predictable demand patterns through manufacturer requirements and regulatory preferences. Enhanced security standards offering substantial opportunities for tamper evident labels systems and integrated applications provide foundational dynamics while allowing suppliers to secure pharmaceutical manufacturing agreements and consumer goods partnerships.
These trends are particularly valuable for first-mover suppliers and premium security development that require substantial innovation investments without immediate cost advantages.
Integration of Advanced Application Technologies and Supply Chain Systems
Modern tamper evident labels suppliers and packaging manufacturers are establishing advanced distribution networks and centralized production facilities that improve application efficiency through system standardization and economies of scale. Integration of automated application systems, high-precision printing technology, and coordinated supply chain management enables more efficient label operation across multiple manufacturing locations.
Advanced application concepts also support next-generation pharmaceutical packaging including specialized authentication integration, manufacturer cluster optimization, and regional label supply networks that optimize system-level economics while enabling comprehensive security monitoring across packaging regions, with USA developments increasingly adopting collaborative manufacturing models to reduce individual operator costs and accelerate adoption.
Analysis of USA Tamper Evident Labels Demand by Key Region

| Region | CAGR (2025 to 2035) |
|---|---|
| West | 7.20% |
| Northeast | 6.80% |
| South | 6.50% |
| Midwest | 6.30% |
The USA tamper evident labels demand is witnessing robust growth, supported by rising security requirements, expanding regulatory initiatives, and the deployment of advanced authentication technologies across regions. West leads the nation with a 7.20% CAGR, reflecting progressive packaging innovation trends, substantial pharmaceutical development, and early adoption of premium security systems.
Northeast follows with a 6.80% CAGR, driven by extensive regulatory compliance, favorable consumer safety demographics, and concentration of pharmaceutical operations that enhance application development. South grows at 6.50%, as supply chain modernization and operational efficiency opportunities increasingly drive tamper evident label deployment. Midwest demonstrates growth at 6.30%, supported by expanding manufacturing facilities and regional safety initiatives.
West Leads National Growth with Innovation and Premium Packaging Applications
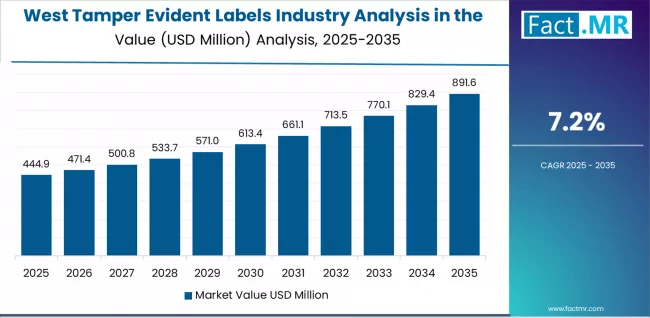
Demand for tamper evident labels in West is projected to exhibit exceptional growth with a CAGR of 7.20% through 2035, driven by progressive safety preferences, substantial packaging innovation creating premium security opportunities, and concentration of pharmaceutical industry advancement across California, Washington and surrounding states.
As the dominant region with extensive pharmaceutical infrastructure and safety-focused regulatory policies, West's emphasis on comprehensive supply chain excellence and security leadership is creating significant demand for tamper evident label systems with proven performance and reliable authentication potential. Major pharmaceutical manufacturers and label suppliers are establishing comprehensive security development programs to support innovation advancement and premium authentication deployment across diverse applications.
- Packaging innovation trends and security precision preferences are requiring comprehensive quality management strategies and authentication solutions, driving demand for tamper evident label systems with demonstrated safety enhancement capabilities and permanent integrity assurance throughout diverse packaging operations.
- Innovation ecosystem strength and security capital availability are supporting deployment of next-generation authentication technologies and novel application pathways that enhance production viability, reduce compliance costs, and create new opportunities across high-performance and pharmaceutical applications, positioning West as a national packaging innovation leadership region.
Northeast Demonstrates Strong Potential with Pharmaceutical Infrastructure
Demand for tamper evident labels in Northeast is expanding at a CAGR of 6.80%, supported by extensive pharmaceutical manufacturing facilities including large-scale security systems, compliance applications, and packaging companies generating concentrated demand favorable for label systems. The region's operational characteristics, featuring substantial regulatory infrastructure and safety requirements ideal for security integration, provide operational advantages.
Compliance expertise concentrated in New York, Massachusetts, and regional packaging innovation corridors facilitates application development and quality management. Tamper evident label suppliers and manufacturers are implementing comprehensive efficiency strategies to serve expanding safety-focused requirements throughout Northeast.
- Pharmaceutical concentration and favorable application economics are creating opportunities for specialized tamper evident label suppliers that can integrate security systems with existing packaging manufacturing operations.
- Safety positioning and quality awareness are building regional competitive advantages in packaging applications, enabling comprehensive efficiency development and manufacturer cluster enhancement that meets production targets while accessing performance pricing opportunities.
South Maintains Strong Growth with Supply Chain Expansion
Demand for tamper evident labels in South is growing at a CAGR of 6.50%, driven by substantial manufacturing facilities from pharmaceutical operations, processing equipment, and regional packaging companies requiring compliance pathways.
The region's industrial base, supporting critical supply chain operations, is increasingly adopting security technologies to maintain competitiveness while meeting regulatory expectations. Manufacturers and tamper evident label suppliers are investing in compliance integration systems and regional supply infrastructure to address growing production management requirements.
- Supply chain modernization imperatives and regulatory competitiveness concerns are facilitating adoption of tamper evident label technologies that enable continued operations while achieving compliance enhancement across packaging operations, processing equipment, and manufacturing facilities.
- Security precision opportunities including regional supply chain development and operational utilization for enhanced packaging integrity environments are creating unique regional advantages and diversified application types throughout South manufacturing operations.
Midwest Shows Progressive Adoption with Packaging Integrity Modernization
Demand for tamper evident labels in Midwest is advancing at a CAGR of 6.30%, supported by expanding manufacturing facilities, regional operational development including packaging and processing operations, and growing emphasis on security solutions across the region.
Packaging integrity modernization and operational facility expansion are driving consideration of tamper evident label systems as compliance enhancement pathways. Packaging companies and label suppliers are developing regional capabilities to support emerging operational deployment requirements.
- Manufacturing infrastructure expansion and operational diversification are creating economic drivers for compliance technologies and tamper evident label deployment across packaging and operational facilities seeking competitive differentiation pathways.
- Regional operational cooperation and coordinated supply chain development are establishing consistent compliance environments and shared operational infrastructure that support multi-state operational projects throughout Midwest manufacturing operations.
Competitive Landscape of USA Tamper Evident Labels Demand
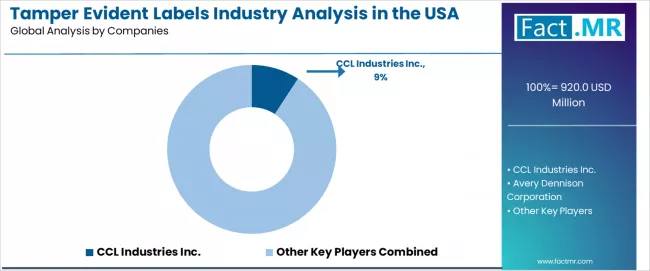
USA tamper evident labels demand is defined by competition among specialized security developers, packaging companies, and integrated solution providers, with major label distributors maintaining significant influence through manufacturing resources and application development capabilities. Companies are investing in tamper evident label advancement, production infrastructure optimization, quality control network structures, and comprehensive integration services to deliver effective, reliable, and scalable security management solutions across USA pharmaceutical manufacturing and packaging applications.
Strategic partnerships, manufacturing infrastructure development, and first-mover application execution are central to strengthening competitive positioning and presence across high-security, premium, and pharmaceutical packaging applications.
CCL Industries Inc., internationally recognized security leader, leads with 9.20% share, offering comprehensive advanced tamper evident label solutions including manufacturing, processing, and distribution services with focus on pharmaceutical applications, integrity stability reliability, and quality optimization across USA operations. Avery Dennison Corporation, operating with extensive USA presence, provides integrated security solutions leveraging tamper evident label expertise, high-performance development, and manufacturing management capabilities.
3M Company delivers full-service tamper evident label production including security development, processing optimization, and system integration serving USA and international pharmaceutical manufacturing projects. UPM Raflatac emphasizes comprehensive quality solutions with integrated label capabilities, high-security control, and performance features leveraging pharmaceutical sector expertise. Brady Corporation offers tamper evident label application development and integration optimization operations for commercial and pharmaceutical applications across USA operations.
USA Tamper Evident Labels Demand - Stakeholder Contribution Framework
The USA tamper evident labels demand is evolving as a cornerstone of security system innovation supporting pharmaceutical manufacturing, packaging operations, compliance applications, and high-security compliance. With the demand projected to exceed USD 1,498 million by 2035, growth is fueled by demand for security systems, operational efficiency, quality control, and mission-critical tamper evident labels for next-generation applications.
The sector's value creation depends on collaborative engagement among government institutions, industry bodies, manufacturers, research organizations, and investors to strengthen domestic production capacity, secure supply chains, and accelerate innovation across applications from pharmaceutical manufacturing operations to packaging production.
How Governments Could Accelerate Development and Demand Competitiveness?
- Federal Security Research Initiatives: Expand R&D funding for tamper evident label systems, quality control, and advanced security technology through agencies such as FDA, DHS, and NIST to enhance performance and domestic technological capabilities.
- Tax Incentives for Pharmaceutical Manufacturing: Offer targeted tax credits for facilities investing in advanced label systems, security manufacturing, and high-security technologies that reduce production costs and operational complexity.
- Domestic Supply Chain Development: Support manufacturing programs and supply resilience strategies for security materials, label components, and quality control verification to mitigate import dependence and price volatility.
- Standards and Certification Policies: Introduce standardized testing frameworks for security performance, authentication capabilities, and high-security compliance to streamline approval and boost operational competitiveness.
- Pharmaceutical and Packaging Applications: Integrate tamper evident labels in government procurement and security infrastructure modernization programs to strengthen domestic production and create long-term demand stability.
- High-Security Compliance Mandates: Promote responsible tamper evident label deployment through safety protocols, operational management systems, and authentication verification procedures.
How Industry Bodies Could Strengthen Sector Coordination and Technical Leadership?
- Unified Performance Standards: Develop consensus-based security, authentication, and high-security performance benchmarks to ensure interoperability and operational competitiveness.
- Workforce Development Programs: Create technical training pathways for packaging engineers, quality control specialists, and system operators to meet rising skill requirements.
- Collaborative R&D Networks: Establish joint programs linking universities, manufacturers, and research labs to drive innovation in label systems, quality control, and security technologies.
- Performance Data Repositories: Build centralized databases for security testing data, operational metrics, and performance effectiveness indicators to accelerate integration optimization.
- Demand Outreach and Awareness: Promote tamper evident labels' role in security efficiency, compliance readiness, and pharmaceutical innovation through coordinated industry campaigns.
How Technology and Material Suppliers Could Capture Value and Drive Innovation?
- Advanced Security Control Development: Invest in high-precision and label control processing that improves security efficiency and system reliability.
- High-Security System Integration: Develop integrated operational systems and hybrid verification platforms for quality applications in pharmaceutical manufacturing and packaging operations.
- Supply Chain Modernization: Implement digital traceability and predictive logistics to secure material delivery and maintain just-in-time production.
- Partnership with Research Institutions: Collaborate with universities and government labs on advanced security control, high-performance authentication formulations suitable for demanding environments.
- Operational Optimization: Introduce efficient material processing and reprocessing of operational materials into reusable systems for cost-efficient operations.
How Manufacturers Could Optimize Operational Efficiency and Demand Expansion?
- Smart Production Facilities: Integrate AI-driven monitoring, precision security control, and real-time process analytics to enhance throughput and reduce operational complexity.
- Scale-up of High-Value Applications: Focus on pharmaceutical manufacturing systems, packaging operations, and high-security platforms for operational systems, security distribution, and quality applications.
- Collaborative Supply Ecosystems: Develop long-term partnerships with material suppliers, processing manufacturers, and application integrators to ensure consistent quality.
- Pilot-to-Mass Production Transition: Use modular production designs and phased commercialization to balance R&D intensity with production scalability.
- Process Cost Optimization: Invest in automated application systems and operational processing technologies to lower production costs and operational complexity.
How Pharmaceutical Manufacturing Companies Could Lead Cross-Sector Tamper Evident Label Integration?
- Pharmaceutical and Packaging Integration: Deploy advanced tamper evident labels in manufacturing systems, packaging operations, and high-security applications for enhanced operational capability.
- Manufacturing Equipment Enhancement: Incorporate tamper evident labels in operational systems and security management tools to improve quality and authentication efficiency.
- Distribution and Supply Chain Management: Apply tamper evident label systems in packaging fulfillment, manufacturing management, and quality monitoring to support operational effectiveness.
- Operational and Performance Testing Expansion: Create regional testing facilities to evaluate system performance, security reliability, and operational effectiveness for manufacturing users.
- Packaging Efficiency Initiatives: Reuse and optimize tamper evident label systems through advanced processing programs that reduce operational costs.
How Packaging Companies Could Unlock Application Innovation and Demand Access?
- Manufacturing and Distribution Expansion: Develop premium tamper evident label systems for pharmaceutical processing operations, packaging management, and high-security monitoring meeting security efficiency standards for growing manufacturing demand.
- Pharmaceutical Processing and Packaging Applications: Integrate tamper evident labels into facility management, security processing, and quality monitoring for operational optimization and efficiency enhancement.
- Advanced Operational Systems: Offer tamper evident label platforms and operational solutions enabling packaging automation and performance enhancement in pharmaceutical applications.
- Design for Operational Services: Partner with pharmaceutical processing operators to provide co-engineered tamper evident label systems optimized for cost, reliability, and security effectiveness.
- Digital Platform Development: Launch packaging and operational platforms for standard tamper evident label systems and technical components for pharmaceutical and packaging buyers.
How Investors and Financial Enablers Could Unlock Growth and Technology Scalability?
- Venture Capital for Technology Startups: Support early-stage companies developing advanced tamper evident labels, security systems, and authentication control technologies.
- Infrastructure and Equipment Financing: Provide capital for domestic processing modernization, production equipment expansion, and digital control system upgrades.
- Public-Private Investment Platforms: Create co-financed innovation hubs for tamper evident label R&D that link investors, startups, and government labs.
- Strategic Consolidation Funding: Back mergers uniting fragmented tamper evident label producers to achieve scale, efficiency, and packaging competitiveness.
- Performance-Linked Financing Models: Tie loan terms and investor returns to security metrics, system efficiency, and application performance.
- Technology Financing Programs: Channel innovation-oriented funds into advanced tamper evident label processing and operational infrastructure.
Key Players in USA Tamper Evident Labels Industry
- 3M Company
- CCL Industries Inc.
- Avery Dennison Corporation
- UPM Raflatac
- Brady Corporation
- Seal King
- TydenBrammall
- American Casting & Manufacturing Corp.
- Atlantic Zeiser GmbH
- Mega Fortris Group
- Labelmaster Software Inc.
Scope of the Report
| Item | Value |
|---|---|
| Quantitative Units | USD 1,498.0 Million |
| Type | Void Labels, Tamper-Evident Tapes, Shrink Bands |
| End Use | Pharmaceuticals, Food & Beverage, Cosmetics & Others |
| Regions Covered | West, Northeast, South, Midwest |
| Key Companies Profiled | 3M Company, CCL Industries Inc., Avery Dennison Corporation, UPM Raflatac, Brady Corporation, Seal King, TydenBrammall, American Casting & Manufacturing Corp., Atlantic Zeiser GmbH, Mega Fortris Group, Labelmaster Software Inc. |
| Additional Attributes | Sales by type and end-use segment, regional demand trends across West, Northeast, South, and Midwest, competitive landscape with established label manufacturers and specialized security technology developers, manufacturer preferences for pharma-grade versus food-grade technologies, integration with regulatory compliance programs and supply chain security policies particularly advanced in West region |
USA Tamper Evident Labels Industry by Segments
-
Type :
- Void Labels
- Tamper-Evident Tapes
- Shrink Bands
-
End Use :
- Pharmaceuticals
- Food & Beverage
- Cosmetics & Others
-
Region :
- West
- Northeast
- South
- Midwest
Table of Content
- Executive Summary
- USA Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- USA Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- USA Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Introduction / Key Findings
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- USA
- Market Attractiveness Analysis By Region
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Market Attractiveness Analysis
- By Country
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- Competition Analysis
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: USA Market Value (USD Million) Forecast by Country, 2020 to 2035
List Of Figures
- Figure 1: USA Market Pricing Analysis
Figure 2: USA Market Value (USD Million) Forecast 2020 to 2035
Figure 3: USA Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
Figure 4: USA Market Y to o to Y Growth Comparison by Region, 2025 to 2035
Figure 5: USA Market Attractiveness Analysis by Region
Figure 6: USA Market Incremental Dollar Opportunity, 2025 to 2035
Figure 7: USA Market Value Share and BPS Analysis by Country, 2025 and 2035
Figure 8: USA Market - Tier Structure Analysis
Figure 9: USA Market - Company Share Analysis
- FAQs -
How big is the tamper evident labels industry analysis in the USA in 2025?
The global tamper evident labels industry analysis in the USA is estimated to be valued at USD 920.0 million in 2025.
What will be the size of tamper evident labels industry analysis in the USA in 2035?
The market size for the tamper evident labels industry analysis in the USA is projected to reach USD 1,498.6 million by 2035.
How much will be the tamper evident labels industry analysis in the USA growth between 2025 and 2035?
The tamper evident labels industry analysis in the USA is expected to grow at a 5.0% CAGR between 2025 and 2035.
What are the key product types in the tamper evident labels industry analysis in the USA?
The key product types in tamper evident labels industry analysis in the USA are void labels, tamper-evident tapes and shrink bands.
Which end use segment to contribute significant share in the tamper evident labels industry analysis in the USA in 2025?
In terms of end use, pharmaceuticals segment to command 42.0% share in the tamper evident labels industry analysis in the USA in 2025.