Biologics Manufacturing Market
Biologics Manufacturing Market Size and Share Forecast Outlook 2025 to 2035
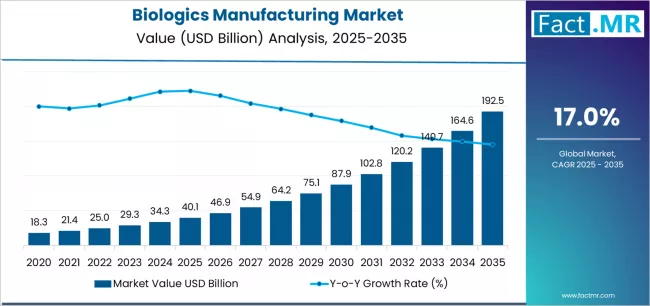
Biologics manufacturing market is projected to grow from USD 40.1 billion in 2025 to USD 192.5 billion by 2035, at a CAGR of 17.0%. Contract Manufacturing will dominate with a 64.2% market share, while monoclonal antibodies (mabs) will lead the modality segment with a 40.4% share.
Biologics Manufacturing Market Forecast and Outlook 2025 to 2035
The global biologics manufacturing market is projected to reach USD 192.5 billion by 2035, recording an absolute increase of USD 152.4 billion over the forecast period. The market is valued at USD 40.1 billion in 2025 and is set to rise at a CAGR of 17.0% during the assessment period.
The scope for biologics manufacturing is expected to grow by approximately 4.8 times during the same period, supported by increasing prevalence of chronic diseases requiring biologic therapeutics worldwide, driving demand for advanced manufacturing infrastructure and increasing investments in cell and gene therapy production with clinical efficacy across oncology and autoimmune disease applications globally.
Quick Stats for Biologics Manufacturing Market
- Biologics Manufacturing Market Value (2025): USD 40.1 billion
- Biologics Manufacturing Market Forecast Value (2035): USD 192.5 billion
- Biologics Manufacturing Market Forecast CAGR: 17.0%
- Leading Mode of Manufacturing in Biologics Manufacturing Market: Contract Manufacturing (64.2%)
- Key Growth Regions in Biologics Manufacturing Market: North America, Asia Pacific, and Europe
- Top Players in Biologics Manufacturing Market: Samsung Biologics, Lonza, WuXi Biologics, Pfizer Inc., Novartis AG, Amgen Inc., Johnson & Johnson, Bristol-Myers Squibb, AbbVie Inc., Roche
Pharmaceutical companies face mounting pressure to scale biologic production while addressing complex manufacturing requirements and supply chain reliability, with modern biologics manufacturing facilities providing documented operational benefits including enhanced productivity, improved quality control, and flexible production capabilities compared to traditional small-molecule manufacturing alone.
Rising emphasis on biosimilar development and expanding contract development and manufacturing organization services enabling accessible biologic production create substantial opportunities for manufacturing service providers and biopharmaceutical partners. However, high capital investment requirements and technical complexity may pose obstacles to rapid capacity expansion.
The contract manufacturing segment dominates market activity, driven by pharmaceutical companies outsourcing biologic production to specialized contract development and manufacturing organizations possessing advanced bioprocessing expertise across diverse therapeutic modality production worldwide.
Biopharmaceutical companies increasingly recognize strategic advantages of contract manufacturing partnerships, with typical outsourcing arrangements providing flexible capacity access and specialized technical capabilities at optimized cost structures through established service provider networks.
The monoclonal antibodies segment demonstrates robust dominance, supported by extensive clinical applications and proven therapeutic efficacy establishing antibody-based biologics as cornerstone treatments in modern medicine. Oncology emerges as the dominant disease indication category, reflecting substantial biologic therapeutic development addressing cancer treatment and immuno-oncology applications.
Regional dynamics show North America maintaining market leadership, supported by extensive biopharmaceutical industry presence and established contract manufacturing infrastructure. Asia Pacific demonstrates the fastest growth trajectory driven by expanding manufacturing capabilities and cost-competitive production services, while Europe emphasizes biosimilar manufacturing and regulatory excellence.
USA leads country-level growth through strong contract development and manufacturing organization capacity and research and development investments, followed by China supported by national biotechnology programs and CAR-T therapy manufacturing expansion.
The competitive landscape features moderate concentration with Samsung Biologics maintaining market leadership position, while established contract manufacturers including Lonza, WuXi Biologics, and integrated biopharmaceutical companies compete through comprehensive service offerings and advanced bioprocessing capabilities across diverse biologic modalities.
Biologics Manufacturing Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the biologics manufacturing market is projected to expand from USD 40.1 billion to USD 75.4 billion, resulting in a value increase of USD 35.3 billion, which represents 23.2% of the total forecast growth for the period. This phase of development will be shaped by rising demand for cell and gene therapy manufacturing capabilities addressing advanced therapeutic modality requirements, capacity expansion programs in contract manufacturing facilities with single-use bioreactor adoption and continuous bioprocessing technologies, as well as expanding integration with automated manufacturing systems and digital process analytics. Companies are establishing competitive positions through investment in large-scale bioreactor installations, advanced purification technologies, and strategic geographic expansion across established biopharmaceutical clusters, emerging manufacturing hubs, and biosimilar production centers.
From 2029 to 2035, the market is forecast to grow from USD 75.4 billion to USD 192.5 billion, adding another USD 117.1 billion, which constitutes 76.8% of the overall expansion. This period is expected to be characterized by the expansion of specialized manufacturing applications, including personalized cell therapy production and mRNA therapeutic manufacturing platforms tailored for pandemic response and precision medicine, strategic collaborations between contract manufacturers and gene therapy developers, and an enhanced focus on artificial intelligence-driven process optimization and predictive quality control systems. The growing emphasis on decentralized manufacturing models and rising adoption of modular production facilities addressing regional supply security will drive demand for flexible biologics manufacturing solutions across diverse therapeutic categories.
Biologics Manufacturing Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 40.1 billion |
| Market Forecast Value (2035) | USD 192.5 billion |
| Forecast CAGR (2025-2035) | 17.0% |
Why is the Biologics Manufacturing Market Growing?
The biologics manufacturing market grows by enabling biopharmaceutical companies to deliver complex therapeutic proteins and cellular products while addressing production scalability requirements and regulatory compliance imperatives without exclusive reliance on internal manufacturing infrastructure.
Pharmaceutical developers face mounting pressure to advance pipeline candidates through clinical development and commercialization while managing capital-intensive manufacturing investments and specialized technical expertise requirements, with modern contract manufacturing organizations typically providing comprehensive bioprocessing services including cell line development for expression optimization, process development for yield improvement, and commercial-scale production for market supply.
The biopharmaceutical industry's need for flexible manufacturing capacity creates demand for contract services that can provide rapid capacity access, specialized expertise, and regulatory compliance support without compromising product quality or development timelines.
Biopharmaceutical company adoption and clinical pipeline growth drive utilization of contract manufacturing services, integrated manufacturing facilities, and specialized cell and gene therapy production platforms, where manufacturing capabilities directly impact drug development success and commercial supply reliability.
The increasing biologic drug approvals globally, with regulatory agencies authorizing numerous monoclonal antibodies and advanced therapies annually, create expanding production requirements across therapeutic categories.
Rising complexity of biologic modalities including cell therapies and gene therapies enables sophisticated manufacturing approaches supporting personalized medicine and regenerative therapeutics. Supply chain vulnerabilities and pandemic-related disruptions may challenge production continuity and capacity availability among diverse pharmaceutical companies with varying manufacturing strategies.
Segmental Analysis
The market is segmented by mode of manufacturing, modality, disease indication, and region. By mode of manufacturing, the market is divided into contract manufacturing and in-house manufacturing. Based on modality, the market is categorized into monoclonal antibodies, biosimilar & recombinant proteins, vaccines, cell & gene therapies, RNA-based therapeutics, and others.
By disease indication, the market includes oncology, autoimmune disorders, infectious diseases, neurological disorders, cardiovascular disorders, and other disease indications. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Mode of Manufacturing, Which Segment Accounts for the Dominant Market Share?
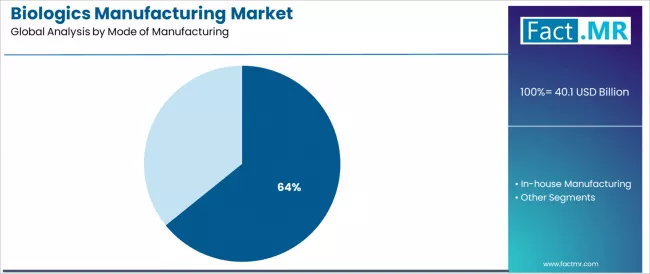
The contract manufacturing segment represents the dominant force in the biologics manufacturing market, capturing 64.2% of the total market share in 2025. This established manufacturing mode encompasses solutions featuring outsourced biologic production services and specialized bioprocessing capabilities, including advanced offerings combining upstream process development, downstream purification optimization, and fill-finish operations that enable superior pharmaceutical company flexibility and reduced capital requirements across monoclonal antibody production, cell therapy manufacturing, and biosimilar development applications worldwide.
The contract manufacturing segment's market leadership stems from its comprehensive value proposition, with services capable of addressing fundamental biopharmaceutical industry needs including rapid capacity access without facility investment, specialized technical expertise across modalities, and regulatory compliance support while maintaining excellent quality standards and production scalability across clinical and commercial supply requirements.
Within the contract manufacturing category, large-scale biologics production demonstrates dominant utilization, driven by pharmaceutical companies seeking commercial manufacturing capacity for marketed products and late-stage clinical candidates. These contract development and manufacturing organizations provide essential infrastructure for companies lacking internal manufacturing capabilities or experiencing capacity constraints.
The in-house manufacturing segment maintains substantial market share at 35.8%, serving integrated biopharmaceutical companies requiring proprietary manufacturing control and vertical integration strategies. These companies maintain dedicated facilities for strategic products and core manufacturing competencies while potentially outsourcing overflow capacity or specialized modalities.
Key strategic advantages driving the contract manufacturing segment include:
- Capital efficiency mechanisms enabling biopharmaceutical companies to avoid substantial facility investment and focus resources on research and development activities
- Specialized expertise access providing advanced bioprocessing knowledge and regulatory experience across diverse biologic modalities without internal capability development
- Enhanced production flexibility characteristics allowing variable capacity utilization and rapid scaling based on clinical development needs and commercial demand fluctuations
- Risk mitigation advantages distributing manufacturing across multiple facilities and geographic locations while maintaining supply chain resilience and business continuity
By Modality, Which Segment Accounts for the Largest Market Share?
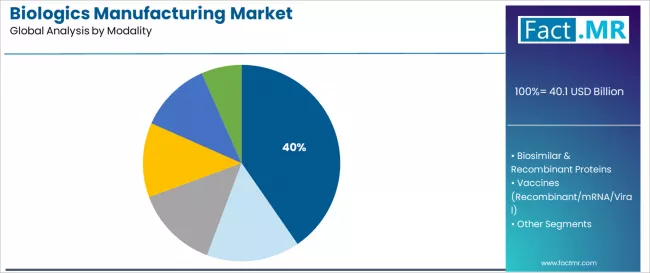
Monoclonal antibodies dominate the biologics manufacturing modality landscape with a 40.4% market share in 2025, reflecting the critical role of antibody-based therapeutics in supporting established clinical efficacy and extensive therapeutic applications across disease categories. The monoclonal antibodies segment's market leadership is reinforced by mature manufacturing processes, well-characterized production systems, and proven commercial success establishing antibodies as the predominant biologic therapeutic class.
Within this segment, therapeutic monoclonal antibodies for oncology and autoimmune diseases represent dominant production volumes, driven by multiple blockbuster products and continuous pipeline development. These antibodies benefit from established Chinese hamster ovary cell expression systems and standardized purification processes supporting reliable manufacturing.
The cell and gene therapies segment represents a rapidly growing modality category with 22.7% market share, demonstrating expansion through advanced therapeutic applications requiring specialized manufacturing approaches. These therapies benefit from regulatory support for regenerative medicine and personalized treatment paradigms despite manufacturing complexity.
Biosimilar and recombinant proteins maintain meaningful presence through generic biologics development and established protein therapeutics including insulin and growth factors. Vaccines demonstrate growing adoption through recombinant and mRNA platform technologies, while RNA-based therapeutics represent emerging manufacturing requirements.
Key market dynamics supporting modality segmentation include:
- Monoclonal antibody manufacturing dominance driven by proven clinical efficacy and established bioprocessing infrastructure
- Cell and gene therapy expansion trends require specialized manufacturing facilities and personalized production approaches
- Integration of platform technologies enabling standardized processes across multiple product candidates within modality classes
- Growing emphasis on continuous manufacturing and single-use systems reducing facility footprint and capital requirements
By Disease Indication, Which Segment Accounts for a Significant Market Share?

Oncology represents a leading disease indication segment in the biologics manufacturing market with a 38.5% market share in 2025, reflecting the fundamental importance of cancer therapeutics in driving biologic drug development and manufacturing demand. The oncology segment demonstrates consistent growth driven by immuno-oncology advancement, targeted antibody therapies, and CAR-T cell therapy manufacturing requirements.
The segment's strength stems from extensive pipeline development in cancer therapeutics including checkpoint inhibitors, antibody-drug conjugates, and cellular immunotherapies. Manufacturing requirements span traditional antibody production through specialized autologous cell therapy manufacturing requiring patient-specific processing.
Within indication applications, autoimmune disorders demonstrate substantial adoption at 19.3% market share through chronic disease management requiring long-term biologic therapy. Infectious diseases maintain presence through vaccine manufacturing and antiviral antibody production, while neurological disorders represent growing requirements for protein replacement and gene therapies.
Key indication dynamics include:
- Oncology dominance accelerating through immuno-oncology innovation and personalized cell therapy manufacturing
- Autoimmune disorder applications addressing rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis with established antibody therapeutics
- Infectious disease requirements expanding through pandemic response capabilities and prophylactic antibody manufacturing
- Neurological disorder emphasis supporting rare disease therapies and gene therapy manufacturing for inherited conditions
What drives In-House Manufacturing Presence?
In-house manufacturing emerges as a significant mode segment in the biologics manufacturing market with approximately 35.8% market share in 2025, reflecting strategic priorities for integrated biopharmaceutical companies maintaining proprietary manufacturing capabilities and vertical integration strategies. The in-house manufacturing segment demonstrates consistent presence driven by control requirements for flagship products, intellectual property protection priorities, and supply security considerations.
The segment's market strength stems from large biopharmaceutical companies operating dedicated manufacturing networks supporting global commercial supply. These integrated facilities provide complete control over production processes, quality systems, and supply chain management supporting strategic product portfolios.
Within in-house manufacturing applications, blockbuster monoclonal antibody production demonstrates substantial capacity utilization through high-volume commercial manufacturing. Companies maintain dedicated facilities for products generating substantial revenue while potentially outsourcing lower-volume products or clinical supply.
Key segment characteristics include:
- Strategic control priorities driving in-house manufacturing for core products and proprietary technologies
- Intellectual property protection addressing manufacturing process know-how and formulation details
- Supply security considerations ensuring reliable capacity for critical commercial products
- Vertical integration strategies optimizing manufacturing costs for high-volume products through dedicated facilities
What Characterizes Cell and Gene Therapy Manufacturing?
Cell and gene therapies represent a rapidly growing modality segment in the biologics manufacturing market with a 22.7% market share in 2025, reflecting specialized manufacturing requirements for advanced therapeutic products addressing previously untreatable genetic diseases and cancer applications. The cell and gene therapy segment demonstrates robust expansion driven by regulatory approvals, clinical success stories, and investment in specialized manufacturing infrastructure.
Within cell and gene therapy applications, autologous CAR-T cell manufacturing demonstrates significant activity through personalized cancer immunotherapy production requiring patient-specific processing. Allogeneic cell therapies maintain growing presence through off-the-shelf product development enabling standardized manufacturing approaches.
The segment benefits from technological advancement in viral vector production, cell processing automation, and analytical methods supporting quality control. Gene therapy manufacturing spans ex vivo cell modification and in vivo gene delivery requiring distinct production platforms and purification strategies.
Key modality dynamics include:
- Autologous cell therapy manufacturing requiring distributed processing facilities near treatment centers
- Viral vector production driving demand for specialized manufacturing capacity and purification expertise
- Process automation adoption addressing labor-intensive manual processing and ensuring consistent product quality
- Regulatory framework evolution supporting accelerated approval pathways and manufacturing flexibility for rare disease therapies
What are the Drivers, Restraints, and Key Trends of the Biologics Manufacturing Market?
The market is driven by three concrete demand factors tied to biopharmaceutical industry evolution. First, increasing biologic drug pipeline development creates expanding manufacturing capacity requirements, with biopharmaceutical companies advancing numerous monoclonal antibodies and cell therapies through clinical development requiring scalable production capabilities, necessitating widespread contract manufacturing utilization. Second, growing biosimilar market expansion drives manufacturing demand as patent expiration of originator biologics enables generic competition, with biosimilar production requiring specialized facilities and regulatory validation demonstrating manufacturing equivalence by 2030. Third, advancing manufacturing technologies including continuous bioprocessing and single-use systems enable improved production efficiency approaches that enhance productivity while reducing facility footprint and capital investment requirements.
Market restraints include high capital expenditure requirements and long facility construction timelines that can challenge rapid capacity expansion and market responsiveness, particularly for specialized modalities where manufacturing infrastructure remains limited and industry competition for capacity proves intense. Technical complexity and regulatory compliance requirements pose another significant obstacle, as biologics manufacturing depends on maintaining process consistency, demonstrating comparability, and meeting stringent quality standards, potentially affecting production yields and regulatory approval timelines. Supply chain vulnerabilities and raw material dependencies create additional barriers for reliable manufacturing, demanding comprehensive vendor qualification and multiple sourcing strategies addressing critical reagent availability and quality consistency.
Key trends indicate accelerated automation adoption in developed markets, particularly North America and Europe, where manufacturers demonstrate increasing implementation of robotic systems, artificial intelligence-driven process control, and advanced process analytics enabling real-time quality monitoring and predictive maintenance. Decentralized manufacturing trends toward regional production facilities and modular cleanroom systems enable pandemic response capabilities and personalized therapy production addressing local patient populations. However, the market thesis could face disruption if significant advances in alternative therapeutic modalities or breakthrough manufacturing technologies substantially alter production economics and capacity requirements in biopharmaceutical industry manufacturing strategies.
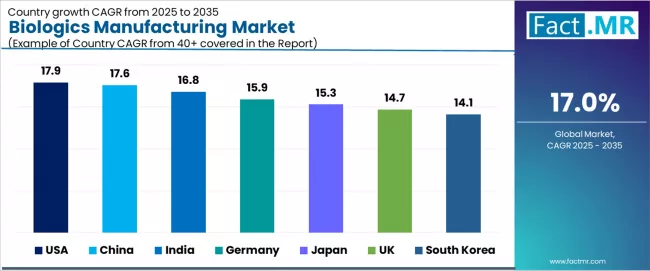
Analysis of the Biologics Manufacturing Market by Key Countries
| Country | CAGR (2025 to 2035) |
|---|---|
| USA | 17.9% |
| China | 17.6% |
| India | 16.8% |
| Germany | 15.9% |
| Japan | 15.3% |
| UK | 14.7% |
| South Korea | 14.1% |
The global biologics manufacturing market is expanding rapidly, with USA leading at a 17.9% CAGR through 2035, driven by strong contract development and manufacturing organization capacity, substantial research and development investments, and established biopharmaceutical industry clusters. China follows at 17.6%, supported by national biotechnology programs, CAR-T therapy manufacturing growth, and government incentives for domestic biologic production. India records 16.8%, reflecting biosimilar expansion, low-cost manufacturing advantages, and growing contract service capabilities.
Germany advances at 15.9%, leveraging European Union biotech funding and precision medicine emphasis. Japan posts 15.3%, focusing on induced pluripotent stem cell technologies and regenerative therapy leadership, while UK grows steadily at 14.7%, anchored by biobank infrastructure and Medicines and Healthcare products Regulatory Agency support. South Korea demonstrates 14.1% growth, driven by Samsung Biologics capacity scale-up and contract manufacturing expansion.
How is USA Leading Global Market Expansion?
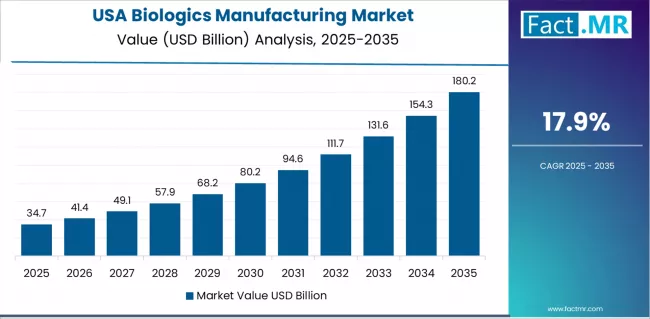
USA demonstrates the strongest growth potential in the biologics manufacturing market with a CAGR of 17.9% through 2035. The country's leadership position stems from extensive contract development and manufacturing organization infrastructure, comprehensive biopharmaceutical industry presence, and substantial venture capital investment supporting biotech innovation.
Growth is concentrated in major biopharmaceutical clusters including Boston-Cambridge, San Francisco Bay Area, San Diego, Research Triangle, and Greater Philadelphia, where contract manufacturers and integrated pharmaceutical companies operate advanced bioprocessing facilities supporting commercial and clinical supply.
Manufacturing infrastructure through purpose-built facilities, technology parks, and specialized cell therapy production centers expands capacity across therapeutic modalities. The country's established regulatory expertise and Food and Drug Administration collaboration provide strong momentum for manufacturing process validation and product approval, including comprehensive support across modality-specific guidance development.
Key market factors:
- Contract manufacturing concentration in major clusters with established bioprocessing expertise and skilled workforce availability
- Biopharmaceutical innovation ecosystem supporting continuous pipeline development and manufacturing demand growth
- Venture capital investment enabling biotech company formation and contract manufacturing service utilization
- Regulatory framework leadership establishing global standards for biologic manufacturing and process validation
Why is China Emerging as a High-Growth Market?
In major biomanufacturing hubs including Shanghai, Suzhou, Beijing, and Wuxi, the adoption of biologics manufacturing capabilities is accelerating across contract service providers and domestic pharmaceutical companies, driven by national biotechnology strategy and healthcare modernization initiatives. The market demonstrates strong growth momentum with a CAGR of 17.6% through 2035, linked to government support for biopharmaceutical industry development and increasing emphasis on innovative drug manufacturing capacity.
Chinese contract manufacturers are expanding large-scale production facilities and developing cell and gene therapy capabilities to serve domestic and international pharmaceutical companies. The country's cost-competitive manufacturing combined with advancing technical capabilities creates ongoing demand for outsourcing partnerships, while domestic biologic drug development drives internal manufacturing capacity investment.
Key development areas:
- National biotechnology programs providing funding and policy support for manufacturing infrastructure development
- Contract manufacturing expansion through companies like WuXi Biologics establishing global-scale production capacity
- CAR-T therapy manufacturing growth addressing domestic cancer treatment demand and clinical trial requirements
- Biosimilar development driving manufacturing capacity for generic biologic production serving large patient populations
What drives USA’s Market Leadership?
USA’s market expansion is driven by established contract manufacturing industry, continuous capacity expansion programs, and technological innovation in bioprocessing systems and automation platforms. The country demonstrates robust growth potential with a CAGR of 17.9% through 2035, supported by pharmaceutical company outsourcing trends and specialized manufacturing requirements for advanced therapies.
American contract manufacturers face capacity utilization opportunities related to biologic drug approval growth, biosimilar competition driving manufacturing efficiency, and cell therapy commercialization requiring distributed production networks. However, established biopharmaceutical clusters and comprehensive service provider ecosystem create stable baseline demand for manufacturing capacity, particularly for monoclonal antibody production where commercial supply requirements drive sustained capacity investment and technology advancement.
Market characteristics:
- Contract manufacturing dominance with major service providers operating multiple large-scale facilities
- Advanced therapy manufacturing emphasis addressing gene therapy and cell therapy production requirements
- Technology leadership in continuous manufacturing and single-use systems reducing production costs
- Regulatory expertise supporting complex manufacturing process validation and comparability demonstration
How does Germany Demonstrate European Leadership?
The market in Germany leads in biologics manufacturing based on European Union biopharmaceutical industry presence and comprehensive regulatory framework supporting biosimilar development and advanced therapy manufacturing. The country shows strong potential with a CAGR of 15.9% through 2035, driven by precision medicine emphasis and contract manufacturing capabilities in major regions.
German manufacturers are operating advanced bioprocessing facilities and developing cell and gene therapy production capabilities supporting European pharmaceutical industry and export markets. Manufacturing infrastructure through established pharmaceutical companies and contract service providers expands capacity across Baden-Württemberg, Bavaria, and North Rhine-Westphalia regions.
Leading market segments:
- Biosimilar manufacturing leadership with multiple companies producing generic biologics for European markets
- Contract manufacturing services providing flexible capacity for European pharmaceutical companies
- Advanced therapy manufacturing addressing cell therapy and gene therapy production requirements
- Quality standards emphasis ensuring robust manufacturing processes and regulatory compliance
What positions India for Biosimilar Leadership?
In major pharmaceutical manufacturing hubs including Hyderabad, Bangalore, Ahmedabad, and Mumbai, biosimilar manufacturing capabilities are expanding across domestic pharmaceutical companies, driven by generic biologics market opportunities and cost-competitive production advantages. The market shows meaningful growth potential with a CAGR of 16.8% through 2035, linked to established pharmaceutical manufacturing expertise and expanding biologic production infrastructure.
Indian companies are developing biosimilar portfolios and establishing manufacturing facilities supporting domestic supply and global market export. The country's cost advantages and technical capabilities create ongoing opportunities for contract manufacturing services targeting international pharmaceutical companies.
Market development factors:
- Biosimilar manufacturing expertise with multiple Indian companies developing generic biologic portfolios
- Cost-competitive production enabling attractive pricing for global biosimilar markets
- Contract manufacturing expansion providing services for international pharmaceutical companies
- Regulatory framework evolution supporting biosimilar development and manufacturing validation
How does Japan show Regenerative Medicine Leadership?
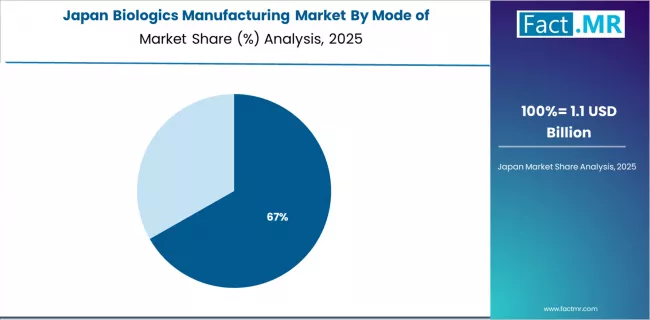
Japan's biologics manufacturing market demonstrates sophisticated capabilities focused on induced pluripotent stem cell technologies and regenerative medicine applications, with documented leadership in cellular therapy manufacturing and research translation.
The country maintains steady growth momentum with a CAGR of 15.3% through 2035, driven by government support for regenerative medicine commercialization and advanced manufacturing infrastructure. Major biopharmaceutical clusters in Kobe, Osaka, and Tokyo showcase specialized manufacturing facilities where cell therapy production and antibody manufacturing support domestic pharmaceutical industry and clinical research programs.
Key market characteristics:
- Induced pluripotent stem cell technology leadership driving regenerative medicine manufacturing development
- Government support through regenerative medicine regulations and fast-track approval pathways
- Academic-industry collaboration supporting technology transfer and manufacturing scale-up
- Quality manufacturing emphasis ensuring robust processes and regulatory compliance standards
What characterizes UK's Infrastructure Advantages?
In major biopharmaceutical centers including Cambridge, Oxford, London, and Manchester, biologics manufacturing capabilities are expanding through contract service providers and integrated pharmaceutical companies, driven by biobank infrastructure and clinical research excellence.
The market demonstrates solid growth potential with a CAGR of 14.7% through 2035, linked to cell and gene therapy development and Medicines and Healthcare products Regulatory Agency regulatory support. The manufacturers are developing advanced therapy manufacturing capabilities and providing contract services supporting European pharmaceutical industry. The country's research infrastructure and regulatory expertise create ongoing opportunities for specialized manufacturing applications.
Key development areas:
- Biobank infrastructure supporting access to biological materials for research and manufacturing
- Cell and gene therapy manufacturing addressing advanced therapeutic development and clinical supply
- Regulatory framework leadership through Medicines and Healthcare products Regulatory Agency guidance on innovative therapies
- Academic clusters supporting technology development and manufacturing process innovation
What drives South Korea's Manufacturing Scale-Up?
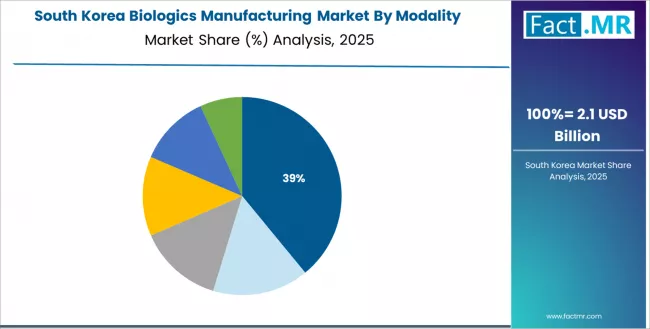
In major industrial centers including Incheon, Songdo, and Seoul, biologics manufacturing capacity is expanding through Samsung Biologics and domestic biopharmaceutical companies, driven by contract manufacturing business growth and government biotechnology support. The market demonstrates meaningful growth potential with a CAGR of 14.1% through 2035, linked to large-scale capacity investment and technology advancement.
South Korean manufacturers are operating some of the world's largest biologics production facilities and serving international pharmaceutical companies through contract manufacturing services. The country's manufacturing expertise and investment capacity create ongoing opportunities for market share expansion in global contract manufacturing industry.
Key development areas:
- Samsung Biologics capacity leadership operating multiple large-scale manufacturing facilities
- Contract manufacturing focus serving global pharmaceutical companies through flexible capacity offerings
- Technology investment supporting advanced bioprocessing systems and automation platforms
- Government support through biotechnology development programs and regulatory framework enhancement
Europe Market Split by Country
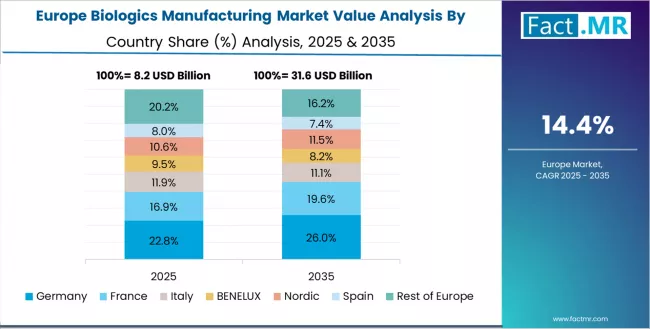
The biologics manufacturing market in Europe is projected to grow from USD 10.1 billion in 2025 to USD 48.3 billion by 2035, registering a CAGR of 16.8% over the forecast period. Germany is expected to maintain its leadership position with a 32.4% market share in 2025, adjusting to 32.1% by 2035, supported by its extensive biopharmaceutical industry infrastructure, comprehensive biosimilar manufacturing capabilities, and established contract service provider networks serving major European markets.
Switzerland follows with a 24.7% share in 2025, projected to reach 25.1% by 2035, driven by integrated biopharmaceutical company headquarters and advanced manufacturing facilities. UK holds a 18.6% share in 2025, expected to maintain 19.0% by 2035 through cell and gene therapy manufacturing development and regulatory framework leadership.
France commands a 13.8% share, while Italy accounts for 7.2% in 2025. The Rest of Europe region is anticipated to gain momentum, expanding its collective share from 3.3% to 3.6% by 2035, attributed to increasing biologics manufacturing investment in Ireland, Belgium, and Denmark implementing advanced bioprocessing facilities.
Competitive Landscape of the Biologics Manufacturing Market

The biologics manufacturing market features approximately 20-25 meaningful players with moderate concentration, where the top three companies control roughly 22-28% of global contract manufacturing market share through established large-scale facilities, comprehensive service offerings, and extensive pharmaceutical company relationships. Competition centers on capacity availability, technical expertise, and quality track record rather than price competition alone.
Market leaders include Samsung Biologics, Lonza, and WuXi Biologics, which maintain competitive advantages through extensive manufacturing capacity, advanced bioprocessing capabilities, and deep expertise in commercial-scale biologic production, creating high pharmaceutical company confidence in reliable supply and regulatory compliance.
These companies leverage ongoing capacity expansion investments and strategic technology partnerships to defend market positions while expanding into specialized modalities including cell and gene therapy manufacturing.
Challengers encompass integrated biopharmaceutical companies including Pfizer Inc., Novartis AG, and Amgen Inc., which operate substantial in-house manufacturing networks supporting proprietary product portfolios while potentially offering contract manufacturing services for third-party products.
Major pharmaceutical companies, including Johnson & Johnson, Bristol-Myers Squibb, and AbbVie Inc., maintain dedicated biologics manufacturing facilities supporting commercial supply for marketed products and clinical material production for pipeline candidates.
Emerging contract manufacturers and regional service providers create competitive pressure through specialized capabilities and geographic advantages, particularly in high-growth markets including China and India, where cost-competitive manufacturing and expanding technical expertise provide opportunities for pharmaceutical company partnerships and biosimilar production contracts.
Market dynamics favor companies that combine proven manufacturing track record with flexible capacity models addressing variable demand patterns and emerging modality requirements. Strategic emphasis on continuous manufacturing implementation, automation advancement, and digital process analytics enables differentiation in increasingly competitive biologics manufacturing markets across established and emerging regions.
Global Biologics Manufacturing Market - Stakeholder Contribution Framework
Biologics manufacturing represents a critical biopharmaceutical industry capability that enables drug developers to deliver complex therapeutic proteins and cellular products while addressing production scalability requirements and regulatory compliance imperatives without exclusive reliance on internal infrastructure investment, typically providing comprehensive bioprocessing services including process development for optimization, commercial manufacturing for supply reliability, and quality assurance for regulatory compliance compared to internal facility construction alone while ensuring improved capital efficiency and accelerated market access.
With the market projected to grow from USD 40.1 billion in 2025 to USD 192.5 billion by 2035 at a 17.0% CAGR, these solutions offer compelling advantages for biopharmaceutical development, contract service applications, and diverse therapeutic modalities requiring specialized production capabilities. Scaling manufacturing capacity and technological advancement requires coordinated action across regulatory policy, quality standards, contract manufacturers, pharmaceutical companies, and innovation ecosystem development.
How Could Governments Spur Local Development and Adoption?
- Biomanufacturing Infrastructure Development: Include biologics manufacturing in national biotechnology strategies, providing targeted support for facility investment through tax incentives and supporting workforce development through specialized training programs.
- Regulatory Framework Excellence: Implement science-based biologics approval pathways, provide clear guidance for manufacturing process validation, and establish harmonized international standards facilitating product registration and market access.
- Research & Innovation Funding: Create public-private partnerships for bioprocessing technology development, support academic research in upstream and downstream processing optimization, and provide grants for continuous manufacturing implementation.
- Workforce Development Programs: Fund specialized biotechnology education programs, support apprenticeship opportunities in biomanufacturing facilities, and establish centers of excellence for process development training.
- Supply Chain Security: Establish strategic reserves for critical biologics, support domestic raw material production capabilities, and create frameworks addressing pandemic response manufacturing flexibility.
How Could Industry Bodies Support Market Development?
- Manufacturing Standards & Quality: Define best practices for biologics production across modalities, establish standardized quality metrics and process analytical technology implementation, and create benchmarking databases supporting operational excellence.
- Technical Training & Certification: Develop comprehensive bioprocessing training curricula, establish professional certification programs for manufacturing personnel, and create continuing education frameworks supporting technology advancement adoption.
- Supply Chain Coordination: Lead initiatives addressing raw material standardization, establish vendor qualification frameworks, and create collaborative approaches to supply security and shortage prevention.
- Technology Advancement: Support collaborative research on next-generation bioprocessing systems, facilitate knowledge sharing on continuous manufacturing implementation, and promote automation and digitalization best practices.
How Could Manufacturers and Technology Players Strengthen the Ecosystem?
- Capacity Expansion Programs: Develop large-scale manufacturing facilities with flexible multiproduct capability, implement single-use systems reducing changeover time and contamination risk, and establish modular facilities enabling rapid capacity deployment.
- Technology Innovation: Advance continuous bioprocessing systems improving productivity and reducing facility footprint, develop automation platforms minimizing manual operations and ensuring process consistency, and implement artificial intelligence-driven process optimization enabling real-time quality control.
- Service Excellence: Provide comprehensive technical support from process development through commercial manufacturing, offer regulatory expertise supporting filing preparation and agency interactions, and deliver supply chain management ensuring reliable product delivery.
- Specialized Capability Development: Build cell and gene therapy manufacturing infrastructure addressing advanced modality requirements, develop viral vector production capacity supporting gene therapy pipeline growth, and establish mRNA manufacturing platforms enabling pandemic response and vaccine production.
How Could Pharmaceutical Companies Navigate Manufacturing Decisions?
- Strategic Sourcing Evaluation: Assess core competency requirements determining in-house versus outsourced manufacturing strategies, evaluate contract manufacturer capabilities including capacity availability and technical expertise, and establish partnership frameworks balancing control requirements with capital efficiency.
- Technology Transfer Excellence: Develop robust process characterization supporting reliable technology transfer, implement comprehensive documentation systems ensuring knowledge capture and regulatory compliance, and establish collaborative relationships with contract manufacturers facilitating successful process implementation.
- Supply Chain Risk Management: Diversify manufacturing sources across multiple facilities and geographic regions, establish strategic inventory positions mitigating supply disruptions, and implement contingency planning addressing capacity constraints and quality issues.
- Quality Assurance Integration: Maintain oversight of contract manufacturing operations through regular audits and performance monitoring, establish clear quality agreements defining responsibilities and specifications, and implement change control processes ensuring product consistency throughout lifecycle.
How Could Investors and Financial Enablers Unlock Value?
- Capacity Investment Financing: Provide capital for contract manufacturers expanding large-scale production facilities, support technology implementation including continuous manufacturing and automation systems, and finance geographic expansion establishing regional manufacturing presence.
- Technology Innovation Support: Back companies developing breakthrough bioprocessing technologies including novel expression systems and purification platforms, invest in digitalization solutions enabling process analytics and predictive maintenance, and support automation companies developing robotic systems for biologics manufacturing.
- Biosimilar Manufacturing Funding: Finance biosimilar development programs requiring manufacturing process development and validation, support emerging market manufacturers establishing cost-competitive production capabilities, and back integrated biosimilar companies developing manufacturing and commercial capabilities.
- Advanced Therapy Infrastructure: Support cell and gene therapy manufacturing facility development addressing specialized production requirements, finance viral vector manufacturing capacity supporting gene therapy pipeline growth, and back companies developing platform technologies enabling standardized manufacturing approaches.
Key Players in the Biologics Manufacturing Market
- Samsung Biologics Co., Ltd.
- Lonza Group AG
- WuXi Biologics (Cayman) Inc.
- Pfizer Inc.
- Novartis AG
- Amgen Inc.
- Johnson & Johnson
- Bristol Myers Squibb Company
- AbbVie Inc.
- F. Hoffmann-La Roche Ltd.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 40.1 Billion |
| Mode of Manufacturing | Contract Manufacturing, In-house Manufacturing |
| Modality | Monoclonal Antibodies (mAbs), Biosimilar & Recombinant Proteins, Vaccines (Recombinant/mRNA/Viral), Cell & Gene Therapies, RNA-based Therapeutics, Others |
| Disease Indication | Oncology, Autoimmune Disorders, Infectious Diseases, Neurological Disorders, Cardiovascular Disorders, Other Disease Indications |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | USA, China, India, Germany, Japan, UK, South Korea, and 40+ countries |
| Key Companies Profiled | Samsung Biologics, Lonza, WuXi Biologics, Pfizer Inc., Novartis AG, Amgen Inc., Johnson & Johnson, Bristol-Myers Squibb, AbbVie Inc., Roche (F. Hoffmann-La Roche Ltd.) |
| Additional Attributes | Dollar sales by mode of manufacturing and modality categories, regional capacity trends across Asia Pacific, Europe, and North America, competitive landscape with contract manufacturers and integrated biopharmaceutical companies, manufacturing specification requirements and quality control standards, integration with process analytical technology and continuous manufacturing systems, innovations in single-use technologies and automation platforms, and development of specialized applications with cell therapy manufacturing capabilities and viral vector production expertise. |
Biologics Manufacturing Market by Segments
-
Mode of Manufacturing :
- Contract Manufacturing
- In-house Manufacturing
-
Modality :
- Monoclonal Antibodies (mAbs)
- Biosimilar & Recombinant Proteins
- Vaccines (Recombinant/mRNA/Viral)
- Cell & Gene Therapies
- RNA-based Therapeutics
- Others
-
Disease Indication :
- Oncology
- Autoimmune Disorders
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Disorders
- Other Disease Indications
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Mode of Manufacturing
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Mode of Manufacturing, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Mode of Manufacturing, 2025 to 2035
- Contract Manufacturing
- In-house Manufacturing
- Y to o to Y Growth Trend Analysis By Mode of Manufacturing, 2020 to 2024
- Absolute $ Opportunity Analysis By Mode of Manufacturing, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Modality
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Modality, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Modality, 2025 to 2035
- Monoclonal Antibodies (mAbs)
- Biosimilar & Recombinant Proteins
- Vaccines (Recombinant/mRNA/Viral)
- Cell & Gene Therapies
- RNA-based Therapeutics
- Others
- Y to o to Y Growth Trend Analysis By Modality, 2020 to 2024
- Absolute $ Opportunity Analysis By Modality, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Disease Indication
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Disease Indication, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Disease Indication, 2025 to 2035
- Oncology
- Autoimmune Disorders
- Infectious Diseases
- Neurological Disorders
- Cardiovascular Disorders
- Other Disease Indications
- Y to o to Y Growth Trend Analysis By Disease Indication, 2020 to 2024
- Absolute $ Opportunity Analysis By Disease Indication, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- By Country
- Market Attractiveness Analysis
- By Country
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Mode of Manufacturing
- By Modality
- By Disease Indication
- Competition Analysis
- Competition Deep Dive
- Samsung Biologics Co., Ltd.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Lonza Group AG
- WuXi Biologics (Cayman) Inc.
- Pfizer Inc.
- Novartis AG
- Amgen Inc.
- Johnson & Johnson
- Bristol Myers Squibb Company
- AbbVie Inc.
- F. Hoffmann-La Roche Ltd.
- Samsung Biologics Co., Ltd.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Mode of Manufacturing, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Disease Indication, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Mode of Manufacturing, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Disease Indication, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Mode of Manufacturing, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Disease Indication, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Mode of Manufacturing, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Disease Indication, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Mode of Manufacturing, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by Disease Indication, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Mode of Manufacturing, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by Disease Indication, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Mode of Manufacturing, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by Disease Indication, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Mode of Manufacturing, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Modality, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by Disease Indication, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Mode of Manufacturing, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Mode of Manufacturing, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Mode of Manufacturing
- Figure 6: Global Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Modality
- Figure 9: Global Market Value Share and BPS Analysis by Disease Indication, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Disease Indication, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Disease Indication
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Mode of Manufacturing, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Mode of Manufacturing, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Mode of Manufacturing
- Figure 26: North America Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Modality
- Figure 29: North America Market Value Share and BPS Analysis by Disease Indication, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Disease Indication, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Disease Indication
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Mode of Manufacturing, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Mode of Manufacturing, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Mode of Manufacturing
- Figure 36: Latin America Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Modality
- Figure 39: Latin America Market Value Share and BPS Analysis by Disease Indication, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Disease Indication, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Disease Indication
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Mode of Manufacturing, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Mode of Manufacturing, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Mode of Manufacturing
- Figure 46: Western Europe Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Modality
- Figure 49: Western Europe Market Value Share and BPS Analysis by Disease Indication, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by Disease Indication, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by Disease Indication
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Mode of Manufacturing, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Mode of Manufacturing, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Mode of Manufacturing
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Modality
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by Disease Indication, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by Disease Indication, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by Disease Indication
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Mode of Manufacturing, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Mode of Manufacturing, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Mode of Manufacturing
- Figure 66: East Asia Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Modality
- Figure 69: East Asia Market Value Share and BPS Analysis by Disease Indication, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by Disease Indication, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by Disease Indication
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Mode of Manufacturing, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Mode of Manufacturing, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Mode of Manufacturing
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Modality
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by Disease Indication, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by Disease Indication, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by Disease Indication
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Mode of Manufacturing, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Mode of Manufacturing, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Mode of Manufacturing
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Modality, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Modality, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Modality
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by Disease Indication, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by Disease Indication, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by Disease Indication
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the biologics manufacturing market in 2025?
The global biologics manufacturing market is estimated to be valued at USD 40.1 billion in 2025.
What will be the size of biologics manufacturing market in 2035?
The market size for the biologics manufacturing market is projected to reach USD 192.5 billion by 2035.
How much will be the biologics manufacturing market growth between 2025 and 2035?
The biologics manufacturing market is expected to grow at a 17.0% CAGR between 2025 and 2035.
What are the key product types in the biologics manufacturing market?
The key product types in biologics manufacturing market are contract manufacturing and in-house manufacturing.
Which modality segment to contribute significant share in the biologics manufacturing market in 2025?
In terms of modality, monoclonal antibodies (mabs) segment to command 40.4% share in the biologics manufacturing market in 2025.