Bioengineered Protein Drugs Market
Bioengineered Protein Drugs Market Size and Share Forecast Outlook 2025 to 2035
The Global Bioengineered Protein Drugs Market Is Forecasted To Reach USD 14.7 Billion In 2025, And Further To USD 37.0 Billion By 2035, Expanding At A 9.7% CAGR. Bioengineered Protein Drugs, Particularly Monoclonal Antibodies, Will Dominate The Product Market, With Oncology Being The Primary Application.
Bioengineered Protein Drugs Market Size and Share Forecast Outlook 2025 to 2035
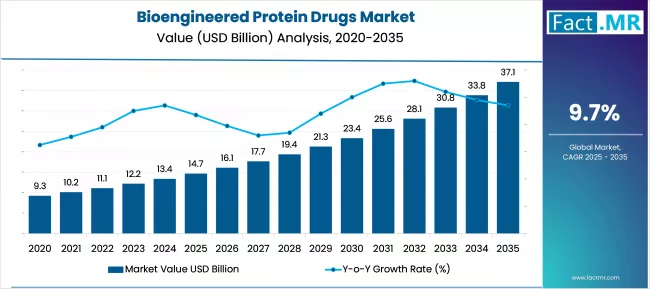
The global bioengineered protein drugs market is projected to increase from USD 14.7 billion in 2025 to USD 37.0 billion by 2035, with a CAGR of 9.7% during the forecast period. The bioengineered protein drugs market is being driven by the increasing prevalence of chronic and genetic disorders, the adoption of targeted therapies, and advances in protein engineering. Growing patient demand for effective biologics and personalized medicine drives global market expansion.
Quick Stats of Bioengineered Protein Drugs Market
- Bioengineered Protein Drugs Market Size (2025): USD 14.7 billion
- Projected Bioengineered Protein Drugs Market Size (2035): USD 37.0 billion
- Forecast CAGR of Bioengineered Protein Drugs Market (2025 to 2035): 9.7%
- Leading Product Type Segment of Bioengineered Protein Drugs Market: Monoclonal Antibodies
- Leading Application Segment of Bioengineered Protein Drugs Market: Oncology
- Key Growth Regions of Bioengineered Protein Drugs Market: Canada, Türkiye, South Korea
- Prominent Players in the Bioengineered Protein Drugs Market: Novartis AG, Panacea Biotec, ProBioGen AG, Reliance Life Science Pvt. Ltd., Sanofi, Roche, and Others.
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 14.7 billion |
| Industry Size (2035F) | USD 37.0 billion |
| CAGR (2025-2035) | 9.7% |
The global Bioengineered Protein Drugs market is forecasted to expand from USD 14.7 billion in 2025 to USD 37.0 billion by 2035, at a CAGR of 9.7%, driven by the rising prevalence of chronic and non-communicable diseases (NCDs) worldwide.
In September 2022, the World Health Organization (WHO) stated that NCDs caused 41 million deaths per year, accounting for 74% of global mortality, with 17.9 million deaths from cardiovascular diseases, 9.3 million from cancer, 4.1 million from chronic respiratory diseases, and 2.0 million from diabetes. This highlights the growing need for advanced biologic therapies to effectively manage diseases. The market is expected to grow to USD 16.1 billion in 2026 and USD 17.6 billion in 2027, driven by rising healthcare awareness and patient demand for targeted therapies.
The market is expected to be worth USD 19.4 billion by 2028, rising to USD 21.2 billion in 2029 and USD 23.3 billion in 2030 as advances in protein engineering and biotechnology expand treatment options. The effectiveness of monoclonal antibodies and fusion proteins is driving adoption, particularly in the oncology and autoimmune segments.
Market growth is expected to continue, reaching USD 25.6 billion in 2031 and USD 28.0 billion in 2032, driven by regulatory support in countries such as Canada, South Korea, and Türkiye, which streamline biologics approvals and promote biosimilar development. Further expansion is expected to reach USD 30.8 billion in 2033 and USD 33.7 billion in 2034, reflecting the growing integration of personalised medicine and precision therapies into clinical practice.
By 2035, the market is expected to be worth USD 37.0 billion, driven by continued R&D investments, ageing populations, and the increased adoption of advanced protein-based drugs in hospitals, speciality clinics, and biotech research hubs worldwide. The combination of disease burden, technological innovation, and favorable regulatory environments positions the Bioengineered Protein Drugs market for significant growth over the next decade.
Key Bioengineered Protein Drugs Market Dynamics
The Bioengineered Protein Drugs market is expanding rapidly, driven by the rising prevalence of chronic and genetic disorders, which has increased demand for advanced therapeutic solutions. Continuous advances in protein engineering and biotechnology have enabled the development of highly effective and targeted therapies, and the growing trend toward personalized medicine is accelerating adoption. These factors, taken together, establish bioengineered protein drugs as an essential component of modern healthcare.
The market faces several challenges that may impede growth. High development and manufacturing costs limit accessibility, and stringent regulatory approval processes delay product launches. Furthermore, the inherent stability and storage challenges of protein-based drugs limit logistical and operational options for both manufacturers and healthcare providers.
Rising Prevalence of Chronic and Genetic Disorders
The rising global burden of chronic diseases such as diabetes, cardiovascular disease, and cancer has increased the demand for novel treatments. Genetic disorders, such as cystic fibrosis, hemophilia, and rare metabolic diseases, necessitate advanced protein-based interventions that can meet unmet medical needs. This growing patient population is driving pharmaceutical companies to focus on bioengineered proteins.
Healthcare systems around the world are recognizing the value of early intervention and long-term management of chronic diseases. Bioengineered protein drugs, with their specificity and therapeutic efficacy, are increasingly regarded as superior alternatives to traditional medicines. This shift has accelerated R&D investment and expanded clinical applications.
Advancements in Protein Engineering and Biotechnology
Rapid technological advancements in protein design, computational biology, and gene editing have revolutionized drug discovery. CRISPR, artificial intelligence, and advanced expression systems now enable precise modifications to protein structures, resulting in increased efficacy and fewer side effects. These tools enable developers to create next-generation protein therapeutics with optimal functionality.
Biotechnology companies use recombinant DNA technology and synthetic biology to efficiently scale production. Improved cell lines and bioprocessing methods reduce development time while maintaining quality. Such breakthroughs not only improve therapeutic outcomes, but also establish a competitive pipeline of new drugs targeting a variety of disease pathways.
Growing Demand for Personalized and Targeted Therapies
Patients and clinicians are increasingly looking for personalized treatments that take into account genetic, environmental, and lifestyle factors. Bioengineered proteins enable therapies that can be designed to target specific biomarkers, resulting in greater precision and fewer side effects than traditional drugs. This personalization increases treatment adherence and improves outcomes in complex diseases.
Oncology, immunology, and rare diseases have emerged as the primary areas where targeted protein drugs are making a significant impact. Pharmaceutical companies are collaborating with diagnostic companies to integrate companion diagnostics, ensuring that the correct patient receives the appropriate therapy. This trend is pushing the market towards precision medicine models.
High Development and Manufacturing Costs
Developing bioengineered protein drugs requires complex R&D, specialized facilities, and highly skilled expertise, resulting in significantly higher costs than conventional small-molecule drugs. Investment in clinical trials, preclinical studies, and adherence to stringent global standards increases financial pressure. These expenses frequently prevent smaller biotech firms from entering the market.
Protein drug production necessitates advanced bioprocessing systems, purification techniques, and stringent quality control protocols. Establishing and maintaining such infrastructure is capital-intensive, resulting in high production costs. As a result, affordability and pricing challenges persist, particularly in emerging markets, which limits broad adoption despite the therapeutic benefits.
Stringent Regulatory Approval Processes
Regulatory authorities must rigorously evaluate bioengineered protein drugs to ensure their safety, efficacy, and quality. This includes long clinical trial phases, extensive documentation, and strict adherence to changing international guidelines. The complexity frequently causes approval timelines to be longer than those for traditional drugs.
Delays in approvals can have a significant impact on market entry, reducing competitive advantage and increasing financial risk. Companies must make significant investments in regulatory compliance, pharmacovigilance, and post-marketing surveillance. Although these frameworks protect patients, they also create barriers that may discourage innovation and slow the adoption of potentially life-saving therapies.
Limited Stability and Storage Challenges of Protein-Based Drugs
Protein-based therapeutics are highly sensitive to temperature, pH, and light exposure, which can lead to degradation or loss of efficacy. Ensuring stability during production, transportation, and storage necessitates advanced formulation and packaging solutions, which frequently add to operational complexity. This fragility creates challenges for global distribution.
Cold chain logistics become critical to preserving drug potency, particularly in areas with inadequate infrastructure. Any compromise in handling can render treatments ineffective, affecting patient outcomes and generating waste. Overcoming these constraints remains a priority for manufacturers, as stability issues impede the accessibility and widespread commercialization of protein drugs
Analyzing the Bioengineered Protein Drugs Market by Key Regions
North America currently dominates the Bioengineered Proteins Drugs market, owing to the high prevalence of chronic diseases, advanced healthcare infrastructure, and the presence of major pharmaceutical companies. The United States, in particular, is a significant market for bioengineered proteins, accounting for a sizable share of the global market.
Increased investment in biotechnology research and development, favorable reimbursement policies, and the availability of advanced healthcare facilities are key drivers of market growth in North America.
Europe is another important market for Bioengineered Proteins Drugs, owing to government initiatives to support biotechnology research, favorable reimbursement policies, and the rising prevalence of chronic diseases. Europe's major markets for bioengineered proteins include Germany, France, and the United Kingdom.
The Asia Pacific region is expected to experience the fastest growth rate during the forecast period, owing to rising healthcare expenditure, increased awareness of bioengineered proteins, and the growing prevalence of chronic diseases in countries such as China and India. The growing middle class and the increased availability of advanced healthcare facilities are also driving market growth in the region.
Country-Wise Outlook
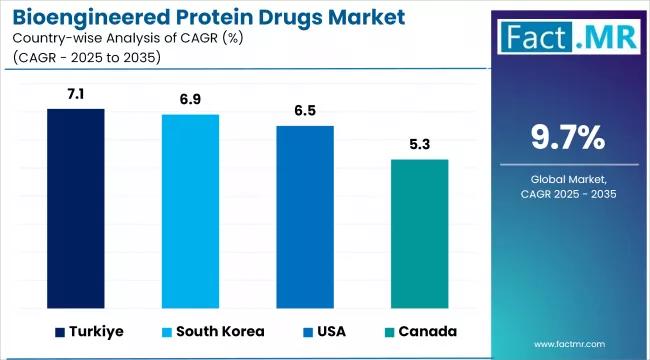
| Country | CAGR (2025-2035) |
|---|---|
| Canada | 5.3% |
| Türkiye | 7.1% |
| South Korea | 6.9% |
Canada Strengthens Bioengineered Protein Drugs Market through Strong Life Sciences Ecosystem
Canada has emerged as a promising market for bioengineered protein drugs, due to its robust healthcare infrastructure and strong life sciences ecosystem. The rising prevalence of chronic illnesses such as diabetes, cancer, and autoimmune disorders is driving up demand for advanced biologics. This need is exacerbated by the country's aging population, which forces healthcare systems to seek targeted and long-term therapeutic solutions.
Scientific advancements are transforming the landscape. Canadian biotech companies and academic institutions are using protein engineering to create safer and more effective therapeutics. Ontario and Quebec are particularly active in developing innovative biologics, while Vancouver is known for its biotech startups that focus on protein-based therapies. These clusters ensure a steady pipeline of novel candidates for commercialization.
Regulatory frameworks play an important role. Health Canada is continually refining its biologics guidelines, with a recent emphasis on risk-based review processes to expedite approvals while ensuring patient safety. Biosimilars are receiving increased attention, with draft updates expected to reduce clinical trial requirements and encourage greater market participation. This regulatory evolution is yielding an environment that strikes a balance between innovation and affordability.
- Growing chronic disease and aging population boost biologics demand
- Biotech hubs in Ontario, Quebec, and Vancouver drive innovation
- Health Canada regulations support faster approvals and biosimilar adoption
Türkiye's Bioengineered Protein Drugs Market Thrives on Rising Prevalence of Cancer
Türkiye’s bioengineered protein drugs market is at a transformative stage as the country seeks to reduce its reliance on imported biologics while building its domestic life sciences capabilities.
The rising prevalence of cancer, metabolic disorders, and autoimmune diseases has heightened demand for innovative therapies, making protein-based drugs increasingly important in treatment protocols across major hospitals and specialty clinics. This trend is particularly visible in urban centers like Istanbul and Ankara, where advanced healthcare facilities drive adoption.
The Turkish Medicines and Medical Devices Agency has been aligning regulations with European Union standards, introducing updates that provide more transparent pathways for biosimilars while maintaining strict quality and safety checks. This evolving framework not only encourages foreign entrants but also strengthens the credibility of domestic producers. Long approval timelines and pricing constraints remain challenges that companies must navigate carefully. Government and organizational initiatives have supported this transition.
Recent national programs have prioritized biomanufacturing capacity development, while collaborations between health authorities and academic institutions are focusing on training skilled professionals in protein drug development. These steps, combined with payer-driven policies to control biologics costs, are shaping Türkiye’s path toward becoming more self-sufficient in bioengineered protein drugs.
- Growing demand for protein therapies
- EU-aligned regulations support biosimilars
- Government boosts biomanufacturing capacity
South Korea Advances in Bioengineered Protein Drugs Market with Focus on Aging Population and Increasing Healthcare Spending
South Korea's bioengineered protein drug market is expanding rapidly, driven by the increasing prevalence of chronic diseases such as diabetes, cancer, and autoimmune disorders. The aging population and increasing healthcare spending are driving up demand for advanced biologics and targeted therapies. Protein-based drugs are increasingly being used in treatment protocols by hospitals and specialty clinics, while patients are increasingly seeking personalized medicine options.
The government has implemented policies that encourage domestic biopharmaceutical development. The Ministry of Health and Welfare, in collaboration with the Korea Food and Drug Administration (KFDA), has streamlined the approval process for biosimilars and advanced protein therapeutics, encouraging innovation while maintaining strict safety and efficacy standards.
South Korean biotechnology companies are making significant investments in protein engineering and biomanufacturing infrastructure. Seoul, Busan, and Daejeon research hubs are collaborating with universities and medical centers to accelerate R&D, with a focus on developing novel biologics and biosimilars.
The market also benefits from public-private partnerships and initiatives aimed at increasing local production capacity, decreasing reliance on imported biologics, and bolstering South Korea's position as a competitive player in the global protein drug market.
- Rising chronic diseases drive protein drug demand
- Supportive policies boost domestic R&D
- Biotech hubs strengthen local production
Category-wise Analysis
Monoclonal Antibodies to Exhibit Leading Share by Product Type
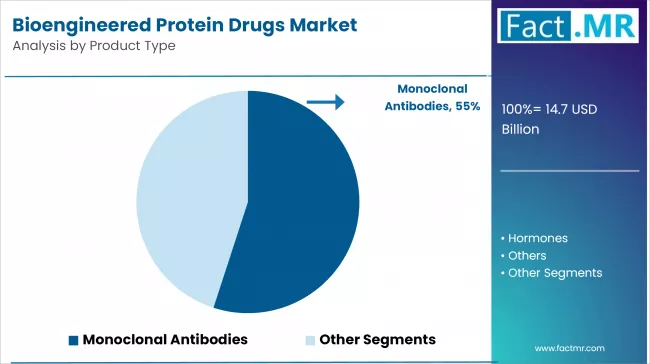
The monoclonal antibodies segment dominates the Bioengineered Protein Drugs market, owing to their targeted therapeutic action and broad applicability across various diseases.
Due to their ability to specifically recognize and bind antigens, monoclonal antibodies are widely used to treat cancers, autoimmune disorders, and infectious diseases, increasing treatment efficacy while minimizing side effects. Their precision and proven clinical outcomes make them popular in hospitals and specialty clinics worldwide.
Furthermore, advances in antibody engineering, such as the development of bispecific antibodies and antibody-drug conjugates, are broadening their therapeutic applications.
Strong R&D pipelines, combined with increased healthcare awareness and the prevalence of chronic and genetic diseases, reinforce monoclonal antibodies' position as a key driver of growth in the bioengineered protein drugs market.
- Targeted therapy for multiple diseases
- Advanced antibody innovations expand use
- R&D and chronic disease drive growth
Oncology to Exhibit Leading by Application
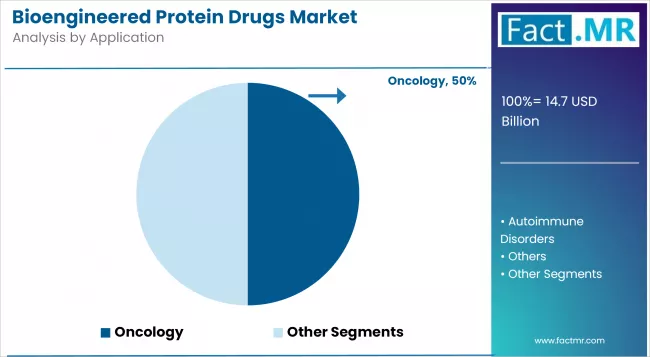
The oncology segment is one of the most prominent categories in the Bioengineered Protein Drugs market, driven by the increasing global incidence of cancer and the growing demand for targeted therapies.
Bioengineered protein drugs, such as monoclonal antibodies and fusion proteins, play a crucial role in oncology by selectively targeting cancer cells, minimising damage to healthy tissues, and enhancing patient outcomes.
Hospitals, cancer centers, and specialty clinics increasingly rely on these therapies to complement conventional treatments like chemotherapy and radiation. Moreover, advancements in protein engineering and immuno-oncology have expanded treatment options, including checkpoint inhibitors and CAR-T therapies.
Ongoing clinical research, coupled with increasing awareness among patients and healthcare providers, is boosting adoption. Personalized cancer care and precision medicine trends further reinforce oncology as a high-growth segment in the bioengineered protein drugs market.
- Rising cancer prevalence drives targeted therapy demand
- Advances in immuno-oncology expand treatment options
- Personalized care boosts adoption in oncology
Competitive Analysis
The Bioengineered Proteins Drugs market is competitive, with several major pharmaceutical companies and numerous smaller biotech firms. The market is fiercely competitive, with companies focusing on the development of new bioengineered proteins and the improvement of existing ones.
Key factors driving market competition include increased investment in research and development (R&D), the availability of advanced research facilities, and the growing demand for effective and targeted treatments for chronic diseases. Furthermore, stringent regulatory requirements for the approval of bioengineered proteins, as well as high production costs, pose significant challenges for market players.
Major pharmaceutical companies such as Roche, Amgen, and Pfizer are among the top players in the Bioengineered Protein Drugs market. These companies have a strong market presence, with a diverse portfolio of bioengineered proteins and significant R&D investments.
Roche, for example, is a market leader with a strong focus on oncology and a diverse portfolio of bioengineered proteins for treating various types of cancer. Amgen is another major player, known for its innovative bioengineered proteins used to treat conditions like anemia and osteoporosis. Pfizer, with its strong market presence, offers a diverse range of bioengineered proteins for the treatment of chronic diseases, including diabetes and rheumatoid arthritis.
Key Players in the Market
- Abbott Laboratories
- Amgen Inc.
- Bayer AG
- Biocon Ltd.
- Dr. Reddy’s Laboratories
- Eli Lilly and Company
- F. Hoffmann – La Roche Ltd.
- Fresenius Kabi
- GlaxoSmithKline plc
- Johnson & Johnson (Janssen)
- Merck & Co. Inc.
- Novartis AG
- Panacea Biotec
- ProBioGen AG
- Reliance Life Science Pvt. Ltd.
- Sanofi
- Roche
- AbbVie
- Pfizer
Recent Developments
- In September 2024, Apeiron Biologics was acquired for $100 million by Ligand Pharmaceuticals Inc., a biopharmaceutical company based in the United States. Apeiron Biologics intends to strengthen Ligand's portfolio by granting it royalty rights to Qarziba (dinutuximab beta), the only approved immunotherapy for high-risk neuroblastoma, which is currently marketed in over 35 countries. Apeiron Biologics AG is an Austria-based biotechnology company that specializes in developing novel immunotherapies for cancer and other diseases.
- In April 2022, Satellite Bio introduces a novel approach to bioengineering tissues. This company specializes in synthetic biology, regenerative medicine, cell therapy, and tissue engineering.
Segmentation of Bioengineered Protein Drugs Market
-
By Product Type :
- Monoclonal Antibodies
- Hormones
- Others
-
By Application :
- Oncology
- Autoimmune Disorders
- Others
-
By Region :
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia & Pacific
- Middle East & Africa
Table of Content
- Executive Summary
- Global Market Outlook
- Demand-side Trends
- Supply-side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020-2024 and Forecast, 2025-2035
- Historical Market Size Value (USD Billion) & Units Analysis, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Projections, 2025-2035
- Y-o-Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020-2024 and Forecast 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Product Type
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By Product Type, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By Product Type, 2025-2035
- Monoclonal Antibodies
- Hormones
- Others
- Y-o-Y Growth Trend Analysis By Product Type, 2020-2024
- Absolute $ Opportunity Analysis By Product Type, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By Application, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By Application, 2025-2035
- Oncology
- Autoimmune Disorders
- Others
- Y-o-Y Growth Trend Analysis By Application, 2020-2024
- Absolute $ Opportunity Analysis By Application, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Region
- Introduction
- Historical Market Size Value (USD Billion) & Units Analysis By Region, 2020-2024
- Current Market Size Value (USD Billion) & Units Analysis and Forecast By Region, 2025-2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia & Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- U.S.
- Canada
- Mexico
- By Product Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Application
- Key Takeaways
- Latin America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Product Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Application
- Key Takeaways
- Western Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Germany
- U.K.
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Europe
- By Product Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Application
- Key Takeaways
- Eastern Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltics
- Rest of Eastern Europe
- By Product Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Application
- Key Takeaways
- East Asia Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- China
- Japan
- South Korea
- By Product Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Application
- Key Takeaways
- South Asia & Pacific Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia & Pacific
- By Product Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Application
- Key Takeaways
- Middle East & Africa Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Product Type
- By Application
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Application
- Key Takeaways
- Key Countries Market Analysis
- U.S.
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- U.K.
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Nordic
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- BENELUX
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Balkan & Baltics
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Application
- U.S.
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product Type
- By Application
- Competition Analysis
- Competition Deep Dive
- Abbott Laboratories
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Amgen Inc.
- Bayer AG
- Biocon Ltd.
- Dr. Reddy’s Laboratories
- Eli Lilly and Company
- F. Hoffmann – La Roche Ltd.
- Fresenius Kabi
- GlaxoSmithKline plc
- Johnson & Johnson (Janssen)
- Merck & Co. Inc.
- Novartis AG
- Panacea Biotec
- ProBioGen AG
- Reliance Life Science Pvt. Ltd.
- Sanofi
- Roche
- AbbVie
- Pfizer
- Abbott Laboratories
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Billion) Forecast by Region, 2020 to 2035
- Table 2: Global Market Units Forecast by Region, 2020 to 2035
- Table 3: Global Market Value (USD Billion) Forecast by Product Type, 2020 to 2035
- Table 4: Global Market Units Forecast by Product Type, 2020 to 2035
- Table 5: Global Market Value (USD Billion) Forecast by Application, 2020 to 2035
- Table 6: Global Market Units Forecast by Application, 2020 to 2035
- Table 7: North America Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 8: North America Market Units Forecast by Country, 2020 to 2035
- Table 9: North America Market Value (USD Billion) Forecast by Product Type, 2020 to 2035
- Table 10: North America Market Units Forecast by Product Type, 2020 to 2035
- Table 11: North America Market Value (USD Billion) Forecast by Application, 2020 to 2035
- Table 12: North America Market Units Forecast by Application, 2020 to 2035
- Table 13: Latin America Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 14: Latin America Market Units Forecast by Country, 2020 to 2035
- Table 15: Latin America Market Value (USD Billion) Forecast by Product Type, 2020 to 2035
- Table 16: Latin America Market Units Forecast by Product Type, 2020 to 2035
- Table 17: Latin America Market Value (USD Billion) Forecast by Application, 2020 to 2035
- Table 18: Latin America Market Units Forecast by Application, 2020 to 2035
- Table 19: Western Europe Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 20: Western Europe Market Units Forecast by Country, 2020 to 2035
- Table 21: Western Europe Market Value (USD Billion) Forecast by Product Type, 2020 to 2035
- Table 22: Western Europe Market Units Forecast by Product Type, 2020 to 2035
- Table 23: Western Europe Market Value (USD Billion) Forecast by Application, 2020 to 2035
- Table 24: Western Europe Market Units Forecast by Application, 2020 to 2035
- Table 25: Eastern Europe Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 26: Eastern Europe Market Units Forecast by Country, 2020 to 2035
- Table 27: Eastern Europe Market Value (USD Billion) Forecast by Product Type, 2020 to 2035
- Table 28: Eastern Europe Market Units Forecast by Product Type, 2020 to 2035
- Table 29: Eastern Europe Market Value (USD Billion) Forecast by Application, 2020 to 2035
- Table 30: Eastern Europe Market Units Forecast by Application, 2020 to 2035
- Table 31: East Asia Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 32: East Asia Market Units Forecast by Country, 2020 to 2035
- Table 33: East Asia Market Value (USD Billion) Forecast by Product Type, 2020 to 2035
- Table 34: East Asia Market Units Forecast by Product Type, 2020 to 2035
- Table 35: East Asia Market Value (USD Billion) Forecast by Application, 2020 to 2035
- Table 36: East Asia Market Units Forecast by Application, 2020 to 2035
- Table 37: South Asia & Pacific Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 38: South Asia & Pacific Market Units Forecast by Country, 2020 to 2035
- Table 39: South Asia & Pacific Market Value (USD Billion) Forecast by Product Type, 2020 to 2035
- Table 40: South Asia & Pacific Market Units Forecast by Product Type, 2020 to 2035
- Table 41: South Asia & Pacific Market Value (USD Billion) Forecast by Application, 2020 to 2035
- Table 42: South Asia & Pacific Market Units Forecast by Application, 2020 to 2035
- Table 43: Middle East & Africa Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 44: Middle East & Africa Market Units Forecast by Country, 2020 to 2035
- Table 45: Middle East & Africa Market Value (USD Billion) Forecast by Product Type, 2020 to 2035
- Table 46: Middle East & Africa Market Units Forecast by Product Type, 2020 to 2035
- Table 47: Middle East & Africa Market Value (USD Billion) Forecast by Application, 2020 to 2035
- Table 48: Middle East & Africa Market Units Forecast by Application, 2020 to 2035
List Of Figures
- Figure 1: Global Market Units Forecast 2020 to 2035
- Figure 2: Global Market Pricing Analysis
- Figure 3: Global Market Value (USD Billion) Forecast 2020 to 2035
- Figure 4: Global Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 5: Global Market Y-o-Y Growth Comparison by Product Type, 2025 to 2035
- Figure 6: Global Market Attractiveness Analysis by Product Type
- Figure 7: Global Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 8: Global Market Y-o-Y Growth Comparison by Application, 2025 to 2035
- Figure 9: Global Market Attractiveness Analysis by Application
- Figure 10: Global Market Value (USD Billion) Share and BPS Analysis by Region, 2025 and 2035
- Figure 11: Global Market Y-o-Y Growth Comparison by Region, 2025 to 2035
- Figure 12: Global Market Attractiveness Analysis by Region
- Figure 13: North America Market Incremental $ Opportunity, 2025 to 2035
- Figure 14: Latin America Market Incremental $ Opportunity, 2025 to 2035
- Figure 15: Western Europe Market Incremental $ Opportunity, 2025 to 2035
- Figure 16: Eastern Europe Market Incremental $ Opportunity, 2025 to 2035
- Figure 17: East Asia Market Incremental $ Opportunity, 2025 to 2035
- Figure 18: South Asia & Pacific Market Incremental $ Opportunity, 2025 to 2035
- Figure 19: Middle East & Africa Market Incremental $ Opportunity, 2025 to 2035
- Figure 20: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 21: North America Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 22: North America Market Y-o-Y Growth Comparison by Product Type, 2025 to 2035
- Figure 23: North America Market Attractiveness Analysis by Product Type
- Figure 24: North America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 25: North America Market Y-o-Y Growth Comparison by Application, 2025 to 2035
- Figure 26: North America Market Attractiveness Analysis by Application
- Figure 27: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 28: Latin America Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 29: Latin America Market Y-o-Y Growth Comparison by Product Type, 2025 to 2035
- Figure 30: Latin America Market Attractiveness Analysis by Product Type
- Figure 31: Latin America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 32: Latin America Market Y-o-Y Growth Comparison by Application, 2025 to 2035
- Figure 33: Latin America Market Attractiveness Analysis by Application
- Figure 34: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 35: Western Europe Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 36: Western Europe Market Y-o-Y Growth Comparison by Product Type, 2025 to 2035
- Figure 37: Western Europe Market Attractiveness Analysis by Product Type
- Figure 38: Western Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 39: Western Europe Market Y-o-Y Growth Comparison by Application, 2025 to 2035
- Figure 40: Western Europe Market Attractiveness Analysis by Application
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 42: Eastern Europe Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 43: Eastern Europe Market Y-o-Y Growth Comparison by Product Type, 2025 to 2035
- Figure 44: Eastern Europe Market Attractiveness Analysis by Product Type
- Figure 45: Eastern Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 46: Eastern Europe Market Y-o-Y Growth Comparison by Application, 2025 to 2035
- Figure 47: Eastern Europe Market Attractiveness Analysis by Application
- Figure 48: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 49: East Asia Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 50: East Asia Market Y-o-Y Growth Comparison by Product Type, 2025 to 2035
- Figure 51: East Asia Market Attractiveness Analysis by Product Type
- Figure 52: East Asia Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 53: East Asia Market Y-o-Y Growth Comparison by Application, 2025 to 2035
- Figure 54: East Asia Market Attractiveness Analysis by Application
- Figure 55: South Asia & Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 56: South Asia & Pacific Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 57: South Asia & Pacific Market Y-o-Y Growth Comparison by Product Type, 2025 to 2035
- Figure 58: South Asia & Pacific Market Attractiveness Analysis by Product Type
- Figure 59: South Asia & Pacific Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 60: South Asia & Pacific Market Y-o-Y Growth Comparison by Application, 2025 to 2035
- Figure 61: South Asia & Pacific Market Attractiveness Analysis by Application
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: Middle East & Africa Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 64: Middle East & Africa Market Y-o-Y Growth Comparison by Product Type, 2025 to 2035
- Figure 65: Middle East & Africa Market Attractiveness Analysis by Product Type
- Figure 66: Middle East & Africa Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 67: Middle East & Africa Market Y-o-Y Growth Comparison by Application, 2025 to 2035
- Figure 68: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 69: Global Market - Tier Structure Analysis
- Figure 70: Global Market - Company Share Analysis
- FAQs -
What is the Global Bioengineered Protein Drugs Market size in 2025?
The bioengineered protein drugs market is valued at USD 14.7 billion in 2025.
Who are the Major Players Operating in the Bioengineered Protein Drugs Market?
Prominent players in the market include Novartis AG, Panacea Biotec, ProBioGen AG, Reliance Life Science Pvt. Ltd., Sanofi, and Roche.
What is the Estimated Valuation of the Bioengineered Protein Drugs Market by 2035?
The market is expected to reach a valuation of USD 37.0 billion by 2035.
What Value CAGR Did the Bioengineered Protein Drugs Market Exhibit over the Last Five Years?
The historic growth rate of the bioengineered protein drugs market is 9.7% from 2020-2024.