Topical Drugs CDMO Market
Topical Drugs CDMO Market Size and Share Forecast Outlook 2025 to 2035
Topical drugs CDMO market is projected to grow from USD 8.0 billion in 2025 to USD 15.3 billion by 2035, at a CAGR of 6.7%. Semi-solid Formulations will dominate with a 66.5% market share, while contract manufacturing will lead the service segment with a 59.0% share.
Topical Drugs CDMO Market Forecast and Outlook 2025 to 2035
The global topical drugs CDMO market is projected to reach USD 15.3 billion by 2035, recording an absolute increase of USD 7.3 billion over the forecast period. The market is valued at USD 8.0 billion in 2025 and is set to rise at a CAGR of 6.7% during the assessment period.
Quick Stats for Topical Drugs CDMO Market
- Topical Drugs CDMO Market Value (2025): USD 8.0 billion
- Topical Drugs CDMO Market Forecast Value (2035): USD 15.3 billion
- Topical Drugs CDMO Market Forecast CAGR: 6.7%
- Leading Formulation Type in Topical Drugs CDMO Market: Semi-solid Formulations (66.5%)
- Key Growth Regions in Topical Drugs CDMO Market: North America, Europe, and Asia Pacific
- Top Players in Topical Drugs CDMO Market: Lubrizol Life Science, Cambrex Corporation, Contract Pharmaceuticals Ltd (CPL), Bora Pharmaceutical CDMO, Ascendia Pharmaceuticals, Pierre Fabre Group, Piramal Pharma Solutions, DPT Laboratories, MedPharm, PCI Pharma Services
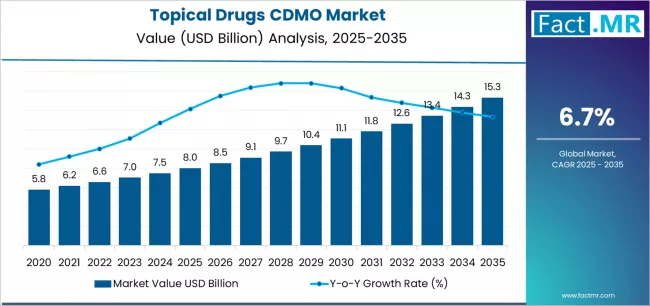
The market is expected to grow by approximately 1.9 times during the same period, supported by increasing pharmaceutical outsourcing trends and expanding dermatology therapeutic pipeline worldwide, driving demand for specialized formulation expertise and increasing investments in advanced manufacturing facilities with enhanced quality standards across semi-solid and transdermal drug development applications globally.
Pharmaceutical companies and emerging biopharmaceutical firms face mounting pressure to accelerate time-to-market and optimize production costs while managing complex formulation requirements and regulatory compliance obligations, with modern topical CDMO services providing documented advantages including specialized formulation capabilities, scalable manufacturing infrastructure, and comprehensive regulatory support compared to in-house production alternatives.
Rising dermatology drug approvals and expanding contract manufacturing adoption across emerging economies create substantial opportunities for CDMO service providers and pharmaceutical partners. However, regulatory complexity across markets and quality control standardization challenges may pose obstacles to seamless global manufacturing network integration.
The semi-solid formulations segment dominates market activity, driven by extensive therapeutic applications and established manufacturing expertise enabling effective dermatological drug delivery worldwide. Pharmaceutical clients increasingly utilize CDMO partners for cream and gel formulations, with typical service offerings providing specialized compounding capabilities and validated manufacturing processes through established quality management systems.
The contract manufacturing segment demonstrates robust market presence, supported by cost optimization imperatives and capacity constraints enabling pharmaceutical companies to leverage external manufacturing infrastructure in modern pharmaceutical outsourcing. Pharmaceutical companies emerge as the dominant end-use segment, reflecting extensive outsourcing adoption for topical product portfolios. Semi-solid formulations maintain market leadership among dosage forms, while contract manufacturing services gain momentum through efficiency advantages.
Regional dynamics show North America maintaining market leadership, supported by extensive pharmaceutical industry presence and advanced formulation technology requirements across USA and Canadian markets. Europe demonstrates strong market positioning driven by established CDMO infrastructure and comprehensive regulatory expertise, while Asia Pacific emphasizes cost-competitive manufacturing and expanding R&D capabilities.
India leads country-level growth through fastest expanding CDMO base and competitive manufacturing advantages, followed by China supported by low-cost manufacturing combined with R&D investment boost.
The competitive landscape features moderate concentration with Lubrizol Life Science maintaining market leadership position, while established players including Cambrex Corporation, Contract Pharmaceuticals Ltd, and Bora Pharmaceutical CDMO compete through specialized formulation capabilities and comprehensive service portfolios across diverse topical drug development and manufacturing applications.
Topical Drugs CDMO Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the topical drugs CDMO market is projected to expand from USD 8.0 billion to USD 10.6 billion, resulting in a value increase of USD 2.6 billion, which represents 35.6% of the total forecast growth for the period. This phase of development will be shaped by rising demand for complex dermatology formulations and specialized manufacturing capabilities in oncology and immunology topical applications, service innovation in transdermal delivery systems with enhanced permeation technologies, as well as expanding integration with pharmaceutical development programs and regulatory consulting services. Companies are establishing competitive positions through investment in advanced manufacturing suites with controlled environments, specialized analytical capabilities, and strategic capacity expansion across North American and European production facilities supporting pharmaceutical client requirements.
From 2029 to 2035, the market is forecast to grow from USD 10.6 billion to USD 15.3 billion, adding another USD 4.7 billion, which constitutes 64.4% of the overall expansion. This period is expected to be characterized by the expansion of specialized service applications, including biosimilar topical formulations and personalized dermatology compounding tailored for precision medicine approaches, strategic collaborations between CDMO providers and pharmaceutical innovators, and an enhanced focus on sustainable manufacturing practices and green chemistry implementation. The growing emphasis on emerging markets manufacturing and rising adoption of continuous manufacturing technologies will drive demand for comprehensive CDMO solutions across diverse pharmaceutical development and commercial production requirements.
Topical Drugs CDMO Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 8.0 billion |
| Market Forecast Value (2035) | USD 15.3 billion |
| Forecast CAGR (2025-2035) | 6.7% |
Why is the Topical Drugs CDMO Market Growing?
The topical drugs CDMO market grows by enabling pharmaceutical and biopharmaceutical companies to access specialized manufacturing expertise and achieve production flexibility while optimizing capital allocation without extensive facility investment requirements. Drug developers and pharmaceutical manufacturers face mounting pressure to accelerate product launches and maintain manufacturing quality while managing capacity constraints and regulatory complexity, with modern CDMO partnerships typically providing superior formulation development capabilities, validated manufacturing processes, and comprehensive regulatory documentation compared to building in-house infrastructure, making contract manufacturing adoption essential for efficient pharmaceutical operations.
The pharmaceutical industry's need for specialized dermatology expertise and scalable production capabilities creates demand for comprehensive CDMO services that can provide formulation optimization, ensure regulatory compliance, and support commercial manufacturing without compromising quality standards or market timelines. Increasing pharmaceutical outsourcing trends and dermatology pipeline expansion drive adoption across pharmaceutical development departments, commercial operations, and emerging biotech companies, where manufacturing flexibility has direct impact on product launch success and market competitiveness.
The growing complexity of topical formulations including novel delivery systems and combination products requires specialized expertise beyond typical pharmaceutical manufacturing capabilities. Rising focus on core competencies enables pharmaceutical companies to concentrate on drug discovery and commercialization while leveraging external manufacturing partners for production operations. However, intellectual property concerns and quality control oversight challenges may restrict complete outsourcing adoption among companies requiring proprietary manufacturing knowledge protection and stringent quality assurance for brand reputation preservation.
Segmental Analysis
The market is segmented by formulation, service, end use, and region. By formulation, the market is divided into semi-solid formulations, liquid formulations, solid formulations, and transdermal products. Based on service, the market is categorized into contract manufacturing and contract development.
By end use, the market includes pharmaceutical companies, biopharmaceutical companies, and others. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Formulation, Which Segment Accounts for the Dominant Market Share?
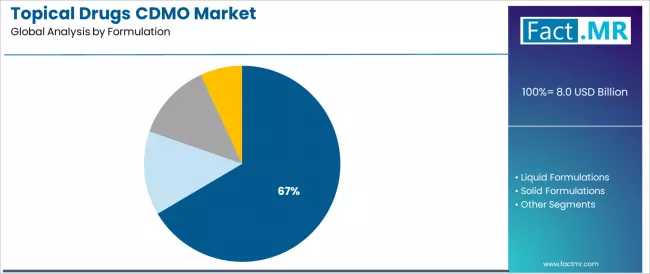
The semi-solid formulations segment represents the dominant force in the topical drugs CDMO market, capturing 66.5% market share in 2025. This established formulation category encompasses solutions featuring versatile drug delivery characteristics and extensive therapeutic applications, including advanced cream formulations, specialized gel systems, and therapeutic ointments that enable superior dermatological drug absorption and patient compliance across diverse skin conditions and treatment protocols worldwide.
The semi-solid formulations segment's market leadership stems from its fundamental role in dermatology therapeutics, with products capable of providing controlled drug release, enhanced skin penetration, and favorable patient acceptability while maintaining excellent stability profiles and manufacturing scalability standards across commercial production requirements.
The liquid formulations segment maintains a substantial 17.0% market share, serving pharmaceutical companies requiring solutions, suspensions, and lotions for specific dermatological applications and patient populations preferring liquid dosage forms. These formulations offer practical advantages for scalp treatments, large body surface applications, and pediatric populations while providing sufficient therapeutic efficacy to support diverse treatment protocols. The liquid segment demonstrates solid market presence, driven by established manufacturing processes and growing demand for spray formulations.
Within the semi-solid category, creams demonstrate particularly strong utilization with 38.0% share of the semi-solid segment, driven by optimal balance between drug delivery efficacy and cosmetic elegance enabling high patient compliance. Gels represent another major sub-segment with 22.0% share supporting targeted drug delivery with enhanced skin feel characteristics, while ointments account for 18.0% through occlusive barrier properties supporting intensive treatment protocols.
Key technical advantages driving the semi-solid formulations segment include:
- Advanced formulation versatility characteristics enabling diverse drug incorporation including hydrophobic and hydrophilic actives across therapeutic categories
- Established manufacturing scalability capabilities allowing efficient production from development batches through commercial volumes without extensive process modifications
- Enhanced patient compliance features enabling optimal cosmetic acceptability and ease of application supporting treatment adherence
- Superior stability profiles providing extended shelf life and predictable drug release characteristics across various storage conditions and climate zones
By Service, Which Segment Accounts for the Largest Market Share?
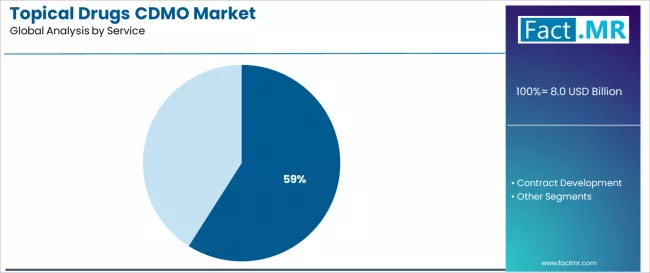
Contract manufacturing dominates the topical drugs CDMO service landscape with approximately 59.0% market share in 2025, reflecting the critical role of production outsourcing in supporting pharmaceutical commercial operations and capacity optimization worldwide. The contract manufacturing segment's market leadership is reinforced by pharmaceutical industry cost pressures, capital allocation optimization, and flexibility requirements necessitating external manufacturing partnerships for established products and market-approved formulations requiring commercial-scale production.
Within this segment, commercial manufacturing represents the core service offering, driven by pharmaceutical companies outsourcing approved product manufacturing to leverage specialized facilities, achieve cost efficiencies, and maintain production flexibility supporting market demand fluctuations. This sub-segment benefits from long-term partnership models and volume-based revenue generation.
The contract development segment represents an important service category with 41.0% market share, demonstrating robust demand through specialized requirements for formulation optimization, analytical method development, and regulatory support enabling pharmaceutical clients to advance pipeline candidates toward commercialization. This segment benefits from innovation-focused partnerships and technology transfer services supporting seamless development-to-manufacturing transitions.
Key market dynamics supporting service growth include:
- Contract manufacturing expansion driven by pharmaceutical outsourcing strategies and capacity optimization requiring flexible production partnerships
- Development service integration enabling comprehensive formulation expertise and regulatory pathway navigation supporting accelerated timelines
- Technology transfer capabilities facilitating efficient scale-up from development through commercial manufacturing with validated processes
- Quality assurance systems providing regulatory compliance and GMP manufacturing standards supporting pharmaceutical client requirements
By End Use, Which Segment Accounts for a Significant Market Share?
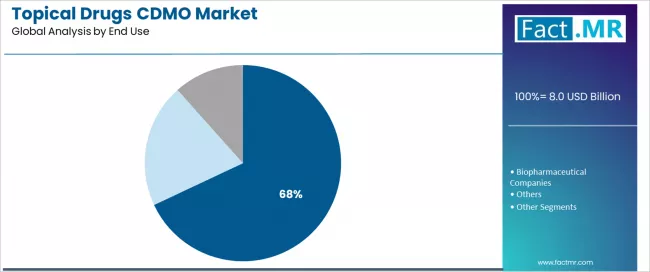
Pharmaceutical companies represent the leading end-use segment in the topical drugs CDMO market with approximately 68.0% market share in 2025, reflecting the extensive outsourcing adoption across established pharmaceutical organizations managing diverse dermatology portfolios. The pharmaceutical companies segment demonstrates consistent demand driven by capacity constraints, cost optimization imperatives, and strategic focus on core competencies including drug discovery and commercialization rather than manufacturing operations.
The biopharmaceutical companies segment emerges as an important end-use category with 24.0% market share in 2025, driven by emerging biotech firms requiring external manufacturing infrastructure and regulatory expertise for specialty dermatology products and innovative formulation platforms. Biopharmaceutical clients leverage CDMO partnerships to access specialized capabilities without capital-intensive facility investments while maintaining development flexibility.
Key end-use dynamics include:
- Pharmaceutical company dominance maintaining strength through established outsourcing strategies and capacity augmentation requirements
- Biopharmaceutical segment growth driving demand for specialized development services and flexible manufacturing supporting pipeline advancement
- Portfolio optimization strategies enabling pharmaceutical companies to focus internal resources on strategic products while outsourcing mature brands
- Risk mitigation emphasis supporting supply chain diversification and manufacturing redundancy through multi-site production networks
What are the Drivers, Restraints, and Key Trends of the Topical Drugs CDMO Market?
The market is driven by three concrete demand factors tied to pharmaceutical efficiency outcomes. First, increasing pharmaceutical outsourcing adoption creates expanding CDMO service demand, with companies seeking cost optimization and capital efficiency requiring comprehensive manufacturing partnerships. Second, growing dermatology drug pipeline and specialty topical approvals drive manufacturing capacity requirements, with novel formulations including oncology topicals and immunomodulators necessitating specialized production expertise by 2030. Third, regulatory complexity and quality requirements enable CDMO value proposition through specialized compliance expertise and validated manufacturing systems that ensure product quality while reducing client regulatory burden and approval timelines.
Market restraints include intellectual property concerns and confidentiality risks that can challenge pharmaceutical company comfort with external manufacturing partnerships, particularly for proprietary formulations and novel delivery technologies requiring protection from competitive intelligence and manufacturing knowledge transfer. Quality control standardization and supply chain reliability pose another significant challenge, as pharmaceutical companies depend on consistent CDMO performance and regulatory compliance, potentially limiting partnership effectiveness when quality systems prove inadequate or manufacturing disruptions impact supply continuity. Regulatory approval delays and technology transfer complexity create additional barriers for efficient outsourcing, demanding extensive validation and regulatory documentation supporting manufacturing site changes and process transfers.
Key trends indicate accelerated Asia Pacific capacity expansion in emerging markets, particularly India and China where cost-competitive manufacturing combines with improving quality standards and regulatory infrastructure supporting global pharmaceutical supply chains. Continuous manufacturing adoption trends toward advanced process technologies enabling real-time quality control and improved manufacturing efficiency that optimize production economics and regulatory compliance. However, the market thesis could face disruption if significant pharmaceutical industry consolidation or major shifts toward in-house manufacturing capabilities reduce external CDMO service demand across established product portfolios.
Analysis of the Topical Drugs CDMO Market by Key Countries
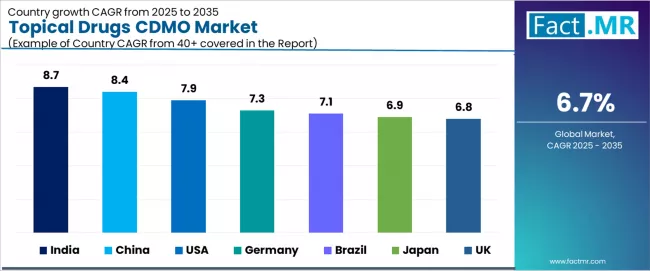
| Country | CAGR (2025-2035) |
|---|---|
| India | 8.7% |
| China | 8.4% |
| USA | 7.9% |
| Germany | 7.3% |
| Brazil | 7.1% |
| Japan | 6.9% |
| UK | 6.8% |
The global topical drugs CDMO market is expanding steadily, with India leading at an 8.7% CAGR through 2035, driven by fastest expanding CDMO base and competitive manufacturing cost advantages. China follows at 8.4%, supported by low-cost manufacturing combined with R&D investment boost. USA records 7.9%, reflecting large patient pool and heavy pharmaceutical outsourcing adoption. Germany advances at 7.3%, anchored by advanced formulation technology demand and quality manufacturing reputation.
Brazil posts 7.1%, focusing on growing cosmetics-therapeutic overlap and regional pharmaceutical expansion, while Japan grows at 6.9%, emphasizing aging population and premium dermatology requirements. UK demonstrates 6.8% growth, driven by strong OTC dermatology uptake and established CDMO infrastructure.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the topical drugs CDMO market with a CAGR of 8.7% through 2035. The country's leadership position stems from rapidly expanding CDMO infrastructure, competitive manufacturing cost advantages, and growing pharmaceutical industry sophistication driving adoption of specialized topical manufacturing services. Growth is concentrated in major pharmaceutical manufacturing hubs, including Hyderabad, Ahmedabad, Mumbai, and Vadodara, where established CDMO facilities expand capacity and upgrade technology to serve global pharmaceutical clients seeking cost-effective production solutions.
Distribution channels through direct pharmaceutical partnerships, international business development offices, and pharmaceutical industry associations expand client accessibility across North American and European markets. The country's fastest expanding CDMO base provides strong momentum for topical manufacturing adoption, including comprehensive regulatory compliance capabilities supporting US FDA, EMA, and other international regulatory authority inspections ensuring global quality standards and market access for pharmaceutical client products manufactured in Indian facilities.
Key market factors:
- Fastest expanding CDMO base concentrated in established pharmaceutical clusters with proven manufacturing expertise and regulatory track records
- Competitive manufacturing advantages through labor cost efficiency and operational expense optimization enabling attractive pricing for pharmaceutical clients
- Regulatory compliance capabilities featuring international GMP standards and successful regulatory inspection histories supporting global market supply
- Technical workforce availability including formulation scientists, quality professionals, and manufacturing specialists supporting service excellence
Why is China Emerging as a High-Growth Market?
In major pharmaceutical manufacturing centers including Shanghai, Jiangsu, Zhejiang, and Beijing, the development of topical drugs CDMO capabilities is accelerating across established facilities and new purpose-built manufacturing sites, driven by pharmaceutical industry modernization and international quality standard adoption. The market demonstrates strong growth momentum with a CAGR of 8.4% through 2035, linked to low-cost manufacturing combined with R&D investment boost supporting comprehensive service capabilities.
Chinese CDMO providers are implementing advanced manufacturing technologies and establishing international quality systems to attract multinational pharmaceutical clients seeking Asia-based production capacity while government pharmaceutical industry support initiatives enable infrastructure investment and technical capability enhancement. The country's massive pharmaceutical market creates substantial domestic demand alongside export manufacturing opportunities, while increasing foreign direct investment in pharmaceutical manufacturing supports technology transfer and best practice adoption elevating Chinese CDMO capabilities toward international parity with established Western providers.
Key development areas:
- Low-cost manufacturing providing competitive pricing advantages and operational efficiency supporting pharmaceutical client cost optimization
- R&D investment boost through government support and private sector funding enabling formulation development capabilities and innovation capacity
- Quality system modernization supporting international GMP compliance and regulatory authority approval for global pharmaceutical supply
- Domestic market growth creating substantial pharmaceutical demand alongside export manufacturing supporting facility utilization optimization
What Drives USA’s Market Resilience?
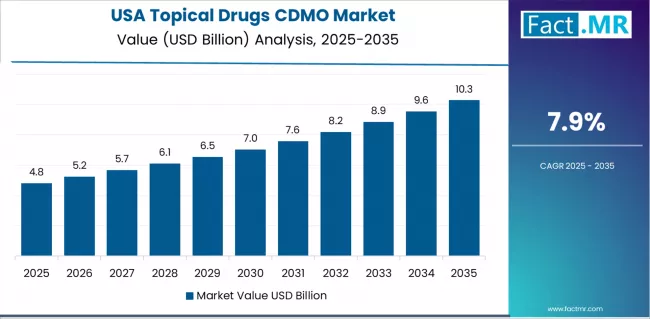
USA’s market expansion is driven by extensive pharmaceutical industry presence, including major brand pharmaceutical companies and emerging specialty pharmaceutical firms requiring domestic and nearshore manufacturing capacity. The country demonstrates steady growth potential with a CAGR of 7.9% through 2035, supported by large patient pool and heavy pharmaceutical outsourcing adoption across dermatology therapeutic categories.
American pharmaceutical companies leverage CDMO partnerships to optimize manufacturing networks, maintain supply chain resilience, and access specialized formulation expertise for complex topical products including transdermal systems and innovative delivery platforms.
The country's established CDMO infrastructure provides comprehensive manufacturing capabilities, while stringent regulatory requirements create demand for experienced partners with proven FDA compliance and inspection readiness. However, higher manufacturing costs compared to emerging markets require value demonstration through specialized capabilities, quality excellence, and regulatory expertise justifying premium pricing for sophisticated formulation development and manufacturing services supporting pharmaceutical client differentiation strategies.
Market characteristics:
- Large patient pool showing substantial dermatology pharmaceutical demand creating commercial manufacturing volume opportunities
- Heavy pharmaceutical outsourcing reflecting industry strategic focus on core competencies and external manufacturing partnership adoption
- Regulatory expertise emphasis providing FDA compliance capabilities and inspection readiness supporting pharmaceutical client regulatory strategies
- Advanced technology capabilities enabling complex formulation development and specialized manufacturing for innovative topical products
How Does Germany Demonstrate Technology Leadership?
The German market leads in advanced formulation technology based on integration with pharmaceutical industry innovation and engineering excellence supporting specialized manufacturing capabilities. The country shows strong potential with a CAGR of 7.3% through 2035, driven by advanced formulation technology demand and quality manufacturing reputation in major regions, including Bavaria, North Rhine-Westphalia, Baden-Württemberg, and Hesse.
German CDMO providers offer sophisticated formulation development services and precision manufacturing for complex topical products requiring specialized expertise including transdermal systems, combination products, and novel delivery platforms. Distribution channels through established pharmaceutical industry relationships and international business development networks expand client accessibility across European and global pharmaceutical markets.
The robust quality management culture ensures consistent manufacturing excellence and regulatory compliance, while engineering expertise supports continuous improvement and process optimization delivering superior pharmaceutical client value through technical innovation and operational excellence.
Leading market segments:
- Advanced formulation technology capabilities enabling complex product development and specialized manufacturing for innovative topical therapies
- Quality manufacturing reputation supporting premium positioning and attracting pharmaceutical clients requiring highest quality standards
- Engineering excellence providing process optimization and continuous improvement supporting manufacturing efficiency and product quality
- Regulatory compliance expertise ensuring comprehensive documentation and inspection readiness for European and international regulatory authorities
What Positions Brazil for Regional Leadership?
Brazil's topical drugs CDMO market demonstrates expanding pharmaceutical manufacturing capabilities focused on regional pharmaceutical industry growth and increasing dermatology-cosmetics convergence supporting specialized topical production. The country maintains solid growth momentum with a CAGR of 7.1% through 2035, driven by growing cosmetics-therapeutic overlap and regional pharmaceutical expansion creating domestic and Latin American market opportunities.
Major pharmaceutical manufacturing centers, including São Paulo, Rio de Janeiro, and Minas Gerais, showcase advancing CDMO capabilities where facilities serve both domestic pharmaceutical companies and international clients seeking Latin American production capacity.
The expanding middle class drives pharmaceutical consumption growth, while cosmetic-pharmaceutical convergence creates opportunities for facilities capable of serving both industries with appropriate quality systems. Brazilian regulatory environment improvements and ANVISA modernization support international pharmaceutical client confidence, while regional trade agreements facilitate pharmaceutical exports across Latin American markets supporting facility utilization and export manufacturing growth.
Key market characteristics:
- Growing cosmetics-therapeutic overlap enabling shared manufacturing capabilities and facility utilization across adjacent product categories
- Regional pharmaceutical expansion supporting domestic industry growth and Latin American export manufacturing opportunities
- Manufacturing infrastructure development through facility upgrades and technology investments enhancing international competitiveness
- Regulatory modernization supporting international standards adoption and pharmaceutical client confidence in Brazilian manufacturing quality
How does Japan Show Premium Quality Leadership?
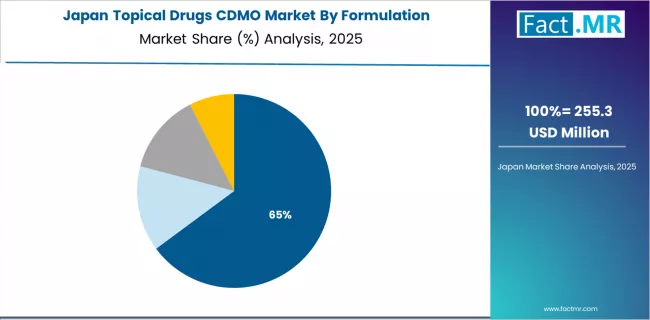
Japan's topical drugs CDMO market demonstrates sophisticated manufacturing excellence focused on premium quality positioning, advanced technology integration, and specialized dermatology expertise supporting domestic and international pharmaceutical clients. The country maintains steady growth momentum with a CAGR of 6.9% through 2035, driven by aging population and premium dermatology requirements creating pharmaceutical demand for specialized topical products. Major pharmaceutical manufacturing regions, including Tokyo, Osaka, Aichi, and Shizuoka, showcase established CDMO capabilities where quality-focused facilities provide comprehensive services from formulation development through commercial manufacturing.
The mature pharmaceutical industry emphasizes quality excellence and regulatory compliance, with Japanese CDMO providers maintaining rigorous manufacturing standards and comprehensive documentation supporting pharmaceutical client requirements. Aging demographics drive dermatology pharmaceutical demand including anti-aging products, wound care formulations, and chronic skin condition treatments, while premium market positioning supports higher-value manufacturing services and specialized formulation expertise commanding premium pricing reflecting superior quality and technical sophistication.
Key market characteristics:
- Aging population driving dermatology pharmaceutical demand and specialized topical product requirements for elderly patient populations
- Premium dermatology emphasis supporting high-value product manufacturing and specialized formulation expertise
- Quality excellence culture ensuring rigorous manufacturing standards and comprehensive quality assurance supporting pharmaceutical client requirements
- Advanced technology integration enabling sophisticated manufacturing processes and analytical capabilities for complex formulation development
What Positions UK for Established Infrastructure Leadership?
The UK's topical drugs CDMO market demonstrates established manufacturing capabilities focused on strong OTC dermatology segment and comprehensive pharmaceutical industry infrastructure supporting domestic and international pharmaceutical clients. The country maintains steady growth momentum with a CAGR of 6.8% through 2035, driven by strong OTC dermatology uptake and established CDMO infrastructure in major regions, including Southeast England, Northwest England, and Scotland.
The UK CDMO providers offer comprehensive services across prescription and OTC topical products, with established facilities serving both domestic pharmaceutical companies and international clients seeking European manufacturing capacity. The robust OTC dermatology market creates substantial manufacturing demand, while post-Brexit regulatory environment requires careful navigation supporting pharmaceutical clients requiring EU and UK market access.
Distribution channels through pharmaceutical industry partnerships and international business development activities expand client accessibility, while established quality systems and regulatory expertise support MHRA compliance and international regulatory authority requirements ensuring pharmaceutical client confidence.
Market development factors:
- Strong OTC dermatology uptake providing substantial manufacturing volume and commercial production opportunities
- Established CDMO infrastructure featuring experienced facilities with proven pharmaceutical manufacturing capabilities
- Regulatory expertise supporting MHRA compliance and international regulatory requirements for pharmaceutical clients
- Pharmaceutical industry integration enabling strong client relationships and comprehensive service offerings
Competitive Landscape of the Topical Drugs CDMO Market
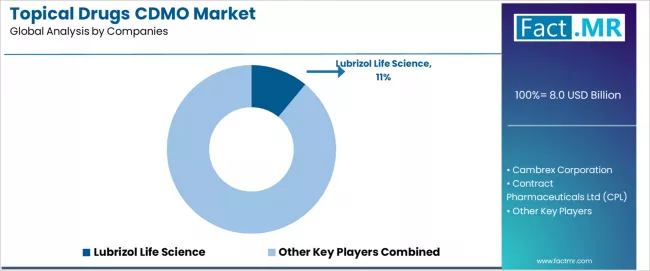
The topical drugs CDMO market features approximately 15-20 meaningful players with moderate concentration, where the top three companies control roughly 20-25% of global market share through established manufacturing infrastructure, specialized formulation expertise, and comprehensive service portfolios. Competition centers on technical capabilities, quality systems, and regulatory track records rather than price competition alone.
Market leaders include Lubrizol Life Science, Cambrex Corporation, and Contract Pharmaceuticals Ltd, which maintain competitive advantages through comprehensive topical formulation expertise, advanced manufacturing facilities, and deep pharmaceutical industry relationships serving diverse client requirements across development and commercial manufacturing, creating high credibility among pharmaceutical and biopharmaceutical clients.
These companies leverage established quality management systems and ongoing capacity investment initiatives to defend market positions while expanding into emerging therapeutic areas including biosimilar topicals and specialty dermatology formulations.
Challengers encompass Bora Pharmaceutical CDMO and Ascendia Pharmaceuticals, which compete through specialized technical capabilities and regional manufacturing presence. Established CDMO providers, including Pierre Fabre Group, Piramal Pharma Solutions, and DPT Laboratories, focus on specific formulation expertise or geographic markets, offering differentiated capabilities in complex formulation development, transdermal systems, and specialized manufacturing technologies.
Regional providers and emerging CDMO companies create competitive pressure through cost-competitive manufacturing and flexible service models, particularly in Asia Pacific markets including India and China where operational efficiency and growing quality standards support global pharmaceutical supply chain integration.
Market dynamics favor companies that combine technical excellence with comprehensive regulatory expertise that addresses the complete development and manufacturing lifecycle from formulation optimization through commercial production and lifecycle management.
Strategic emphasis on continuous manufacturing technologies, sustainable practices, and emerging market capacity expansion enables differentiation in increasingly competitive and globally distributed pharmaceutical CDMO markets across developed and emerging economies.
Global Topical Drugs CDMO Market — Stakeholder Contribution Framework
Topical drugs CDMO services represent a critical pharmaceutical industry enabler that allows drug developers, pharmaceutical companies, and biopharmaceutical firms to access specialized manufacturing expertise and achieve production flexibility while optimizing capital allocation without extensive facility investments, typically providing superior formulation development capabilities, validated manufacturing processes, and comprehensive regulatory support compared to in-house infrastructure development while ensuring accelerated timelines and maintained quality standards.
With the market projected to grow from USD 8.0 billion in 2025 to USD 15.3 billion by 2035 at a 6.7% CAGR, these solutions offer compelling advantages for semi-solid formulation applications, contract manufacturing services, and diverse pharmaceutical client requirements seeking production optimization. Scaling market penetration and service excellence requires coordinated action across regulatory policy, quality standards, CDMO providers, pharmaceutical clients, and technology advancement initiatives.
How Could Governments Spur Local Development and Adoption?
- Manufacturing Infrastructure Support: Provide investment incentives for CDMO facility construction and technology upgrades, establish pharmaceutical manufacturing zones with supportive infrastructure, and create grant programs supporting quality system implementation and regulatory compliance preparation.
- Regulatory Framework Development: Implement streamlined approval processes for manufacturing site changes and technology transfers, establish clear guidance for CDMO regulatory responsibilities, and create regulatory support programs helping emerging market providers achieve international compliance standards.
- Skilled Workforce Development: Fund pharmaceutical manufacturing training programs, support technical education in formulation science and pharmaceutical engineering, and create apprenticeship programs connecting educational institutions with CDMO employers.
- Quality Assurance Standards: Establish national GMP standards aligned with international requirements, create inspection programs ensuring manufacturing quality, and support accreditation systems recognizing compliant facilities and qualified service providers.
- Trade Policy Support: Implement favorable tariff structures for pharmaceutical manufacturing equipment imports, establish intellectual property protections supporting client confidentiality, and create trade agreements facilitating pharmaceutical product movement across international markets.
How Could Industry Bodies Support Market Development?
- Technical Standards Development: Define best practices for topical drug manufacturing, establish formulation development guidelines and analytical method standards, and create benchmarking frameworks supporting quality excellence and continuous improvement.
- Quality Assurance Programs: Develop industry certification programs for CDMO facilities, establish audit protocols and inspection standards, and create knowledge sharing platforms disseminating best practices across service providers.
- Regulatory Guidance: Lead development of regulatory position papers addressing CDMO responsibilities, provide training on regulatory requirements and compliance strategies, and facilitate regulatory authority dialogue supporting clear expectations and consistent oversight.
- Professional Development: Run certification programs for pharmaceutical manufacturing professionals, offer technical training on advanced manufacturing technologies and quality systems, and create networking forums connecting CDMO providers with pharmaceutical clients.
How Could CDMO Providers and Technology Companies Strengthen the Ecosystem?
- Technical Excellence: Develop advanced formulation capabilities addressing complex delivery systems, invest in analytical infrastructure supporting comprehensive characterization, and maintain state-of-the-art manufacturing facilities with appropriate environmental controls and process capabilities.
- Quality Systems: Implement robust quality management systems exceeding regulatory requirements, establish comprehensive documentation practices supporting client regulatory filings, and maintain inspection readiness through regular internal audits and continuous improvement programs.
- Regulatory Expertise: Build comprehensive regulatory intelligence capabilities, provide client support for regulatory submissions and inspections, and maintain direct communication channels with regulatory authorities supporting efficient approval processes.
- Innovation Investment: Develop continuous manufacturing capabilities, implement advanced process analytical technologies, and create sustainable manufacturing practices supporting environmental stewardship and operational efficiency.
How Could Pharmaceutical Companies Navigate the Market?
- Strategic Partnership Development: Establish long-term CDMO relationships supporting portfolio needs, conduct comprehensive capability assessments and quality audits, and create collaborative frameworks enabling efficient technology transfer and ongoing communication.
- Quality Oversight: Implement rigorous supplier qualification programs, maintain active quality agreement management and performance monitoring, and conduct regular audits ensuring sustained compliance and manufacturing excellence.
- Risk Management: Develop supply chain diversification strategies including multiple manufacturing sites, establish contingency plans addressing potential disruptions, and maintain appropriate inventory strategies supporting supply continuity.
- Intellectual Property Protection: Implement comprehensive confidentiality agreements and intellectual property safeguards, conduct technology transfer with appropriate controls protecting proprietary knowledge, and maintain oversight ensuring manufacturing knowledge remains appropriately protected.
How Could Investors and Financial Enablers Unlock Value?
- Capacity Expansion Financing: Provide growth capital for established companies like Lubrizol Life Science and emerging providers to fund facility construction, equipment procurement, and technology implementation supporting client demand.
- Technology Investment: Back companies developing advanced manufacturing technologies including continuous processing systems, automation platforms, and analytical capabilities that enhance operational efficiency and quality assurance.
- Geographic Expansion Support: Finance emerging market CDMO development in India, China, and other regions establishing cost-competitive manufacturing capacity with international quality standards.
- Consolidation Opportunities: Support strategic acquisitions creating comprehensive service networks, enable technology acquisition expanding capability portfolios, and facilitate market consolidation creating scale advantages and geographic coverage optimization.
Key Players in the Topical Drugs CDMO Market
- Lubrizol Life Science
- Cambrex Corporation
- Contract Pharmaceuticals Ltd (CPL)
- Bora Pharmaceutical CDMO
- Ascendia Pharmaceuticals
- Pierre Fabre Group
- Piramal Pharma Solutions
- DPT Laboratories
- MedPharm
- PCI Pharma Services
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 8.0 Billion |
| Formulation | Semi-solid Formulations, Liquid Formulations, Solid Formulations, Transdermal Products |
| Service | Contract Manufacturing, Contract Development |
| End Use | Pharmaceutical Companies, Biopharmaceutical Companies, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | India, China, USA, Germany, Japan, Brazil, UK, and 40+ countries |
| Key Companies Profiled | Lubrizol Life Science, Cambrex Corporation, Contract Pharmaceuticals Ltd (CPL), Bora Pharmaceutical CDMO, Ascendia Pharmaceuticals, Pierre Fabre Group, Piramal Pharma Solutions, DPT Laboratories, MedPharm, PCI Pharma Services |
| Additional Attributes | Dollar sales by formulation and service categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with CDMO service providers and pharmaceutical manufacturing companies, service specifications and capability requirements, integration with pharmaceutical development programs and regulatory compliance frameworks, innovations in continuous manufacturing and sustainable production practices, and development of specialized applications with complex formulation expertise and advanced delivery system capabilities. |
Topical Drugs CDMO Market by Segments
-
Formulation :
- Semi-solid Formulations
- Liquid Formulations
- Solid Formulations
- Transdermal Products
-
Service :
- Contract Manufacturing
- Contract Development
-
End Use :
- Pharmaceutical Companies
- Biopharmaceutical Companies
- Others
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Formulation
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Formulation, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Formulation, 2025 to 2035
- Semi-solid Formulations
- Liquid Formulations
- Solid Formulations
- Transdermal Products
- Y to o to Y Growth Trend Analysis By Formulation, 2020 to 2024
- Absolute $ Opportunity Analysis By Formulation, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Service
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Service, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Service, 2025 to 2035
- Contract Manufacturing
- Contract Development
- Y to o to Y Growth Trend Analysis By Service, 2020 to 2024
- Absolute $ Opportunity Analysis By Service, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Pharmaceutical Companies
- Biopharmaceutical Companies
- Others
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Formulation
- By Service
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Formulation
- By Service
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Formulation
- By Service
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Formulation
- By Service
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Formulation
- By Service
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Formulation
- By Service
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Formulation
- By Service
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Formulation
- By Service
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Formulation
- By Service
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Formulation
- By Service
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Formulation
- By Service
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Formulation
- By Service
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Formulation
- By Service
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Formulation
- By Service
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Formulation
- By Service
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Formulation
- By Service
- By End Use
- Competition Analysis
- Competition Deep Dive
- Lubrizol Life Science
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Cambrex Corporation
- Contract Pharmaceuticals Ltd (CPL)
- Bora Pharmaceutical CDMO
- Ascendia Pharmaceuticals
- Pierre Fabre Group
- Piramal Pharma Solutions
- DPT Laboratories
- MedPharm
- PCI Pharma Services
- Lubrizol Life Science
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Formulation, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Service, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Formulation, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Service, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Formulation, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Service, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Formulation, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Service, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Formulation, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Service, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Formulation, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Service, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Formulation, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Service, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Formulation, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Service, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Formulation, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Formulation, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Formulation
- Figure 6: Global Market Value Share and BPS Analysis by Service, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Service, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Service
- Figure 9: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by End Use
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Formulation, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Formulation, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Formulation
- Figure 26: North America Market Value Share and BPS Analysis by Service, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Service, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Service
- Figure 29: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by End Use
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Formulation, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Formulation, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Formulation
- Figure 36: Latin America Market Value Share and BPS Analysis by Service, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Service, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Service
- Figure 39: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by End Use
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Formulation, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Formulation, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Formulation
- Figure 46: Western Europe Market Value Share and BPS Analysis by Service, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Service, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Service
- Figure 49: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by End Use
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Formulation, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Formulation, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Formulation
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Service, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Service, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Service
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Formulation, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Formulation, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Formulation
- Figure 66: East Asia Market Value Share and BPS Analysis by Service, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Service, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Service
- Figure 69: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by End Use
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Formulation, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Formulation, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Formulation
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Service, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Service, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Service
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Formulation, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Formulation, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Formulation
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Service, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Service, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Service
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the topical drugs CDMO market in 2025?
The global topical drugs CDMO market is estimated to be valued at USD 8.0 billion in 2025.
What will be the size of topical drugs CDMO market in 2035?
The market size for the topical drugs CDMO market is projected to reach USD 15.3 billion by 2035.
How much will be the topical drugs CDMO market growth between 2025 and 2035?
The topical drugs CDMO market is expected to grow at a 6.7% CAGR between 2025 and 2035.
What are the key product types in the topical drugs CDMO market?
The key product types in topical drugs CDMO market are semi-solid formulations, liquid formulations, solid formulations and transdermal products.
Which service segment to contribute significant share in the topical drugs CDMO market in 2025?
In terms of service, contract manufacturing segment to command 59.0% share in the topical drugs CDMO market in 2025.