Critical Care Biomarker Market
Critical Care Biomarker Market Size and Share Forecast Outlook 2026 to 2036
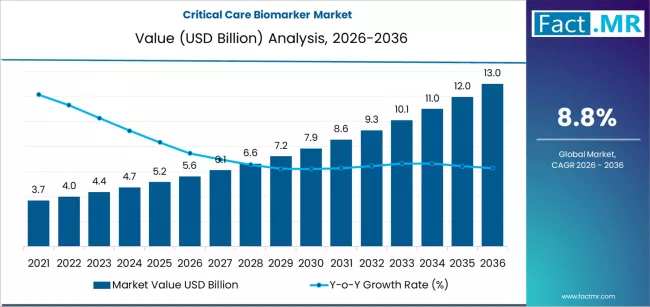
Critical care biomarker market is projected to grow from USD 5.6 billion in 2026 to USD 13.0 billion by 2036, at a CAGR of 8.8%. Cardiac biomarkers will dominate with a 29.4% market share, while early diagnosis will lead the application segment with a 37.8% share.
Critical Care Biomarker Market Forecast and Outlook 2026 to 2036
The global critical care biomarkers market is projected to total USD 5.61 billion in 2026, advancing to USD 13.02 billion by 2036 at an 8.8% CAGR. This substantial growth trajectory is driven by the escalating need for rapid, precise diagnostic tools in high-acuity medical environments.
Key Takeaways from the Critical Care Biomarker Market
- Market Value for 2026: USD 5.61 Billion
- Market Value for 2036: USD 13.02 Billion
- Forecast CAGR 2026 to 2036: 8.8%
- Leading Product Segment (2026): Cardiac Biomarkers (29.4%)
- Leading Application Segment (2026): Early Diagnosis (37.8%)
- Leading End User Segment (2026): ICU Laboratories (48.3%)
- Key Growth Countries: India (11.8% CAGR), China (11.6% CAGR), Brazil (11.1% CAGR), USA (9.9% CAGR), France (9.8% CAGR), UK (9.7% CAGR), Germany (9.6% CAGR)
- Key Players: Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers, Beckman Coulter, bioMérieux
Biomarkers for cardiac injury, sepsis, and organ dysfunction are transitioning from supportive evidence to central decision-making tools, enabling clinicians to navigate time-sensitive interventions with greater confidence. Integration of these tests into streamlined clinical protocols directly addresses the imperative to improve patient outcomes, reduce hospital stays, and optimize resource utilization within intensive care units.
Advancements in assay technology, particularly the development of high-sensitivity troponins and molecular panels for pathogen detection, are expanding the diagnostic repertoire at the point of care. The convergence of biomarker data with electronic health records and clinical decision support systems is creating a more dynamic and personalized approach to critical care management.
This evolution positions biomarker analysis not merely as a diagnostic function but as a foundational component of digitalized, protocol-driven intensive care, essential for managing the growing complexity and cost pressures within acute care settings worldwide.
Critical Care Biomarker Market
| Metric | Value |
|---|---|
| Market Value (2026) | USD 5.61 Billion |
| Market Forecast Value (2036) | USD 13.02 Billion |
| Forecast CAGR 2026 to 2036 | 8.8% |
Category
| Category | Segments |
|---|---|
| Product | Cardiac Biomarkers, Sepsis Biomarkers, Coagulation Biomarkers, Renal Injury Biomarkers, Respiratory Biomarkers |
| Application | Early Diagnosis, Risk Stratification, Treatment Monitoring, Prognosis Assessment |
| End User | ICU Laboratories, Central Hospital Labs, Point-of-Care Settings |
| Region | North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, Middle East & Africa |
Which Macro Healthcare Imperatives are Accelerating Biomarker Integration in Acute Care?
| Industry Development | Impact |
|---|---|
| Advances in Point-of-Care Testing | Enables real-time, bedside decision-making, drastically reducing turnaround time for life-saving interventions in sepsis and cardiac care. |
| Integration of AI with Diagnostic Data | Enhances the predictive power of biomarker panels by analyzing trends and correlating with patient vitals, improving early warning scores. |
| Global Sepsis Management Initiatives | Drives standardized adoption of biomarker panels like procalcitonin and lactate for rapid identification and protocol activation. |
| Pressure to Reduce ICU Length of Stay | Promotes biomarkers for treatment monitoring and prognosis, facilitating timely de-escalation of care and step-down unit transfers. |
Segmental Analysis
By Product, Which Biomarker Class Addresses the Most Time-Sensitive Critical Condition?
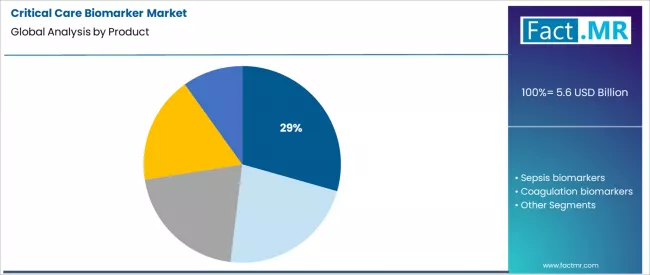
Cardiac biomarkers command a leading 29.4% share. This dominance is anchored in the universal and urgent need to diagnose acute myocardial infarction within emergency and critical care settings. High-sensitivity troponin assays have become the cornerstone for rule-in/rule-out protocols, directly influencing immediate therapeutic pathways.
The high volume of patients presenting with chest pain or cardiac symptoms in emergency departments and ICUs ensures consistent, high-volume demand. Continuous innovation toward even faster and more specific assays sustains this segment’s central role.
By Application, Where is Speed of Result Most Directly Linked to Clinical Outcome?
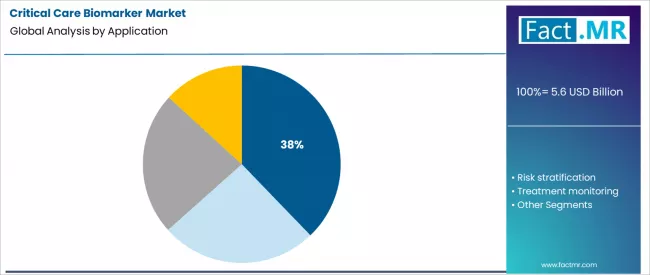
Early diagnosis constitutes the primary application segment at 37.8%. In critical care, the timeliness of a diagnosis is frequently as crucial as its accuracy. Rapid identification of conditions like sepsis, acute kidney injury, or myocardial infarction through biomarker signatures allows for the immediate initiation of targeted, protocol-driven therapy.
This application’s growth is fueled by the development of bundled biomarker tests and the integration of testing into fast-track clinical algorithms designed to improve morbidity and mortality statistics.
By End User, Which Setting Demands the Highest Throughput and Fastest Turnaround?
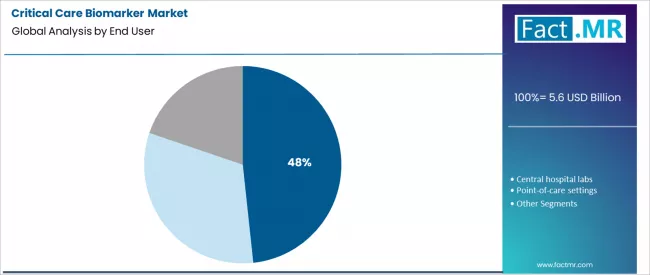
ICU laboratories hold a dominant 48.3% share. These specialized, on-site labs are engineered to support the intensive care unit’s need for rapid, around-the-clock test results. They balance the high-volume processing of central labs with the urgent timing requirements of point-of-care testing.
Proximity to clinicians facilitates immediate consultation on complex results and allows for batch testing of multiple parameters from a single sample, which is essential for managing multi-organ dysfunction in critically ill patients.
What Forces are Propelling and Constraining the Integration of Biomarkers in Critical Care?
Unequivocal clinical need for rapid, objective data to guide time-sensitive treatment decisions in complex patients principally drives expansion. The adoption of biomarker-driven clinical protocols for sepsis, cardiac care, and trauma improves standardized outcomes and reduces diagnostic ambiguity.
A significant restraint involves the high costs of novel biomarker panels and advanced testing platforms. Budget pressures in hospital systems can delay the adoption of newer, more informative tests, especially in resource-constrained settings, creating a disparity in care standards.
A major opportunity lies in developing multiplexed biomarker panels on integrated cartridge-based systems. These panels can deliver a comprehensive organ dysfunction profile from a single micro-sample, vastly improving diagnostic efficiency and providing a holistic view of patient status at admission.
The key trend is the seamless integration of continuous biomarker data with digital patient monitors and clinical decision support software. This creates a dynamic, data-rich patient portrait, moving from intermittent snapshots to a real-time streaming diagnostic environment that predicts clinical deterioration before overt symptoms appear.
Analysis of the Critical Care Biomarker Market by Key Countries
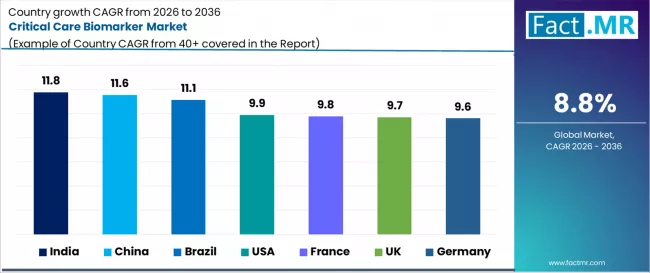
| Country | CAGR 2026 to 2036 |
|---|---|
| USA | 9.9% |
| Germany | 9.6% |
| China | 11.6% |
| India | 11.8% |
| Brazil | 11.1% |
| France | 9.8% |
| UK | 9.7% |
What underpins USA's adoption of Advanced Biomarker Panels in Emergency and ICU Settings?
A high-density network of advanced trauma centers, cardiac care units, and a strong culture of protocol-based medicine support a 9.9% CAGR. Reimbursement structures that recognize the value of rapid diagnostics for conditions like sepsis encourage adoption.
The presence of leading diagnostic corporations ensures early access to next-generation assays. Demand is particularly strong for multiplexed panels that streamline the workup of critically ill patients with unclear presentations.
How does Germany's Hub-and-Spoke Hospital System and Quality Focus Shape Demand?
Germany's 9.6% CAGR reflects its structured hospital system where complex cases centralize in university hospital ICUs equipped with sophisticated laboratory infrastructure. These hubs drive demand for comprehensive biomarker menus to support specialized intensive care.
A rigorous focus on quality management and adherence to evidence-based treatment guidelines necessitates reliable, high-performance testing. Collaborations between industry and clinical researchers are common for validating new biomarkers in specific German patient cohorts.
Which Factors Fuel China's High-Growth Trajectory in Hospital-Based Testing?
China's 11.6% CAGR is driven by massive investments in healthcare infrastructure, including the construction of new, large-scale tertiary hospitals with modern ICUs. National health policies emphasizing the improvement of critical care outcomes create top-down demand for advanced diagnostic tools.
The scale of the patient population allows for rapid clinical validation studies. Growth concentrates on high-volume cardiac and sepsis biomarkers that can be deployed across expanding hospital networks.
Why is India's Healthcare Expansion a Primary Catalyst for Biomarker Adoption?
India's leading 11.8% CAGR is propelled by the rapid growth of private hospital chains offering advanced critical care services. Increasing medical tourism and rising domestic expectations for quality care elevate the standard for diagnostic support in ICUs.
The demand driver stems from the need for cost-effective yet reliable testing solutions that can operate reliably in varied settings. This environment fosters partnerships for localized manufacturing and the development of tiered testing solutions.
How is Brazil's Focus on Public Health Emergencies and Hospital Care Influencing Uptake?
Brazil's 11.1% CAGR is linked to the modernization of public hospital ICUs and a high burden of infectious diseases that can lead to sepsis. National protocols for managing severe community-acquired pneumonia and sepsis create structured demand for relevant biomarkers like procalcitonin. The growth trajectory is supported by efforts to reduce mortality in public health emergencies, making rapid diagnostics a strategic priority for health authorities.
What Specific Needs define France's Advanced and Protocol-Driven Critical Care System?
France's 9.8% CAGR operates within a highly standardized and protocol-driven intensive care environment. National recommendations for the management of severe sepsis, ARDS, and cardiac arrest explicitly incorporate biomarker testing.
The network of regional university hospitals serves as early adopters, validating and integrating new tests into standardized care bundles before broader dissemination. This system ensures methodical and evidence-based adoption.
How does the UK's NHS Focus on Clinical Pathways and Efficiency Shape Biomarker Use?
The UK’s 9.7% CAGR is guided by the NHS's emphasis on clinical pathways and evidence-based cost-effectiveness. Adoption is strongest where biomarkers demonstrably improve patient flow, such as rapid rule-out protocols for myocardial infarction in emergency departments or guiding antibiotic stewardship in sepsis.
National Institute for Health and Care Excellence guidance plays a pivotal role in endorsing specific biomarker tests, after which implementation follows through commissioned pathways within NHS trusts.
Competitive Landscape of the Critical Care Biomarker Market
Large, integrated in-vitro diagnostics corporations that provide end-to-end systems dominate the competitive environment. Companies like Roche, Abbott, Siemens, and Beckman Coulter compete through comprehensive instrument platforms installed in central and ICU laboratories, coupled with proprietary biomarker assays.
Success hinges on menu expansion with high-medical-value tests, securing regulatory clearances for new indications, and demonstrating improved clinical outcomes and operational efficiencies to hospital administrators. Firms like bioMérieux compete strongly in the niche of sepsis and infectious disease biomarkers. The trend is toward providing not just assays, but integrated IT solutions that connect biomarker results directly to electronic health records and clinical alerts.
Key Players in the Critical Care Biomarker Market
- Abbott Laboratories
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers
- Beckman Coulter
- bioMérieux
- Others
Scope of Report
| Items | Metrics |
|---|---|
| Quantitative Units | USD Billion |
| Product | Cardiac Biomarkers, Sepsis Biomarkers, Coagulation Biomarkers, Renal Injury Biomarkers, Respiratory Biomarkers |
| Application | Early Diagnosis, Risk Stratification, Treatment Monitoring, Prognosis Assessment |
| End User | ICU Laboratories, Central Hospital Labs, Point-of-Care Settings |
| Key Countries | India, China, Brazil, USA, France, UK, Germany |
| Key Companies | Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers, Beckman Coulter, bioMérieux |
| Additional Analysis | Clinical utility analysis of biomarker-guided protocols versus standard care; total cost of care impact studies; analysis of pre-analytical variables in critical care sampling; review of emerging multi-omics biomarkers for sepsis subtyping; integration requirements for biomarker data in digital ICU platforms. |
Market by Segments
-
Product :
- Cardiac Biomarkers
- Sepsis Biomarkers
- Coagulation Biomarkers
- Renal Injury Biomarkers
- Respiratory Biomarkers
-
Application :
- Early Diagnosis
- Risk Stratification
- Treatment Monitoring
- Prognosis Assessment
-
End User :
- ICU Laboratories
- Central Hospital Labs
- Point-of-Care Settings
-
Region :
-
North America
- USA
- Canada
-
Latin America
- Brazil
- Mexico
- Argentina
- Rest of Latin America
-
Western Europe
- Germany
- France
- UK
- Italy
- Spain
- BENELUX
- Rest of Western Europe
-
Eastern Europe
- Poland
- Russia
- Czech Republic
- Rest of Eastern Europe
-
East Asia
- China
- Japan
- South Korea
- Rest of East Asia
-
South Asia & Pacific
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia & Pacific
-
Middle East & Africa
- GCC Countries
- South Africa
- Turkiye
- Rest of MEA
-
Bibliography
- International Sepsis Forum. (2025). Biomarkers in Sepsis: A Practical Guide for the Intensivist. Critical Care Medicine.
- Katz, J. N., & Shah, B. R. (2024). The Evolving Role of Biomarkers in Acute Cardiovascular Care. Journal of the American College of Cardiology.
- Pierrakos, C., & Vincent, J. L. (2023). Biomarkers of organ dysfunction in the critically ill. The Lancet Respiratory Medicine.
- Society of Critical Care Medicine. (2024). Guidelines for the Use of Biomarkers in Critical Illness.
- World Health Organization. (2024). Global report on sepsis: epidemiology, diagnosis and management.
- Zarbock, A., & Koyner, J. L. (2025). Renal Stress Biomarkers: From Discovery to Clinical Implementation in the ICU. Intensive Care Medicine.
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2021 to 2025 and Forecast, 2026 to 2036
- Historical Market Size Value (USD Million) Analysis, 2021 to 2025
- Current and Future Market Size Value (USD Million) Projections, 2026 to 2036
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2021 to 2025 and Forecast 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2026 to 2036
- Cardiac biomarkers
- Sepsis biomarkers
- Coagulation biomarkers
- Renal injury biomarkers
- Respiratory biomarkers
- Cardiac biomarkers
- Y to o to Y Growth Trend Analysis By Product, 2021 to 2025
- Absolute $ Opportunity Analysis By Product, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2026 to 2036
- Early diagnosis
- Risk stratification
- Treatment monitoring
- Prognosis assessment
- Early diagnosis
- Y to o to Y Growth Trend Analysis By Application, 2021 to 2025
- Absolute $ Opportunity Analysis By Application, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By End User
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End User, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End User, 2026 to 2036
- ICU laboratories
- Central hospital labs
- Point-of-care settings
- ICU laboratories
- Y to o to Y Growth Trend Analysis By End User, 2021 to 2025
- Absolute $ Opportunity Analysis By End User, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2021 to 2025
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2026 to 2036
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- USA
- Canada
- Mexico
- By Product
- By Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- By End User
- Key Takeaways
- Latin America Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Product
- By Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- By End User
- Key Takeaways
- Western Europe Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Product
- By Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- By End User
- Key Takeaways
- Eastern Europe Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Product
- By Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- By End User
- Key Takeaways
- East Asia Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- China
- Japan
- South Korea
- By Product
- By Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- By End User
- Key Takeaways
- South Asia and Pacific Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Product
- By Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- By End User
- Key Takeaways
- Middle East & Africa Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Product
- By Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- By End User
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Canada
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Mexico
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Brazil
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Chile
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Germany
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- UK
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Italy
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Spain
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- France
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- India
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- China
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Japan
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- South Korea
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Russia
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Poland
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Hungary
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- South Africa
- Pricing Analysis
- Market Share Analysis, 2025
- By Product
- By Application
- By End User
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product
- By Application
- By End User
- Competition Analysis
- Competition Deep Dive
- Abbott Laboratories
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers
- Beckman Coulter
- bioMérieux
- Others
- Abbott Laboratories
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2021 to 2036
- Table 2: Global Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 3: Global Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 4: Global Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 5: North America Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 6: North America Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 7: North America Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 8: North America Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 10: Latin America Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 11: Latin America Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 12: Latin America Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 14: Western Europe Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 15: Western Europe Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 16: Western Europe Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 20: Eastern Europe Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 22: East Asia Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 23: East Asia Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 24: East Asia Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Product, 2021 to 2036
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by End User, 2021 to 2036
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2021 to 2036
- Figure 3: Global Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 4: Global Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 5: Global Market Attractiveness Analysis by Product
- Figure 6: Global Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 7: Global Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 8: Global Market Attractiveness Analysis by Application
- Figure 9: Global Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 10: Global Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 11: Global Market Attractiveness Analysis by End User
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2026 and 2036
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2026 to 2036
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 23: North America Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 24: North America Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 25: North America Market Attractiveness Analysis by Product
- Figure 26: North America Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 27: North America Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 28: North America Market Attractiveness Analysis by Application
- Figure 29: North America Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 30: North America Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 31: North America Market Attractiveness Analysis by End User
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 33: Latin America Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 35: Latin America Market Attractiveness Analysis by Product
- Figure 36: Latin America Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 38: Latin America Market Attractiveness Analysis by Application
- Figure 39: Latin America Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 40: Latin America Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 41: Latin America Market Attractiveness Analysis by End User
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 43: Western Europe Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 45: Western Europe Market Attractiveness Analysis by Product
- Figure 46: Western Europe Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 48: Western Europe Market Attractiveness Analysis by Application
- Figure 49: Western Europe Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 51: Western Europe Market Attractiveness Analysis by End User
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 55: Eastern Europe Market Attractiveness Analysis by Product
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 58: Eastern Europe Market Attractiveness Analysis by Application
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 61: Eastern Europe Market Attractiveness Analysis by End User
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 63: East Asia Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 65: East Asia Market Attractiveness Analysis by Product
- Figure 66: East Asia Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 68: East Asia Market Attractiveness Analysis by Application
- Figure 69: East Asia Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 70: East Asia Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 71: East Asia Market Attractiveness Analysis by End User
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Product
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by End User
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Product, 2026 and 2036
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Product, 2026 to 2036
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Product
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 91: Middle East & Africa Market Attractiveness Analysis by End User
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the critical care biomarker market in 2026?
The global critical care biomarker market is estimated to be valued at USD 5.6 billion in 2026.
What will be the size of critical care biomarker market in 2036?
The market size for the critical care biomarker market is projected to reach USD 13.0 billion by 2036.
How much will be the critical care biomarker market growth between 2026 and 2036?
The critical care biomarker market is expected to grow at a 8.8% CAGR between 2026 and 2036.
What are the key product types in the critical care biomarker market?
The key product types in critical care biomarker market are cardiac biomarkers, sepsis biomarkers, coagulation biomarkers, renal injury biomarkers and respiratory biomarkers.
Which application segment to contribute significant share in the critical care biomarker market in 2026?
In terms of application, early diagnosis segment to command 37.8% share in the critical care biomarker market in 2026.