Spinal Implants and Devices Market
Spinal Implants and Devices Market Size and Share Forecast Outlook 2025 to 2035
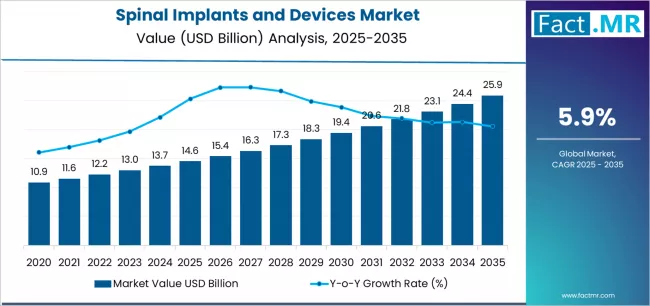
Spinal implants and devices market is projected to grow from USD 14.6 billion in 2025 to USD 25.9 billion by 2035, at a CAGR of 5.9%. Spinal Fusion Devices will dominate with a 58.2% market share, while spinal fusion and fixation technologies will lead the technology segment with a 67.0% share.
Spinal Implants and Devices Market Forecast and Outlook 2025 to 2035
The global spinal implants and devices market is projected to reach USD 25.88 billion by 2035, recording an absolute increase of USD 11.33 billion over the forecast period. The market is valued at USD 14.55 billion in 2025 and is set to rise at a CAGR of 5.9% during the assessment period.
The market is expected to grow by approximately 1.8 times during the same period, supported by increasing prevalence of degenerative disc diseases and spinal disorders among aging populations worldwide, driving demand for advanced surgical solutions and increasing investments in minimally invasive technologies with enhanced patient outcomes across spinal fusion procedures and motion preservation applications globally.
Quick Stats for Spinal Implants and Devices Market
- Spinal Implants and Devices Market Value (2025): USD 14.55 billion
- Spinal Implants and Devices Market Forecast Value (2035): USD 25.88 billion
- Spinal Implants and Devices Market Forecast CAGR: 5.9%
- Leading Product Type in Spinal Implants and Devices Market: Spinal Fusion Devices (58.23%)
- Key Growth Regions in Spinal Implants and Devices Market: Asia Pacific, North America, and Europe
- Top Players in Spinal Implants and Devices Market: Medtronic, Johnson & Johnson, VB Spine, LLC, NuVasive, Zimmer Biomet, Globus Medical, Inc., Alphatec Spine, Inc, Orthofix Holdings, Inc, RTI Surgical Holdings, Ulrich GmbH & Co. KG
Orthopedic surgeons and healthcare facilities face mounting pressure to improve surgical precision and reduce recovery times while addressing complex spinal pathologies and patient quality of life requirements, with modern spinal implant technologies providing documented clinical benefits including improved fusion rates, reduced operative trauma, and faster post-surgical recovery compared to conventional open surgical approaches alone.
Rising adoption of minimally invasive techniques and expanding robotic-assisted spine surgery platforms enabling enhanced surgical accuracy create substantial opportunities for device manufacturers and healthcare providers. However, high device costs and reimbursement challenges may pose obstacles to widespread advanced technology adoption across emerging healthcare markets.
The spinal fusion devices segment dominates market activity, driven by extensive clinical validation supporting degenerative spine disease treatment and traumatic spine injury management across diverse patient populations worldwide. Spine surgeons increasingly recognize the established efficacy of fusion systems, with typical product offerings providing reliable spinal stabilization and arthrodesis achievement at competitive pricing through established orthopedic distribution networks.
The spinal biologics segment demonstrates robust growth potential, supported by rising demand for bone graft substitutes and evidence-based bone healing enhancement integrating biological augmentation in modern fusion approaches. Spinal fusion and fixation technologies emerge as the dominant technical approach, reflecting established surgical protocols and comprehensive instrumentation systems in spine surgery practice. Open surgery represents the leading surgical approach, driven by complex pathology management and surgeon familiarity enabling comprehensive spinal reconstruction.
Regional dynamics show North America maintaining market leadership, supported by high spine surgery volumes and established medical device innovation infrastructure across orthopedic specialty centers. Asia Pacific demonstrates the fastest growth trajectory driven by expanding healthcare infrastructure and increasing surgical procedure adoption accepting advanced spinal treatment options, while Europe emphasizes clinical evidence standards and regulatory compliance for medical device quality assurance.
India leads country-level growth through expanding hospital infrastructure and awareness programs increasing surgeries, followed by China supported by aging population demographics and CFDA approvals for new devices. The competitive landscape features moderate concentration with Medtronic plc maintaining market leadership position at a 15.0% share, while specialized players including Johnson & Johnson, NuVasive, Zimmer Biomet, and Globus Medical compete through differentiated implant technologies and comprehensive surgical technique training programs across diverse spinal applications.
Spinal Implants and Devices Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the spinal implants and devices market is projected to expand from USD 14.55 billion to USD 18.37 billion, resulting in a value increase of USD 3.82 billion, which represents 33.7% of the total forecast growth for the period. This phase of development will be shaped by rising demand for minimally invasive surgical solutions addressing patient preference for reduced recovery times, product innovation in navigation-guided systems with robotic assistance capabilities and three-dimensional printed patient-specific implants, as well as expanding integration with digital surgical planning platforms and intraoperative imaging technologies. Companies are establishing competitive positions through investment in biomaterial research, clinical evidence generation programs, and strategic market expansion across ambulatory surgery centers, specialty spine hospitals, and surgeon training initiatives.
From 2029 to 2035, the market is forecast to grow from USD 18.37 billion to USD 25.88 billion, adding another USD 7.51 billion, which constitutes 66.3% of the overall expansion. This period is expected to be characterized by the expansion of specialized product applications, including motion preservation devices for younger active patients and biologics-enhanced fusion systems tailored for specific spinal pathologies, strategic collaborations between device manufacturers and healthcare systems, and an enhanced focus on value-based care models and clinical outcome tracking initiatives. The growing emphasis on outpatient spine surgery protocols and rising surgeon preference for modular implant systems addressing multiple surgical approaches will drive demand for comprehensive spinal solution portfolios across diverse patient demographics and pathology complexity levels.
Spinal Implants and Devices Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 14.55 billion |
| Market Forecast Value (2035) | USD 25.88 billion |
| Forecast CAGR (2025-2035) | 5.9% |
Why is the Spinal Implants and Devices Market Growing?
The spinal implants and devices market grows by enabling spine surgeons and healthcare facilities to deliver effective surgical treatment for degenerative spine conditions and spinal trauma while addressing patient expectations for improved outcomes and faster recovery without exclusive reliance on conservative management approaches.
Spine surgeons aim to achieve solid fusion rates and restore spinal stability while managing surgical complications, patient comorbidities, and cost containment pressures, with modern spinal implant systems typically providing biomechanically optimized designs enabling immediate post-operative stability, osteoconductive surfaces promoting bone integration, and modular configurations accommodating diverse anatomical variations compared to historical fixation methods alone, making advanced spinal implants essential for contemporary spine surgery practice.
The orthopedic industry's need for clinically proven devices supporting complex spinal reconstruction and fusion procedures creates demand for specialized implant solutions that can provide reliable fixation, enhance biological healing, and support minimally invasive surgical techniques without compromising structural integrity or long-term performance.
Clinical evidence validation and surgeon training supporting implant efficacy drive adoption in hospital operating rooms, ambulatory surgery centers, and specialty spine institutes, where device performance has direct impact on patient outcomes and surgical success rates. The increasing prevalence of degenerative disc disease affecting approximately 40% of adults over age 40 and traumatic spine injuries requiring surgical intervention creates expanding patient populations seeking surgical solutions.
Rising patient awareness about surgical options and healthcare system emphasis on value-based care enable informed treatment decisions and adherence to evidence-based surgical protocols. However, device cost pressures and insurance coverage limitations may limit adoption rates and optimal implementation across healthcare systems serving diverse patient populations with varying financial resources and insurance coverage levels.
Segmental Analysis
The market is segmented by product, technology, surgery type, procedure type, and region. By product, the market is divided into spinal fusion devices (thoracic & lumbar fusion devices, cervical fusion devices), spinal biologics (allografts, xenografts, DBM, BMP, synthetic bone grafts), vertebral compression fracture treatment devices, non-fusion devices, and spinal bone growth stimulators.
Based on technology, the market is categorized into spinal fusion and fixation technologies, vertebral compression fracture treatment (vertebroplasty, kyphoplasty/vertebral augmentation), and motion preservation technologies. By surgery type, the market includes open surgery and minimally invasive surgery. Based on procedure type, the market is divided into discectomy, laminotomy, foraminotomy, corpectomy, and facetectomy. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Product, Which Segment Accounts for the Dominant Market Share?
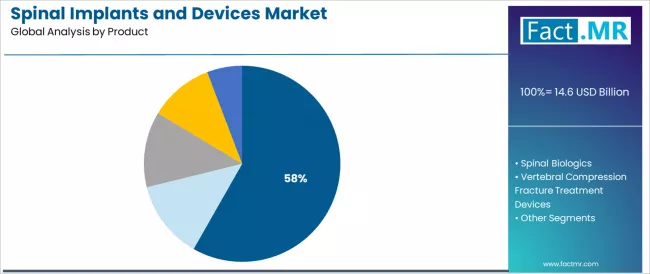
Spinal fusion devices represent the dominant force in the spinal implants and devices market, capturing 58.23% of total market share in 2025. This established product category encompasses solutions featuring comprehensive fixation systems and interbody cage technologies, including pedicle screw-rod constructs for posterior stabilization and interbody fusion cages for anterior and lateral approaches that enable rigid spinal fixation and fusion achievement across degenerative disc disease, spinal stenosis, and deformity correction applications worldwide.
The spinal fusion devices segment's market leadership stems from its extensive clinical evidence base, with solutions capable of addressing diverse spinal pathologies including multi-level degenerative disease, traumatic instability, and deformity requiring arthrodesis while maintaining proven fusion rates exceeding 90% in appropriate patient populations across various spinal regions and surgical approaches.
Within the fusion devices segment, thoracic & lumbar fusion devices represent 65% share driven by high prevalence of lower back pain and lumbar degenerative disease requiring surgical intervention, while cervical fusion devices account for 35% serving neck pain and cervical radiculopathy management.
Spinal biologics maintain a substantial market share at 21.5%, serving surgeons requiring bone graft materials and biological augmentation addressing fusion enhancement and bone healing optimization including allografts for structural support, bone morphogenetic proteins for osteoinductive stimulation, and synthetic bone grafts for osteoconductive scaffolding.
Spinal biologics offer essential biological functionality for surgeons enabling fusion success improvement while avoiding autograft harvest morbidity. The spinal biologics segment demonstrates strong growth potential, driven by expanding clinical evidence supporting biological enhancement and surgeon preference for avoiding autograft complications.
Within the biologics category, allografts represent 30% share providing structural bone grafting, BMP accounts for 20% offering osteoinductive growth factors, xenografts comprise 20%, while DBM and synthetic bone grafts each represent 15% serving specific clinical requirements.
Key clinical advantages driving the spinal fusion devices segment include:
- Advanced biomechanical stability mechanisms with demonstrated load-sharing capabilities enabling immediate post-operative spinal support without external bracing requirements across fusion procedures
- Established surgical technique familiarity allowing surgeon adoption and predictable implantation without extensive training complexity for standard fusion approaches
- Enhanced modular design features enabling intraoperative flexibility and anatomical customization while maintaining system compatibility and inventory efficiency
- Superior clinical evidence profile providing extensive long-term outcomes data and regulatory clearance across various spinal pathologies and patient populations
By Technology, Which Segment Accounts for the Largest Market Share?
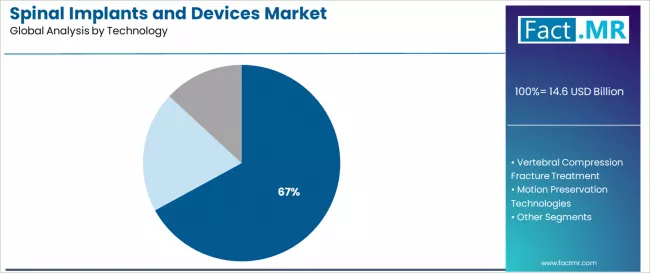
Spinal fusion and fixation technologies dominate the spinal implants technology landscape with a 67.02% market share in 2025, reflecting the fundamental role of arthrodesis procedures in supporting definitive treatment for degenerative spine disease, spinal instability, and deformity correction across contemporary spine surgery practice worldwide.
The segment's market leadership is reinforced by comprehensive instrumentation systems enabling anterior, posterior, and lateral surgical approaches, extensive surgeon training infrastructure supporting technique adoption, and proven clinical outcomes that characterize mainstream spine surgery for structural pathology.
Within this segment, posterior pedicle screw-rod systems represent the primary fixation approach, driven by versatile application across thoracolumbar spine and comprehensive biomechanical stability. This sub-segment benefits from decades of clinical validation and universal surgeon familiarity.
The motion preservation technologies segment represents an important innovation category at 15.8% share, demonstrating steady expansion through specialized requirements for younger active patients seeking alternatives to fusion, disc replacement technologies maintaining segmental motion, and dynamic stabilization systems addressing early degenerative changes. This segment benefits from patient preference for motion-sparing procedures and emerging clinical evidence supporting appropriate patient selection.
Within motion preservation, total disc replacement accounts for 40% share serving single-level disc disease, posterior dynamic devices represent 25%, prosthetic nucleus comprises 20%, and facet replacement accounts for 15% addressing specific anatomical structures. The vertebral compression fracture treatment segment maintains important presence through balloon kyphoplasty and vertebroplasty procedures serving osteoporotic compression fractures and vertebral augmentation requirements in elderly populations.
Key market dynamics supporting technology growth include:
- Fusion technology dominance driven by established clinical evidence and comprehensive pathology coverage, requiring robust fixation and reliable fusion achievement
- Motion preservation advancement trends require careful patient selection criteria and long-term outcome validation for appropriate application expansion
- Integration of minimally invasive approaches enabling reduced surgical trauma and faster recovery across both fusion and non-fusion technologies
- Growing emphasis on patient-specific solutions supporting three-dimensional printed implants and custom instrumentation for complex deformity cases
By Surgery Type, Which Segment Accounts for the Dominant Market Share?
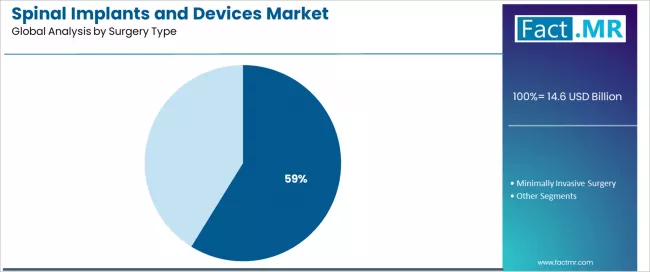
Open surgery dominates the spinal implants surgery type landscape with a 58.81% market share in 2025, reflecting the critical role of traditional open approaches in supporting complex spinal pathology management, comprehensive decompression requirements, and multi-level fusion procedures across spine surgery practice worldwide.
The open surgery segment's market leadership is reinforced by surgeon training foundations emphasizing direct anatomical visualization, comprehensive pathology access enabling thorough neural decompression, and established clinical outcomes that characterize mainstream spine surgery for complex cases.
Within this segment, posterior open approaches represent the primary surgical access, driven by versatile application for decompression and fusion procedures. This sub-segment benefits from universal surgeon competency and straightforward anatomical access.
The minimally invasive surgery segment represents a rapidly growing category at 25.0% share, demonstrating strong expansion through specialized requirements for muscle-sparing approaches, tubular retractor systems enabling targeted decompression, and percutaneous fixation techniques reducing soft tissue trauma. This segment benefits from patient preference for smaller incisions, reduced post-operative pain, and faster recovery enabling earlier return to function.
Key market dynamics supporting surgery type growth include:
- Open surgery resilience driven by complex pathology requirements and surgeon preference for comprehensive visualization, requiring proven techniques and reliable outcomes
- Minimally invasive acceleration trends require specialized instrumentation and learning curve navigation for successful technique adoption
- Integration of navigation guidance enabling minimally invasive accuracy improvement and radiation exposure reduction
- Growing emphasis on outpatient surgery feasibility supporting minimally invasive technique adoption for appropriate single-level cases
By Procedure Type, Which Segment Accounts for a Significant Market Share?
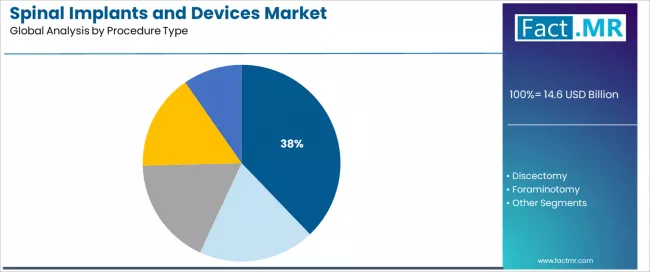
Laminotomy represents a leading procedure type segment in the spinal implants market with a 37.84% market share in 2025, reflecting the fundamental role of decompression procedures in supporting spinal stenosis treatment, nerve root decompression, and canal widening across degenerative spine surgery. The laminotomy segment demonstrates consistent demand driven by the need to address neural compression from degenerative hypertrophy, disc herniation, and bony stenosis across aging patient populations requiring surgical intervention.
The foraminotomy segment emerges as an important procedure category with 25.0% share, driven by the substantial proportion of patients experiencing radicular symptoms from foraminal stenosis requiring targeted nerve root decompression procedures. Patients with radicular leg pain or arm pain from nerve compression require specialized foraminal decompression optimizing nerve root decompression while minimizing structural disruption.
Within procedure applications, discectomy demonstrates significant baseline demand as patients seek disc herniation removal and nerve decompression restoration. Corpectomy and facetectomy address specific pathologies including vertebral body tumors and facet-mediated pain requiring targeted surgical approaches.
Key procedure dynamics include:
- Laminotomy requirements accelerating across aging populations with emphasis on central canal decompression and bilateral nerve root access
- Foraminotomy applications driving demand for targeted approaches and endoscopic techniques enabling minimally invasive foraminal decompression
- Discectomy procedures prioritizing fragment removal and nerve root decompression through various surgical corridors
- Specialized corpectomy and facetectomy techniques emphasizing targeted pathology management for specific clinical indications
What are the Drivers, Restraints, and Key Trends of the Spinal Implants and Devices Market?
Aging global demographics and increasing degenerative disc disease prevalence create expanding surgical candidate populations, with spinal fusion and decompression procedures representing essential interventions for quality of life restoration in comprehensive spine care pathways, requiring widespread device availability.
Advancing minimally invasive surgical techniques drive device innovation and procedure adoption, with navigation-guided systems, tubular retractors, and percutaneous instrumentation demonstrating substantial benefits in reduced operative trauma, shorter hospital stays, and faster functional recovery by 2030.
Improving healthcare access in emerging markets and expanding spine surgery training programs enable surgical capacity growth that increases procedure volumes while meeting rising patient expectations for modern spine care.
Market restraints include high device costs and reimbursement pressures that can challenge hospital budgets and patient access across healthcare systems, particularly when advanced implant systems require USD 5,000-15,000 per surgical level depending on device complexity and biologics utilization with declining reimbursement rates in mature markets.
Surgical complication risks and revision surgery requirements pose another significant concern, as spinal fusion procedures carry inherent risks including pseudarthrosis, adjacent segment degeneration, and hardware failure, potentially affecting patient outcomes and healthcare costs. Regulatory approval complexity and clinical evidence requirements create additional market entry barriers, demanding extensive preclinical testing, clinical trials, and post-market surveillance for new device technologies.
Key trends indicate accelerated robotic-assisted spine surgery adoption in developed markets, particularly United States and Germany, where hospitals demonstrate commitment to surgical precision technologies enabling enhanced pedicle screw placement accuracy, reduced radiation exposure, and improved surgical workflow.
Three-dimensional printing and patient-specific instrumentation trends toward customized implant manufacturing with anatomically matched designs combine preoperative surgical planning with personalized device fabrication that optimizes surgical fit and biomechanical performance. The market could face disruption if significant breakthroughs in biological disc regeneration or injectable fusion technologies provide less invasive alternatives reducing dependence on traditional implant-based surgical approaches.
Analysis of the Spinal Implants and Devices Market by Key Countries
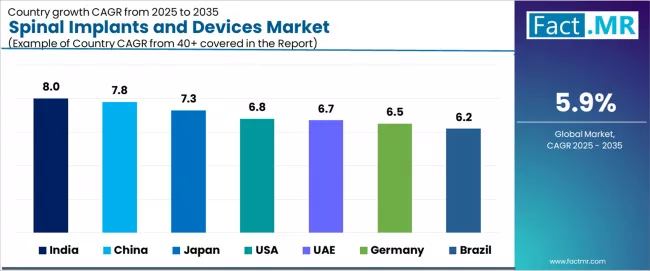
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 8.0% |
| China | 7.8% |
| Japan | 7.3% |
| USA | 6.8% |
| UAE | 6.7% |
| Germany | 6.5% |
| Brazil | 6.2% |
The global spinal implants and devices market is expanding steadily, with India leading at an 8.0% CAGR through 2035, driven by expanding hospital infrastructure, awareness programs increasing surgeries, and growing middle-class healthcare spending. China follows at 7.8%, supported by aging population demographics, rising spinal disorders prevalence, and CFDA approvals for new devices.
Japan records 7.3%, reflecting medical tourism growth, improved healthcare infrastructure, and increasing spine procedures. USA advances at 6.8%, leveraging rising degenerative disc disease, high minimally invasive surgery adoption, and strong research and development investment.
UAE posts 6.7%, focusing on advanced healthcare adoption and government investments in spinal care, while Germany grows steadily at 6.5%, emphasizing aging population needs, regulatory support, and advanced spine centers. Brazil demonstrates 6.2% growth, anchored by spinal trauma from accidents and private hospital expansion.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the spinal implants and devices market with a CAGR of 8.0% through 2035. The country's leadership position stems from rapidly expanding multi-specialty hospital infrastructure across tier-1 and tier-2 cities, increasing awareness about surgical spine treatment options addressing quality of life improvement, and growing medical insurance penetration enabling surgical procedure affordability.
Growth is concentrated in major metropolitan medical centers and emerging healthcare hubs, including Mumbai, Delhi, Bangalore, and Hyderabad, where orthopedic and neurosurgery departments are increasingly performing complex spine surgeries using modern implant technologies for degenerative spine disease and traumatic injury management.
Distribution channels through medical device distributors, hospital procurement systems, and specialty spine centers expand product accessibility across urban medical facilities and tertiary care hospitals. The country's expanding spine surgery training programs with fellowship initiatives provides strong momentum for surgical technique adoption, including comprehensive skill development across surgeons from basic decompression procedures to complex deformity correction surgeries.
Key market factors:
- Hospital infrastructure expansion concentrated in urban centers and medical tourism destinations with advanced spine surgery capabilities
- Surgical awareness growth through patient education initiatives and physician referral network development enabling treatment-seeking behavior
- Comprehensive insurance coverage expansion ecosystem, including government health schemes and private insurance plans covering spine surgery
- Training program development featuring international partnerships and domestic fellowship programs offering advanced spine surgery education
Why is China Emerging as a High-Growth Market?
In major metropolitan centers including Beijing, Shanghai, Guangzhou, and Chengdu, the adoption of advanced spinal implant solutions is accelerating across public hospitals and private spine centers, driven by rapidly aging population with over 280 million people aged 60 and above and increasing prevalence of degenerative spine conditions. The market demonstrates strong growth momentum with a CAGR of 7.8% through 2035, linked to comprehensive healthcare system modernization and increasing focus on orthopedic specialty development.
Chinese spine surgeons are implementing advanced fusion systems and minimally invasive techniques to meet growing patient demand while serving expanding elderly populations requiring surgical intervention. The country's substantial population base creates ongoing demand for spine surgery capacity, while government emphasis on healthcare quality improvement drives adoption of imported and domestic premium implant technologies.
Key development areas:
- Population aging acceleration leading spine surgery demand with emphasis on degenerative disease management and osteoporotic fracture treatment
- Healthcare infrastructure modernization through public hospital upgrades and private spine center development
- Regulatory approval acceleration enabling CFDA clearances for innovative domestic and international devices
- Domestic manufacturing growth alongside international partnerships offering cost-competitive and premium implant solutions
What drives USA’s Market Resilience?
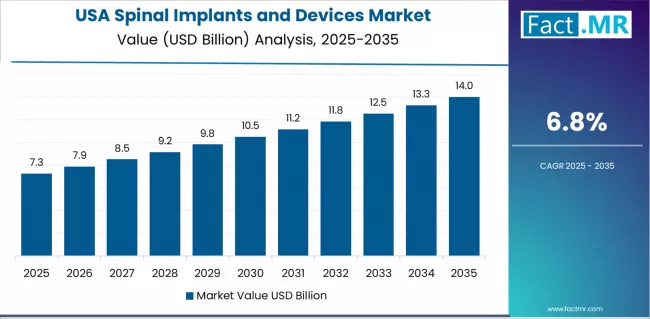
USA’s market expansion is driven by diverse spine pathology prevalence, including degenerative disc disease affecting approximately 40% of adults over 40 and spinal stenosis requiring surgical intervention in aging populations. The country demonstrates steady growth potential with a CAGR of 6.8% through 2035, supported by continuous innovation from established medical device manufacturers and high adoption rates of minimally invasive surgical techniques enabling outpatient procedures.
American spine surgeons face implementation considerations related to value-based care models and bundled payment programs, requiring demonstrated clinical outcomes and cost-effectiveness. However, established spine surgery infrastructure with over 500,000 procedures annually and strong research and development investment exceeding USD 2 billion create stable baseline demand for advanced spinal implants, particularly among academic medical centers and specialty spine practices where innovation adoption drives clinical excellence.
Market characteristics:
- Degenerative spine disease prevalence and aging demographics showing robust surgical demand with substantial procedure volumes
- Minimally invasive surgery leadership varying between coastal medical centers with early adoption and heartland facilities with gradual technique transition
- Future projections indicate continued growth with emphasis on robotic-assisted surgery and artificial intelligence-guided surgical planning
- Growing emphasis on ambulatory surgery centers and outpatient spine surgery supporting cost containment and patient convenience
How does Germany Demonstrate Clinical Excellence Leadership?
The market in Germany leads in evidence-based spine surgery based on comprehensive clinical research infrastructure and rigorous device evaluation standards for enhanced patient safety. The country shows strong potential with a CAGR of 6.5% through 2035, driven by aging population demographics and spine surgeon preferences for clinically validated implant systems in major medical centers, including university hospitals in Munich, Berlin, Hamburg, and Frankfurt.
German spine surgeons are adopting advanced implant technologies through comprehensive clinical study participation and emphasis on long-term outcome tracking for evidence-based practice, particularly in specialized spine centers and academic institutions demanding proven clinical credentials. Distribution channels through established medical device distributors and direct hospital relationships expand coverage across university medical centers and community hospitals.
Leading market segments:
- Academic medical center leadership in major cities implementing comprehensive spine surgery programs and clinical research
- Regulatory compliance emphasis with German medical device regulations and European Union requirements for device safety
- Strategic partnerships between device manufacturers and spine centers advancing clinical evidence generation
- Focus on health technology assessment and outcome documentation addressing healthcare quality standards
What Positions Japan for Technology Adoption Leadership?
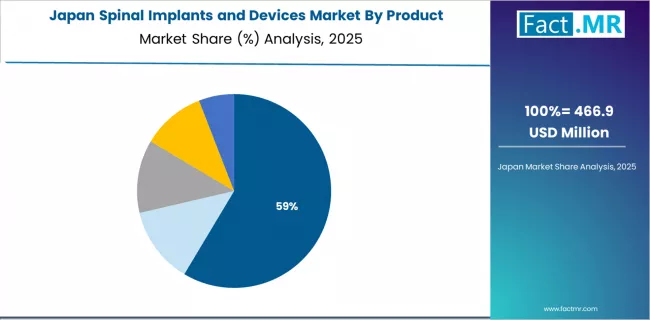
In major metropolitan areas including Tokyo, Osaka, Kyoto, and Fukuoka, spine surgeons are implementing advanced spinal implant technologies through comprehensive training programs and medical device company support, with documented clinical adoption showing substantial acceptance of innovative systems through quality-focused healthcare culture. The market shows steady growth potential with a CAGR of 7.3% through 2035, linked to aging society demographics, improving healthcare infrastructure supporting complex spine procedures, and medical tourism development in major regions.
Spine surgeons are adopting proven technologies with manufacturer clinical support to enhance surgical outcomes while maintaining standards demanded by Japanese healthcare quality expectations and patient safety priorities. The country's sophisticated healthcare system creates ongoing opportunities for premium implant technologies that differentiate through superior clinical performance and comprehensive technical support.
Market development factors:
- Aging population demographics leading spine surgery demand across Japan with emphasis on degenerative disease and osteoporotic fractures
- Healthcare infrastructure advancement providing growth opportunities in regional medical centers and university hospitals
- Medical tourism development through international patient programs and spine center marketing initiatives
- Technology adoption supporting minimally invasive techniques and navigation-guided surgery for precision improvement
How Does UAE Show Healthcare Investment Leadership?
UAE's spinal implants market demonstrates advanced healthcare development focused on attracting international expertise and establishing regional spine surgery excellence, with documented infrastructure investments achieving substantial medical tourism growth through world-class spine centers across Dubai and Abu Dhabi.
The country maintains strong growth momentum with a CAGR of 6.7% through 2035, driven by government healthcare investments and comprehensive medical city development emphasizing specialty care excellence that aligns with national healthcare transformation initiatives.
Major healthcare facilities, including internationally accredited hospitals and specialty spine institutes, showcase advanced adoption of spinal implant technologies where premium devices integrate seamlessly with internationally trained spine surgeon expertise and comprehensive perioperative care programs.
Key market characteristics:
- Government healthcare investment driving facility development with emphasis on specialty spine surgery capabilities
- Medical tourism positioning enabling regional patient attraction and international standard healthcare delivery
- International collaboration supporting surgeon recruitment and technology partnerships with leading device manufacturers
- Premium positioning emphasizing advanced technologies and comprehensive spine surgery services for affluent patient segments
What Characterizes Brazil's Market Development?
In major metropolitan centers including São Paulo, Rio de Janeiro, Brasília, and Belo Horizonte, the adoption of spinal implant solutions is expanding across public and private healthcare sectors, driven by high rates of spinal trauma from traffic accidents and workplace injuries requiring surgical intervention. The market demonstrates moderate growth potential with a CAGR of 6.2% through 2035, linked to comprehensive private hospital sector expansion and increasing insurance coverage supporting surgical procedure accessibility in major urban regions.
Brazilian spine surgeons are implementing modern implant systems and training in advanced techniques to meet trauma care requirements while serving growing populations seeking elective spine surgery for degenerative conditions. The country's substantial trauma burden creates ongoing demand for spinal fixation devices, while expanding private healthcare infrastructure drives adoption of premium implant technologies and minimally invasive surgical capabilities.
Key development areas:
- Trauma care requirements leading spine surgery demand with emphasis on thoracolumbar fracture management and cervical injury treatment
- Private hospital expansion through healthcare sector investment and multi-specialty facility development
- Insurance coverage growth enabling surgical access improvement across middle-class populations
- Training program development supporting spine surgery education and technique advancement for domestic surgeon populations
Europe Market Split by Country

The spinal implants and devices market in Europe is projected to grow from USD 4.1 billion in 2025 to USD 7.2 billion by 2035, registering a CAGR of 6.4% over the forecast period. Germany is expected to maintain its leadership position with a 31.0% market share in 2025, adjusting to 30.5% by 2035, supported by its advanced spine surgery infrastructure, comprehensive clinical research networks, and aging population demographics serving major European markets.
France follows with a 19.5% share in 2025, projected to reach 20.0% by 2035, driven by comprehensive public healthcare system and academic spine center development in major cities implementing advanced surgical techniques. UK holds a 18.0% share in 2025, expected to maintain 18.5% by 2035 through ongoing National Health Service spine surgery programs and private spine center expansion.
Italy commands a 15.5% share, while Spain accounts for 11.0% in 2025. The rest of Europe is anticipated to gain momentum, expanding its collective share from 5.0% to 5.5% by 2035, attributed to increasing spine surgery adoption in Nordic countries and emerging Eastern European healthcare markets implementing modern orthopedic surgery practices.
Competitive Landscape of the Spinal Implants and Devices Market
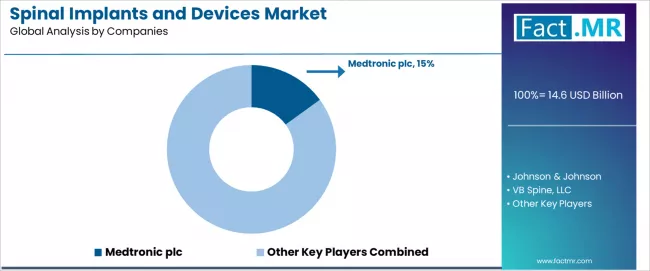
The spinal implants and devices market features approximately 15-25 meaningful players with moderate concentration, where the top three companies control roughly 35-40% of global market share through established product portfolios, comprehensive distribution networks, and extensive surgeon training programs. Competition centers on clinical evidence generation, surgical technique innovation, and surgeon relationship management rather than price competition alone.
Market leaders include Medtronic plc with a 15.0% market share, along with Johnson & Johnson and NuVasive, which maintain competitive advantages through comprehensive spinal implant portfolios spanning fusion systems, biologics, and enabling technologies, extensive clinical evidence supporting device efficacy and safety, and deep relationships with spine surgeons through training programs and research collaborations, creating high credibility among surgical communities seeking reliable implant solutions.
These companies leverage substantial research and development investments and global manufacturing capabilities to defend market positions while expanding into adjacent categories including robotic surgical systems and artificial intelligence-enabled surgical planning platforms.
Challengers encompass established orthopedic companies including Zimmer Biomet and Globus Medical competing through differentiated implant designs and comprehensive fusion portfolios, specialized spine device manufacturers including Alphatec Spine and Orthofix Holdings focusing on specific technology platforms or procedural approaches, and emerging companies including RTI Surgical Holdings emphasizing biologics and tissue-based solutions. Regional players including Ulrich GmbH serve specific geographic markets with established surgeon relationships and localized product offerings.
Emerging medical device startups and technology-focused companies create competitive pressure through innovative surgical approaches and enabling technologies, particularly in minimally invasive instrumentation and motion preservation segments where novel mechanisms and biomaterial advances provide differentiation opportunities.
Market dynamics favor companies that combine proven clinical performance with surgeon education excellence addressing complete surgical solutions from preoperative planning through implantation and post-operative management.
Strategic emphasis on value demonstration through clinical registries, surgeon training infrastructure investment, and modular product systems enabling broad procedural coverage enables differentiation in increasingly competitive spine device segments across developed and emerging markets.
Global Spinal Implants and Devices Market - Stakeholder Contribution Framework
Spinal implants and devices represent a critical medical technology category that enables spine surgeons and healthcare facilities to deliver effective surgical treatment for degenerative spine conditions and spinal trauma while addressing patient expectations for improved outcomes and faster recovery without exclusive conservative management dependency, typically providing biomechanically optimized designs enabling immediate post-operative stability, osteoconductive surfaces promoting bone integration, and modular configurations accommodating diverse anatomical variations compared to historical fixation methods alone while ensuring improved patient functional outcomes and comprehensive surgical success rates.
With the market projected to grow from USD 14.55 billion in 2025 to USD 25.88 billion by 2035 at a 5.9% CAGR, these solutions offer compelling advantages for spinal fusion applications, minimally invasive surgical approaches, and diverse patient populations seeking effective spine surgery solutions. Scaling market adoption and surgical technique advancement requires coordinated action across healthcare policy, clinical evidence development, device manufacturers, spine surgeons, and healthcare systems.
How Could Governments Spur Local Development and Adoption?
- Healthcare Infrastructure Investment: Include spine surgery capabilities in hospital development programs, providing targeted support for specialty spine center establishment and supporting surgical training institutions through fellowship funding and equipment grants.
- Reimbursement Policy Development: Implement fair payment rates for spine surgery procedures, provide technology add-on payments for innovative devices demonstrating superior outcomes, and establish bundled payment models incentivizing value-based spine care delivery.
- Regulatory Framework Optimization: Create efficient medical device approval pathways balancing safety assurance with innovation access, establish clear clinical evidence requirements and post-market surveillance protocols, and develop regional regulatory harmonization facilitating device availability.
- Surgical Training Support: Fund spine surgery fellowship programs expanding surgical workforce capacity, invest in simulation centers and cadaveric training facilities, and support continuing medical education for technique advancement.
- Research & Innovation Funding: Establish grants for spine surgery outcomes research, support device innovation programs and clinical trial infrastructure, and create academic-industry partnerships advancing surgical technique development.
How Could Industry Bodies Support Market Development?
- Clinical Guidelines Development: Define evidence-based treatment algorithms for spinal pathologies across degenerative disease and trauma, establish standardized outcome measurement and registry participation requirements, and create surgical technique recommendations supporting best practice dissemination.
- Quality Standards Promotion: Lead device quality specifications and manufacturing standards, emphasizing material biocompatibility, mechanical performance, and sterilization validation supporting patient safety.
- Surgeon Education Programs: Develop comprehensive training curricula for spinal implant utilization, surgical technique mastery, and complication management through structured educational pathways.
- Outcomes Registry Development: Create spine surgery registries capturing procedure outcomes, device performance, and patient-reported outcomes enabling continuous quality improvement.
How Could Manufacturers and Technology Providers Strengthen the Ecosystem?
- Product Innovation Excellence: Develop next-generation spinal implants with enhanced biomechanical properties, improved biological integration, and modular versatility that address diverse pathologies while simplifying surgical technique.
- Clinical Evidence Generation: Provide comprehensive clinical trial data, registry participation, and long-term outcomes documentation that support surgeon confidence and regulatory submissions aligned with evidence-based practice.
- Surgeon Education Investment: Offer extensive training programs including cadaveric laboratories, surgical technique workshops, and fellowship support that ensure successful device implementation and optimal patient outcomes.
- Technical Service Excellence: Build comprehensive field support capabilities including surgical case coverage, technical consultation, and inventory management that optimize hospital partnerships and surgeon satisfaction.
How Could Spine Surgeons and Healthcare Facilities Navigate the Market?
- Evidence-Based Device Selection: Conduct systematic evaluation of implant technologies comparing clinical outcomes, biomechanical performance, and cost-effectiveness through standardized assessment frameworks.
- Surgical Technique Mastery: Pursue comprehensive training in advanced spine surgery techniques including minimally invasive approaches, navigation guidance, and complex reconstruction through fellowship and continuing education.
- Outcomes Tracking Implementation: Develop registry participation, patient-reported outcome collection, and quality improvement programs that enable continuous surgical performance optimization.
- Value-Based Care Delivery: Implement bundled payment participation, enhanced recovery protocols, and outpatient surgery pathways supporting cost containment while maintaining clinical excellence.
How Could Investors and Financial Enablers Unlock Value?
- Innovation Investment: Back companies developing breakthrough spinal technologies including motion preservation devices, biological fusion enhancement, and robotic surgical systems advancing clinical capabilities.
- Manufacturing Expansion Financing: Provide growth capital for established companies expanding production capacity and geographic market presence supporting increasing global demand.
- Clinical Research Funding: Support clinical trial execution, registry development, and outcomes research generating evidence supporting device adoption and reimbursement.
- Market Access Support: Finance international expansion strategies, regulatory submission programs, and distribution network development enabling global market penetration for innovative devices.
Key Players in the Spinal Implants and Devices Market
- Medtronic plc
- Johnson & Johnson
- VB Spine, LLC
- NuVasive
- Zimmer Biomet Holdings
- Globus Medical, Inc.
- Alphatec Spine, Inc.
- Orthofix Holdings, Inc.
- RTI Surgical Holdings
- Ulrich GmbH & Co. KG
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 14.55 Billion |
| Product | Spinal Fusion Devices (Thoracic & Lumbar Fusion Devices, Cervical Fusion Devices), Spinal Biologics (Allografts, Xenografts, DBM, BMP, Synthetic Bone Grafts), Vertebral Compression Fracture Treatment Devices, Non-fusion Devices, Spinal Bone Growth Stimulators |
| Technology | Spinal Fusion and Fixation Technologies, Vertebral Compression Fracture Treatment (Vertebroplasty, Kyphoplasty/Vertebral Augmentation), Motion Preservation Technologies |
| Surgery Type | Open Surgery, Minimally Invasive Surgery |
| Procedure Type | Discectomy, Laminotomy, Foraminotomy, Corpectomy, Facetectomy |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | India, China, USA, Germany, Japan, UAE, Brazil, and 40+ countries |
| Key Companies Profiled | Medtronic, Johnson & Johnson, VB Spine, LLC, NuVasive, Zimmer Biomet, Globus Medical, Inc., Alphatec Spine, Inc, Orthofix Holdings, Inc, RTI Surgical Holdings, Ulrich GmbH & Co. KG |
| Additional Attributes | Dollar sales by product and technology categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with medical device manufacturers and orthopedic companies, product specifications and clinical performance requirements, integration with minimally invasive surgical platforms and robotic-assisted systems, innovations in biomaterial development and three-dimensional printing technologies, and development of specialized applications with patient-specific implants and navigation-guided surgical capabilities. |
Spinal Implants and Devices Market by Segments
-
Product :
- Spinal Fusion Devices
- Thoracic & Lumbar Fusion Devices
- Cervical Fusion Devices
- Spinal Biologics
- Allografts
- Xenografts
- DBM
- BMP
- Synthetic Bone Grafts
- Vertebral Compression Fracture Treatment Devices
- Non-fusion Devices
- Spinal Bone Growth Stimulators
- Spinal Fusion Devices
-
Technology :
- Spinal Fusion and Fixation Technologies
- Vertebral Compression Fracture Treatment
- Vertebroplasty
- Kyphoplasty/Vertebral Augmentation
- Motion Preservation Technologies
-
Surgery Type :
- Open Surgery
- Minimally Invasive Surgery
-
Procedure Type :
- Discectomy
- Laminotomy
- Foraminotomy
- Corpectomy
- Facetectomy
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2025 to 2035
- Spinal Fusion Devices
- Spinal Biologics
- Vertebral Compression Fracture Treatment Devices
- Non-fusion Devices
- Spinal Bone Growth Stimulators
- Y to o to Y Growth Trend Analysis By Product, 2020 to 2024
- Absolute $ Opportunity Analysis By Product, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Technology
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Technology, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Technology, 2025 to 2035
- Spinal Fusion and Fixation Technologies
- Vertebral Compression Fracture Treatment
- Motion Preservation Technologies
- Y to o to Y Growth Trend Analysis By Technology, 2020 to 2024
- Absolute $ Opportunity Analysis By Technology, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Surgery Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Surgery Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Surgery Type, 2025 to 2035
- Open Surgery
- Minimally Invasive Surgery
- Y to o to Y Growth Trend Analysis By Surgery Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Surgery Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Procedure Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Procedure Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Procedure Type, 2025 to 2035
- Laminotomy
- Discectomy
- Foraminotomy
- Corpectomy
- Facetectomy
- Y to o to Y Growth Trend Analysis By Procedure Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Procedure Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product
- By Technology
- By Surgery Type
- By Procedure Type
- Competition Analysis
- Competition Deep Dive
- Medtronic plc
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Johnson & Johnson
- VB Spine, LLC
- NuVasive
- Zimmer Biomet Holdings
- Globus Medical, Inc.
- Alphatec Spine, Inc.
- Orthofix Holdings, Inc.
- RTI Surgical Holdings
- Ulrich GmbH & Co. KG
- Medtronic plc
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Surgery Type, 2020 to 2035
- Table 5: Global Market Value (USD Million) Forecast by Procedure Type, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 9: North America Market Value (USD Million) Forecast by Surgery Type, 2020 to 2035
- Table 10: North America Market Value (USD Million) Forecast by Procedure Type, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 13: Latin America Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 14: Latin America Market Value (USD Million) Forecast by Surgery Type, 2020 to 2035
- Table 15: Latin America Market Value (USD Million) Forecast by Procedure Type, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: Western Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 18: Western Europe Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 19: Western Europe Market Value (USD Million) Forecast by Surgery Type, 2020 to 2035
- Table 20: Western Europe Market Value (USD Million) Forecast by Procedure Type, 2020 to 2035
- Table 21: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: Eastern Europe Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 23: Eastern Europe Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 24: Eastern Europe Market Value (USD Million) Forecast by Surgery Type, 2020 to 2035
- Table 25: Eastern Europe Market Value (USD Million) Forecast by Procedure Type, 2020 to 2035
- Table 26: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 27: East Asia Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 28: East Asia Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 29: East Asia Market Value (USD Million) Forecast by Surgery Type, 2020 to 2035
- Table 30: East Asia Market Value (USD Million) Forecast by Procedure Type, 2020 to 2035
- Table 31: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 32: South Asia and Pacific Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 33: South Asia and Pacific Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 34: South Asia and Pacific Market Value (USD Million) Forecast by Surgery Type, 2020 to 2035
- Table 35: South Asia and Pacific Market Value (USD Million) Forecast by Procedure Type, 2020 to 2035
- Table 36: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 37: Middle East & Africa Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 38: Middle East & Africa Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 39: Middle East & Africa Market Value (USD Million) Forecast by Surgery Type, 2020 to 2035
- Table 40: Middle East & Africa Market Value (USD Million) Forecast by Procedure Type, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Product
- Figure 6: Global Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Technology
- Figure 9: Global Market Value Share and BPS Analysis by Surgery Type, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Surgery Type, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Surgery Type
- Figure 12: Global Market Value Share and BPS Analysis by Procedure Type, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Procedure Type, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Procedure Type
- Figure 15: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 16: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 17: Global Market Attractiveness Analysis by Region
- Figure 18: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 23: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 24: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 25: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 26: North America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Product
- Figure 29: North America Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Technology
- Figure 32: North America Market Value Share and BPS Analysis by Surgery Type, 2025 and 2035
- Figure 33: North America Market Y to o to Y Growth Comparison by Surgery Type, 2025 to 2035
- Figure 34: North America Market Attractiveness Analysis by Surgery Type
- Figure 35: North America Market Value Share and BPS Analysis by Procedure Type, 2025 and 2035
- Figure 36: North America Market Y to o to Y Growth Comparison by Procedure Type, 2025 to 2035
- Figure 37: North America Market Attractiveness Analysis by Procedure Type
- Figure 38: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 39: Latin America Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Product
- Figure 42: Latin America Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 43: Latin America Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 44: Latin America Market Attractiveness Analysis by Technology
- Figure 45: Latin America Market Value Share and BPS Analysis by Surgery Type, 2025 and 2035
- Figure 46: Latin America Market Y to o to Y Growth Comparison by Surgery Type, 2025 to 2035
- Figure 47: Latin America Market Attractiveness Analysis by Surgery Type
- Figure 48: Latin America Market Value Share and BPS Analysis by Procedure Type, 2025 and 2035
- Figure 49: Latin America Market Y to o to Y Growth Comparison by Procedure Type, 2025 to 2035
- Figure 50: Latin America Market Attractiveness Analysis by Procedure Type
- Figure 51: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 52: Western Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 53: Western Europe Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 54: Western Europe Market Attractiveness Analysis by Product
- Figure 55: Western Europe Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 56: Western Europe Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 57: Western Europe Market Attractiveness Analysis by Technology
- Figure 58: Western Europe Market Value Share and BPS Analysis by Surgery Type, 2025 and 2035
- Figure 59: Western Europe Market Y to o to Y Growth Comparison by Surgery Type, 2025 to 2035
- Figure 60: Western Europe Market Attractiveness Analysis by Surgery Type
- Figure 61: Western Europe Market Value Share and BPS Analysis by Procedure Type, 2025 and 2035
- Figure 62: Western Europe Market Y to o to Y Growth Comparison by Procedure Type, 2025 to 2035
- Figure 63: Western Europe Market Attractiveness Analysis by Procedure Type
- Figure 64: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 65: Eastern Europe Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 66: Eastern Europe Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 67: Eastern Europe Market Attractiveness Analysis by Product
- Figure 68: Eastern Europe Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 69: Eastern Europe Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 70: Eastern Europe Market Attractiveness Analysis by Technology
- Figure 71: Eastern Europe Market Value Share and BPS Analysis by Surgery Type, 2025 and 2035
- Figure 72: Eastern Europe Market Y to o to Y Growth Comparison by Surgery Type, 2025 to 2035
- Figure 73: Eastern Europe Market Attractiveness Analysis by Surgery Type
- Figure 74: Eastern Europe Market Value Share and BPS Analysis by Procedure Type, 2025 and 2035
- Figure 75: Eastern Europe Market Y to o to Y Growth Comparison by Procedure Type, 2025 to 2035
- Figure 76: Eastern Europe Market Attractiveness Analysis by Procedure Type
- Figure 77: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 78: East Asia Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 79: East Asia Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 80: East Asia Market Attractiveness Analysis by Product
- Figure 81: East Asia Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 82: East Asia Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 83: East Asia Market Attractiveness Analysis by Technology
- Figure 84: East Asia Market Value Share and BPS Analysis by Surgery Type, 2025 and 2035
- Figure 85: East Asia Market Y to o to Y Growth Comparison by Surgery Type, 2025 to 2035
- Figure 86: East Asia Market Attractiveness Analysis by Surgery Type
- Figure 87: East Asia Market Value Share and BPS Analysis by Procedure Type, 2025 and 2035
- Figure 88: East Asia Market Y to o to Y Growth Comparison by Procedure Type, 2025 to 2035
- Figure 89: East Asia Market Attractiveness Analysis by Procedure Type
- Figure 90: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 91: South Asia and Pacific Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 92: South Asia and Pacific Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 93: South Asia and Pacific Market Attractiveness Analysis by Product
- Figure 94: South Asia and Pacific Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 95: South Asia and Pacific Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 96: South Asia and Pacific Market Attractiveness Analysis by Technology
- Figure 97: South Asia and Pacific Market Value Share and BPS Analysis by Surgery Type, 2025 and 2035
- Figure 98: South Asia and Pacific Market Y to o to Y Growth Comparison by Surgery Type, 2025 to 2035
- Figure 99: South Asia and Pacific Market Attractiveness Analysis by Surgery Type
- Figure 100: South Asia and Pacific Market Value Share and BPS Analysis by Procedure Type, 2025 and 2035
- Figure 101: South Asia and Pacific Market Y to o to Y Growth Comparison by Procedure Type, 2025 to 2035
- Figure 102: South Asia and Pacific Market Attractiveness Analysis by Procedure Type
- Figure 103: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 104: Middle East & Africa Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 105: Middle East & Africa Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 106: Middle East & Africa Market Attractiveness Analysis by Product
- Figure 107: Middle East & Africa Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 108: Middle East & Africa Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 109: Middle East & Africa Market Attractiveness Analysis by Technology
- Figure 110: Middle East & Africa Market Value Share and BPS Analysis by Surgery Type, 2025 and 2035
- Figure 111: Middle East & Africa Market Y to o to Y Growth Comparison by Surgery Type, 2025 to 2035
- Figure 112: Middle East & Africa Market Attractiveness Analysis by Surgery Type
- Figure 113: Middle East & Africa Market Value Share and BPS Analysis by Procedure Type, 2025 and 2035
- Figure 114: Middle East & Africa Market Y to o to Y Growth Comparison by Procedure Type, 2025 to 2035
- Figure 115: Middle East & Africa Market Attractiveness Analysis by Procedure Type
- Figure 116: Global Market - Tier Structure Analysis
- Figure 117: Global Market - Company Share Analysis
- FAQs -
How big is the spinal implants and devices market in 2025?
The global spinal implants and devices market is estimated to be valued at USD 14.6 billion in 2025.
What will be the size of spinal implants and devices market in 2035?
The market size for the spinal implants and devices market is projected to reach USD 25.9 billion by 2035.
How much will be the spinal implants and devices market growth between 2025 and 2035?
The spinal implants and devices market is expected to grow at a 5.9% CAGR between 2025 and 2035.
What are the key product types in the spinal implants and devices market?
The key product types in spinal implants and devices market are spinal fusion devices , spinal biologics , vertebral compression fracture treatment devices, non-fusion devices and spinal bone growth stimulators.
Which technology segment to contribute significant share in the spinal implants and devices market in 2025?
In terms of technology, spinal fusion and fixation technologies segment to command 67.0% share in the spinal implants and devices market in 2025.