Shingles Vaccine Market
Shingles Vaccine Market Size and Share Forecast Outlook 2025 to 2035
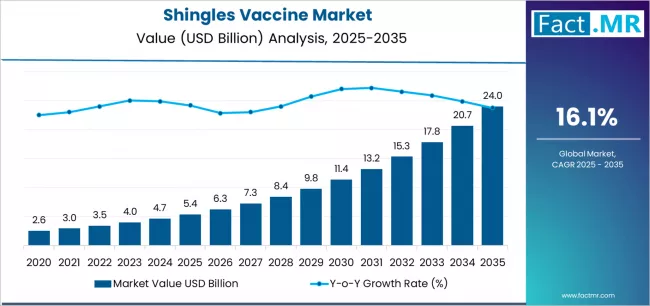
Shingles vaccine market is projected to grow from USD 5.4 billion in 2025 to USD 24.0 billion by 2035, at a CAGR of 16.1%. Shingrix will dominate with a 94.1% market share, while private healthcare settings will lead the end use segment with a 68.3% share.
Shingles Vaccine Market Forecast and Outlook 2025 to 2035
The global shingles vaccine market is set to grow from USD 5.4 billion in 2025 to USD 24.0 billion by 2035, adding USD 18.6 billion in new revenue and advancing at a CAGR of 16.1%. Growth is driven by escalating demand for herpes zoster prevention validation, expanding immunization program infrastructure across regulated markets, and accelerating elderly care requirements among healthcare providers and public health organizations seeking effective disease prevention solutions.
Shingles vaccine is increasingly recognized as essential preventive tools for geriatric care practitioners, offering superior immunogenicity capabilities, long-term protection assurance, and comprehensive efficacy characteristics compared to traditional live attenuated vaccine approaches.
Quick Stats for Shingles Vaccine Market
- Shingles Vaccine Market Value (2025): USD 5.4 billion
- Shingles Vaccine Market Forecast Value (2035): USD 24.0 billion
- Shingles Vaccine Market Forecast CAGR: 16.1%
- Leading Product in Shingles Vaccine Market: Shingrix (94.1%)
- Key Growth Regions in Shingles Vaccine Market: North America, Europe, and Asia Pacific
- Top Players in Shingles Vaccine Market: GlaxoSmithKline (GSK), Pfizer, Merck & Co., CanSinoBIO, Vaccitech
Shingrix vaccines command the largest share in the market, favored in private healthcare and public immunization environments for their established recombinant properties, providing superior protection mechanisms, enhanced immune response capabilities, and regulatory acceptance across diverse patient vaccination applications and demographic populations.
Private healthcare settings remain fundamental in immunization delivery protocols where routine preventive care and adult vaccination programs match patient requirements and disease prevention confidence standards. Government healthcare settings are advancing among end-use categories as specialized public health facility networks expand and immunization campaign infrastructure increases accessibility in community-convenient locations with structured prevention programs.
Geographic concentration demonstrates dynamic growth patterns with aging population centers leading expansion, supported by rising elderly demographic capacity, herpes zoster awareness expansion among healthcare populations, and vaccination program establishment in healthcare centers.
Developed markets demonstrate robust development through established adult immunization quality ecosystems, regulatory framework maturity for recombinant vaccines, and standardized acceptance of preventive vaccination procedures. Competitive advantage is consolidating around efficacy profiles, duration of protection documentation, immunogenicity optimization, and integrated prevention portfolios rather than standalone vaccine formulations alone.
The first half of the decade will witness the market climbing from USD 5.4 billion to approximately USD 11.3 billion, adding USD 5.9 billion in value, which constitutes 32% of the total forecast growth period. This phase will be characterized by the continued dominance of Shingrix methodologies in private healthcare settings, combined with accelerating adoption of recombinant vaccine technologies in government immunization programs where superior efficacy validation and extended protection duration create favorable public health outcomes.
The latter half will witness sustained expansion from USD 11.3 billion to USD 24.0 billion, representing an addition of USD 12.7 billion or 68% of the decade's growth, defined by broadening acceptance of adult immunization protocols and integration of comprehensive vaccination platforms across mainstream healthcare and public health facilities.
Where revenue comes from - Now Vs Next (industry-level view)
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | Shingrix | 94.1% | Product dominance |
| Private Healthcare Settings | 68.3% | Primary delivery | |
| Recombinant Vaccine | 90-95% | Technology preference | |
| Adult Immunization | 85-90% | Target population | |
| Government Settings | 31.7% | Public programs | |
| Future (3-5 yrs) | Government Program Expansion | 38-44% | Coverage increase |
| Emerging Market Penetration | 32-38% | Geographic growth | |
| Combination Vaccines | 22-28% | Multi-disease prevention | |
| Digital Reminder Systems | 26-32% | Compliance enhancement | |
| Pharmacy-Based Vaccination | 35-41% | Access expansion | |
| Employer Wellness Programs | 28-34% | Corporate integration | |
| Next-Generation Formulations | 18-24% | Innovation pipeline |
Shingles Vaccine Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 5.4 billion |
| Market Forecast (2035) ↑ | USD 24.0 billion |
| Growth Rate ★ | 16.1% CAGR |
| Leading Product → | Shingrix |
| Primary End Use → | Private Healthcare Settings |
The market demonstrates exceptional fundamentals with Shingrix capturing a commanding 94.1% share through superior efficacy characteristis, established recombinant vaccine advantages, and proven long-term protection profiles across private and government healthcare applications. Private Healthcare Settings drive primary delivery demand at 68.3% share, supported by established adult immunization infrastructure and preventive care requirements that maintain patient compliance across diverse healthcare segments.
Geographic concentration remains anchored in developed markets with emerging growth leadership through aging population expansion and vaccination awareness infrastructure development, while healthcare systems show accelerated adoption rates driven by disease burden demographics and public health policy procedure preferences.
Imperatives for Stakeholders in Shingles Vaccine Market
Design for efficacy and accessibility, not just disease prevention
- Offer complete immunization solutions: high-efficacy vaccines + cold chain management support + patient education materials + healthcare provider training systems + coverage advocacy platforms.
- Preconfigured vaccination packages: pharmacy administration programs, workplace wellness configurations, senior care facility protocols, and combination immunization schedules for diverse delivery requirements.
Public health readiness for coverage expansion
- Comprehensive efficacy documentation, safety monitoring systems, and program infrastructure (vaccine registry integration, adverse event tracking, population coverage measurement protocols).
Affordability-by-design approach
- Cost-optimized pricing portfolios, flexible reimbursement models, government partnership programs, and transparent health economic value documentation.
Healthcare provider training-focused market penetration
- Established administration technique workshops + comprehensive certification programs (storage requirements, patient counseling, adverse event management); direct provider engagement for relationship development and vaccination confidence building.
Segmental Analysis
The market segments by product into Shingrix, Zostavax, and SKYZoster, representing the evolution from live attenuated formulations toward sophisticated recombinant vaccine technologies with enhanced immunogenicity capabilities, comprehensive efficacy validation, and advanced safety characteristics. The type segmentation divides the market into recombinant vaccine and live attenuated vaccine, reflecting distinct immunization objectives for superior protection and extended duration versus traditional vaccination implementation and established product validation.
The end-use segmentation shows private healthcare settings' commanding 68.3% position, followed by government healthcare settings at 31.7%, demonstrating varied delivery infrastructure levels and immunization program concentration patterns. The segmentation structure reveals shingles vaccine evolution from basic live virus attenuation toward comprehensive recombinant immunization platforms with enhanced efficacy characteristics and multi-dimensional protection duration capabilities, while product diversity spans from adjuvanted recombinant vaccines to traditional live formulations requiring specialized cold chain management.
What Makes Shingrix Command the Largest Share in the Shingles Vaccine Market?

Shingrix commands the leading position in the shingles vaccine market with a 94.1% market share through superior efficacy characteristics, including established clinical trial documentation, extensive real-world effectiveness evidence, and validated protection pathways that enable healthcare providers to achieve predictable disease prevention outcomes across varied elderly patient categories and diverse risk demographics.
The segment benefits from exceptional efficacy advantages through adjuvant system optimization, over 90% protection rates without age-related decline concerns, and proven long-term immunity documentation without requiring booster dose procedures for extended periods. Advanced recombinant technology enables non-live vaccine optimization, immunocompromised patient suitability variation, and two-dose schedule implementation, where protection precision and safety profile excellence represent critical immunization requirements.
The adjuvanted recombinant formulation holds commanding share within the product segment, appealing to healthcare providers seeking superior protection capabilities for high-risk elderly populations. Shingrix products differentiate through proven clinical efficacy profiles, regulatory authority recommendations, and integration with established adult immunization guidelines that enhance vaccination confidence while maintaining optimal prevention outcomes for diverse herpes zoster risk management applications.
Key market characteristics:
- Advanced efficacy properties with over 90% protection rates and sustained immunity for extended duration applications
- Superior safety profiles, enabling immunocompromised patient vaccination and broad population suitability for comprehensive coverage
- Comprehensive regulatory endorsement, including preferential recommendations and guideline inclusion for global immunization programs
Why do Private Healthcare Settings Experience Greater Preference for Acquiring Shingles Vaccines?

Private healthcare settings demonstrate end-use leadership in the shingles vaccine market with a 68.3% share due to widespread adult immunization requirements and established focus on preventive care services, patient-initiated vaccination, and reimbursement-supported programs that maximize disease prevention while maintaining appropriate revenue generation characteristics.
Healthcare providers and patients prioritize private settings for convenient access scheduling, comprehensive patient counseling services, and integration with established primary care workflows that enables coordinated preventive experiences across multiple adult vaccination categories. The segment benefits from substantial insurance coverage availability and patient awareness campaigns that emphasize private practice-based vaccine delivery for routine prevention applications across affluent demographic populations.
Pharmacy-based vaccination programs capture significant share within the private setting segment, demonstrating healthcare system preference for accessible delivery formats. Aging population expansion incorporates shingles vaccination as essential preventive components for wellness programs, while pharmacy vaccination authority increases direct patient access with convenience positioning credentials for comprehensive immunization coverage outcomes.
What are the Drivers, Restraints, and Key Trends of the Shingles Vaccine Market?
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Rising aging population & herpes zoster disease burden (demographic shift, post-herpetic neuralgia cases) | ★★★★★ | Population aging requirements enable shingles vaccine adoption for disease prevention; increasing elderly demographics drive vaccination consumption across global markets and diverse healthcare segments. |
| Driver | Growth in adult immunization programs and preventive care emphasis (healthcare policy, awareness campaigns) | ★★★★★ | Drives demand for effective prevention technologies and evidence-based immunization systems; manufacturers providing superior efficacy outcomes gain competitive advantage in guideline-recommended vaccine segments. |
| Driver | Clinical evidence validation and real-world effectiveness documentation (efficacy studies, protection duration data) | ★★★★☆ | Healthcare providers demand scientifically-validated vaccines and proven protection systems; clinical evidence visibility expanding addressable segments beyond traditional immunization demographics and public health clientele. |
| Restraint | High vaccine costs & reimbursement limitations (pricing barriers, coverage gaps) | ★★★★☆ | Cost-conscious healthcare systems face budget limitations and access constraints, restricting vaccination adoption and affecting market penetration in resource-limited countries and uninsured population segments. |
| Restraint | Cold chain requirements & distribution complexity (storage conditions, logistics challenges) | ★★★☆☆ | Healthcare facilities face infrastructure concerns and handling limitations; increases implementation barriers and affects adoption penetration in rural healthcare settings and developing market vaccination programs. |
| Trend | Government immunization program expansion & coverage mandates (public funding, universal vaccination) | ★★★★★ | Growing public health recognition for systematic prevention approaches and population health improvement beyond individual vaccination decisions; government program integration becomes core market expansion strategy for sustainable growth positioning. |
| Trend | Combination vaccine development & multi-disease prevention (pneumococcal combinations, influenza integration) | ★★★★☆ | Adult immunization evolving beyond single-disease focus toward comprehensive vaccination protocols; combination positioning drives enhanced patient compliance and healthcare system efficiency in sophisticated prevention programs. |
Analysis of the Shingles Vaccine Market by Key Country
The shingles vaccine market demonstrates steady expansion across advanced healthcare systems where demographic aging, preventive medicine uptake, and structured adult immunization pathways influence adoption trajectories. Regional performance reflects the interaction of reimbursement design, population health priorities, and vaccination infrastructure maturity.
Strong performers include the USA, Japan, and Germany due to established immunization frameworks and elderly care programs. Developed markets such as the UK, France, and Canada benefit from systematic primary care structures and public health program consistency. Australia shows accelerated growth supported by national immunisation schedule integration and improved coverage monitoring systems.
A broader regional synthesis indicates that North American and European markets maintain strong adoption trends due to systematic reimbursement design and preventive care investment. Asia Pacific demonstrates measured yet steady growth influenced by aging demographics and expanding program rationalization. Emerging markets show long-term opportunity in awareness-building, public health alignment, and infrastructure development.

| Region/Country | CAGR (2025 to 2035) | Growth Strategy | How to win | What to watch out |
|---|---|---|---|---|
| USA | 15.4% | Medicare integration | Provide comprehensive coverage support | Pricing pressures; policy changes |
| Japan | 14.8% | Elderly care focus | Lead with efficacy positioning | Reimbursement challenges; distribution |
| Germany | 15.1% | Public health programs | Offer systematic delivery solutions | Cost constraints; regulatory complexity |
| UK | 15.6% | NHS inclusion | Deliver value-based programs | Budget limitations; coverage decisions |
| France | 16.3% | Prevention emphasis | Maintain clinical evidence focus | Reimbursement delays; market access |
| Canada | 16.7% | Provincial programs | Push provincial formulary inclusion | Regional variations; pricing negotiations |
| Australia | 17.4% | Immunization schedule | Support national program integration | Access barriers; remote area challenges |
How does the USA Maintain Its Leadership Position in the Shingles Vaccine Market?
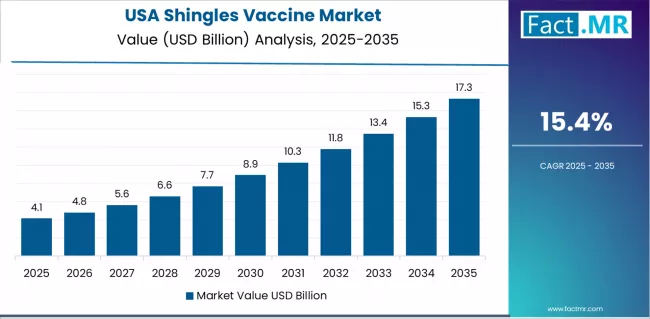
The USA maintains market leadership with an estimated 15.4 % CAGR from 2025 to 2035, supported by comprehensive Medicare Part D coverage structures and widespread adult immunization capacity in primary care practices and retail pharmacies. The market benefits from strong insurance penetration, rising elderly population cohorts, and consistent adoption patterns across both metropolitan and community healthcare systems. Preventive care culture and public health communication reinforce vaccine uptake, particularly among baby boomer segments that engage frequently with primary care and aging care services.
American healthcare providers rely on stable supplier relationships and reimbursement frameworks that support predictable administration volumes. Pharmacy-based vaccination programs and integrated primary care pathways create operational consistency and optimize patient throughput. Continued adoption reflects the alignment of clinical evidence, Medicare billing efficiency, and growing emphasis on real-world protection outcomes in older adults.
Why does Japan Emerge as a Key Aging-Society Driver in the Market?

Japan registers an estimated 14.8 % CAGR through 2035, reflecting a healthcare system shaped by super-aging demographics and a strong preventive medicine culture. Public health centers and large hospital networks in Tokyo, Osaka, and Nagoya increasingly incorporate shingles vaccination into routine elderly care pathways. Growing awareness of herpes zoster burden encourages adoption, particularly in settings focused on geriatric quality of life and community-based disease prevention.
The Japanese market favors platforms with comprehensive safety documentation, validated clinical performance, and long-term effectiveness monitoring. Healthcare system maturity supports the integration of evidence-based immunization methodologies across elderly care environments. Adoption patterns mirror the country’s broader prevention ethos, where structured protocols and robust provider training influence consistent vaccination decisions.
How does Germany Strengthen Its Market Position through Public Health Integration?
Germany demonstrates stable expansion with a projected 15.1 % CAGR from 2025 to 2035, supported by systematic immunization practices and advanced public health governance. The market benefits from established primary care networks, specialized vaccination centers, and rigorous regulatory evaluation. Shingles vaccines align well with Germany’s evidence-driven immunization culture where benefit risk assessments and long-term protection data shape adoption.
Healthcare providers prioritize products with extensive clinical documentation, including immunogenicity data, safety monitoring, and health economic evaluation. Statutory health insurance coverage supports structured adult immunization activities and encourages adherence to national immunization advisory committee recommendations. Aging demographics further strengthen the demand environment and sustain vaccination program continuity.
What explains the UK’s Steady NHS-Driven Growth Curve?
The UK records an estimated 15.6 % CAGR between 2025 and 2035, shaped by NHS program structures and JCVI recommendation frameworks that guide shingles vaccine adoption. The market benefits from efficient primary care integration, established vaccination delivery networks, and community-level program execution. Cost-effectiveness analysis and systematic evaluation influence coverage decisions, which contributes to stable yet controlled expansion.
UK healthcare providers emphasize products supported by comprehensive health economic analysis, including QALY assessment and population benefit documentation. The country’s organized primary care ecosystem ensures consistent access and supports uniform implementation across regions. Growing elderly population groups and established public health culture continue to reinforce demand within the national vaccination framework.
Why does France Demonstrate a Prevention-Focused Growth Pattern?
France achieves an estimated 16.3 % CAGR through 2035, supported by a healthcare system that places strong emphasis on preventive care and structured immunization protocols. General practitioners and specialized vaccination clinics maintain steady uptake, guided by universal healthcare coverage and comprehensive elderly care programs. Shingles vaccines integrate effectively with France’s national vaccination calendar and safety-focused supervision systems.
Providers prioritize evidence-based products that demonstrate clear clinical benefit, validated effectiveness, and strong pharmacovigilance documentation. Post-marketing surveillance and detailed benefit risk assessments influence adoption decisions. Established aging demographics and long-standing prevention culture support ongoing market expansion and alignment with national immunization recommendations.
How does Canada Benefit from Provincial Immunization Pathways?
Canada records one of the stronger growth profiles with an estimated 16.7 % CAGR between 2025 and 2035, supported by provincial immunization programs that shape funding and implementation patterns. The market benefits from harmonized NACI recommendations and progressive provincial formulary inclusion that improves access to shingles vaccines in elderly populations.
Healthcare providers emphasize products that align with NACI guidance and offer provincial program compatibility, including cost-effectiveness justification and implementation support. Provincial diversity contributes to variations in adoption timing, yet national preventive care culture and systematic adult immunization pathways support consistent long-term expansion.
Why does Australia Exhibit the Fastest Growth among Developed Markets?
Australia demonstrates the fastest expansion with a projected 17.4 % CAGR from 2025 to 2035, driven by strong National Immunisation Program alignment and robust preventive health infrastructure. General practice networks and community pharmacy services maintain consistent immunization delivery, supported by national registry integration that enhances coverage tracking and safety monitoring.
Australian providers prioritize ATAGI-recommended products with reliable registry compatibility and validated safety documentation. Federal funding structures encourage adoption across diverse population groups, particularly in aging communities. The country’s universal healthcare system and rising emphasis on preventive care strengthen shingles vaccine utilization and create a favorable long-term demand environment.
Competitive Landscape of the Shingles Vaccine Market

The shingles vaccine market exhibits a highly consolidated competitive structure with approximately 5-10 active players operating across global pharmaceutical networks and regional immunization distribution portfolios. GlaxoSmithKline plc. (GSK) maintains overwhelming market leadership at a 65.0% share, reflecting dominant product portfolio positioning with Shingrix's superior efficacy profile and sophisticated global commercialization strategies.
This competitive landscape demonstrates the maturation of recombinant vaccine technology, where established players leverage extensive clinical trial advantages, comprehensive regulatory approval documentation, and healthcare provider relationship programs to maintain dominant positions, while emerging vaccine developers and regional manufacturers create limited niche opportunities through alternative formulation approaches and geographic market strategies.
Market leadership is maintained through several critical competitive advantages extending beyond manufacturing capabilities and product portfolios. Global distribution networks enable leading players to navigate complex cold chain requirements and access varied healthcare segments including private practices, retail pharmacies, and government immunization programs.
Clinical evidence infrastructure and healthcare provider education program availability represent crucial differentiators in vaccine categories, where decades of immunology expertise, manufacturing scale advantages, and real-world effectiveness frameworks create prescribing preference among evidence-focused healthcare providers.
Production efficiency in adjuvanted vaccine manufacturing facilities, supply chain cold storage management, and regulatory compliance systems separate major pharmaceutical companies from smaller competitors, while comprehensive post-marketing surveillance documentation addressing safety monitoring studies, effectiveness evaluations, and pharmacovigilance data strengthen market position and regulatory authority confidence.
The market demonstrates limited differentiation opportunities given Shingrix's overwhelming efficacy advantages, where legacy live attenuated vaccine methodologies face obsolescence from innovation-focused leaders offering superior protection. However, emerging opportunities exist in combination vaccine categories and next-generation formulations.
Premium positioning through superior clinical outcomes and comprehensive real-world evidence capabilities command dominant market share through proven prevention effectiveness and healthcare system value integration. Specialized product portfolios addressing specific patient populations with targeted efficacy data create positioning opportunities beyond general elderly vaccination competition.
Integrated disease prevention offerings emphasizing complementary adult immunization compatibility, unified healthcare provider support, and cross-indication prevention programs generate healthcare system loyalty and portfolio preferences beyond transactional vaccine purchases.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Global pharmaceutical corporations | Comprehensive vaccine portfolios; global distribution; clinical evidence | Brand recognition; regulatory expertise; provider relationships; manufacturing scale | Pricing flexibility; emerging markets; alternative technologies; access programs |
| Specialized vaccine companies | Innovation expertise; vaccine technology; immunology optimization | Product differentiation; clinical sophistication; formulation innovation; scientific credibility | Distribution infrastructure; market access; healthcare system relationships; global reach |
| Regional manufacturers | Local production; market understanding; regional distribution; pricing flexibility | Affordability positioning; local relationships; regulatory familiarity; cultural alignment | Clinical validation; international recognition; manufacturing scale; advanced technology |
| Government agencies | Program implementation; funding decisions; policy frameworks; coverage mandates | Population reach; systematic delivery; public health mandate; equity focus | Innovation speed; flexibility; individual customization; premium positioning |
| Healthcare systems | Provider networks; patient access; vaccination delivery; quality monitoring | Implementation capability; patient relationships; systematic delivery; outcome tracking | Product development; pricing power; market expansion; innovation leadership |
Key Players in the Shingles Vaccine Market
- GlaxoSmithKline plc. (GSK)
- Pfizer Inc.
- Merck & Co. Inc.
- CanSinoBIO (CanSino Biologics Inc.)
- Vaccitech plc.
- GC Biopharma Corp.
- GeneOne Life Science Inc.
- SK bioscience Co. Ltd.
- AIM Vaccine Co. Ltd.
- Dynavax Technologies Corporation
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 5.4 billion |
| Product | Shingrix, Zostavax, SKYZoster |
| Type | Recombinant Vaccine, Live Attenuated Vaccine |
| End Use | Private Healthcare Settings, Government Healthcare Settings |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | USA, Japan, Germany, UK, France, Canada, Australia, and 15+ additional countries |
| Key Companies Profiled | GlaxoSmithKline (GSK), Pfizer, Merck & Co., CanSinoBIO, Vaccitech, GC Biopharma Corp., Geneone Life Science |
| Additional Attributes | Dollar sales by product and end-use categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with established pharmaceutical corporations and specialized vaccine companies, healthcare provider preferences for Shingrix formulations and private healthcare settings, integration with adult immunization programs and pharmacy vaccination services, innovations in recombinant vaccine technologies and adjuvant platforms, and development of sophisticated disease prevention systems with enhanced efficacy profiles and comprehensive real-world effectiveness documentation frameworks. |
Shingles Vaccine Market by Segments
-
Product :
- Shingrix
- Zostavax
- SKYZoster
-
Type :
- Recombinant Vaccine
- Live Attenuated Vaccine
-
End Use :
- Private Healthcare Settings
- Government Healthcare Settings
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- Japan
- China
- India
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2025 to 2035
- Shingrix
- Zostavax
- SKYZoster
- Y to o to Y Growth Trend Analysis By Product, 2020 to 2024
- Absolute $ Opportunity Analysis By Product, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Private Healthcare Settings
- Government Healthcare Settings
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Product
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product
- By End Use
- Competition Analysis
- Competition Deep Dive
- GlaxoSmithKline plc. (GSK)
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Pfizer Inc.
- Merck & Co. Inc.
- CanSinoBIO (CanSino Biologics Inc.)
- Vaccitech plc.
- GC Biopharma Corp.
- GeneOne Life Science Inc.
- SK bioscience Co. Ltd.
- AIM Vaccine Co. Ltd.
- Dynavax Technologies Corporation
- GlaxoSmithKline plc. (GSK)
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Product
- Figure 6: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by End Use
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 22: North America Market Attractiveness Analysis by Product
- Figure 23: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by End Use
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 29: Latin America Market Attractiveness Analysis by Product
- Figure 30: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 32: Latin America Market Attractiveness Analysis by End Use
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 36: Western Europe Market Attractiveness Analysis by Product
- Figure 37: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 39: Western Europe Market Attractiveness Analysis by End Use
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Product
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 50: East Asia Market Attractiveness Analysis by Product
- Figure 51: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 53: East Asia Market Attractiveness Analysis by End Use
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Product
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Product
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the shingles vaccine market in 2025?
The global shingles vaccine market is estimated to be valued at USD 5.4 billion in 2025.
What will be the size of shingles vaccine market in 2035?
The market size for the shingles vaccine market is projected to reach USD 24.0 billion by 2035.
How much will be the shingles vaccine market growth between 2025 and 2035?
The shingles vaccine market is expected to grow at a 16.1% CAGR between 2025 and 2035.
What are the key product types in the shingles vaccine market?
The key product types in shingles vaccine market are shingrix, zostavax and skyzoster.
Which end use segment to contribute significant share in the shingles vaccine market in 2025?
In terms of end use, private healthcare settings segment to command 68.3% share in the shingles vaccine market in 2025.