Allantoin Market
Allantoin Market Size and Share Forecast Outlook 2025 to 2035
Allantoin market is projected to grow from USD 0.7 billion in 2025 to USD 1.3 billion by 2035, at a CAGR of 6.4%. Cosmetics will dominate with a 42.8% market share, while asia pacific will lead the region segment with a 37.2% share.
Allantoin Market Forecast and Outlook 2025 to 2035
The global allantoin market is projected to grow from USD 0.7 billion in 2025 to approximately USD 1.3 billion by 2035, recording an extraordinary absolute increase of USD 0.6 billion over the forecast period. This translates into a total growth of 83.9%, with the market forecast to expand at a compound annual growth rate (CAGR) of 6.4% between 2025 and 2035.
Quick Stats on Allantoin Market
- Allantoin Market Value (2025): USD 0.7 billion
- Allantoin Market Forecast Value (2035): USD 1.3 billion
- Allantoin Market Forecast CAGR (2025 to 2035): 6.4%
- Leading Application in Allantoin Market: Cosmetics (42.8%)
- Leading Region in Allantoin Market: Asia Pacific (37.2%)
- Key Growth Regions in Allantoin Market: Asia Pacific, Europe, and North America
- Key Players in Allantoin Market: Ashland Global Holdings Inc., Clariant AG, Arkema SA, Luotian Guanghui, Lubon Industry, Huanghua Suntime, Rita Corp, EMD Performance Materials
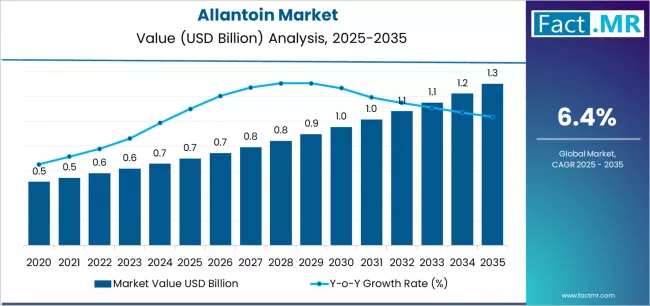
The overall market size is expected to grow by nearly 1.8X during this period, supported by exponential demand for skin-soothing active ingredients, rising adoption of dermatological formulation technologies, and growing emphasis on cosmetic product efficacy enhancement and pharmaceutical ingredient diversification across global personal care operations.
The allantoin market is positioned for substantial expansion, driven by increasing recognition of skin-healing compound importance, growing demand for multifunctional cosmetic actives with enhanced performance standards, and rising adoption of clinically validated ingredients across cosmetic formulation and pharmaceutical development systems globally.
The market demonstrates robust fundamentals supported by expanding skincare product networks, cosmetic formulators' focus on efficacy-driven ingredient protocols and rising recognition of allantoin as a critical active ingredient in achieving enhanced skin regeneration outcomes, irritation reduction capabilities, and moisture retention effectiveness within modern cosmetic technology architectures across diverse personal care applications.
Market growth is underpinned by technological innovations in allantoin synthesis processes, particularly biotechnology-based production techniques and purification technology integration, which offer enhanced ingredient purity, improved skin compatibility profiles, and superior formulation stability with comprehensive clean beauty protocols prevalent in contemporary cosmetic manufacturing practices.
Cosmetic and pharmaceutical producers increasingly prioritize allantoin solutions that deliver optimal balance between dermatological efficacy, formulation versatility, and cost-effectiveness while adhering to increasingly stringent safety standards and regulatory requirements across global beauty markets.
The convergence of premium skincare consumption expansion in developing regions, dermatological product growth in developed economies, and natural ingredient infrastructure development in emerging markets creates multifaceted growth opportunities for allantoin manufacturers and specialty ingredient suppliers.
The cosmetic active ingredient landscape is experiencing transformative changes as formulators adopt sophisticated skin-soothing compounds including synthetic allantoin for pharmaceutical applications, botanical-derived allantoin for natural cosmetics, and innovative encapsulation technologies that enable enhanced ingredient delivery and improved product performance.
These technological advancements are complemented by evolving formulation capabilities encompassing anti-aging cream development for wrinkle reduction, wound healing preparations, and innovative oral care product formulations that significantly improve consumer outcomes and product differentiation.
The integration of clinical validation platforms and dermatological testing protocols further expands credibility of allantoin deployment, particularly benefiting premium cosmetic brands and pharmaceutical manufacturers where ingredient efficacy remains critical for ensuring consumer trust and therapeutic effectiveness.
Between 2025 and 2030, the allantoin market is projected to expand from USD 0.7 billion to USD 0.9 billion, demonstrating strong foundational growth driven by global premium skincare expansion, increasing awareness of dermatological active ingredients, and initial deployment of advanced biotechnology synthesis platforms across cosmetic and pharmaceutical ingredient production. This growth phase establishes market infrastructure, validates allantoin efficacy claims, and creates comprehensive supply chain networks supporting global personal care operations.
From 2030 to 2035, the market is forecast to reach USD 1.3 billion, driven by mature natural cosmetic penetration, next-generation encapsulation technologies requiring sophisticated ingredient delivery systems, and comprehensive integration of clinical validation protocols demanding enhanced dermatological evidence. The growing adoption of clean beauty reformulation programs, along with specialized cosmetic chemist training initiatives, and dermatological product certification expansion will drive demand for comprehensive allantoin solutions with enhanced skin-healing performance outcomes and seamless cosmetic formulation system integration functionality.
Allantoin Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value (2025E) | USD 0.7 billion |
| Forecast Value (2035F) | USD 1.3 billion |
| Forecast CAGR (2025 to 2035) | 6.4% |
Why is the Allantoin Market Growing?
Market expansion is being supported by the exponential increase in premium skincare investment and the corresponding need for clinically validated active ingredients in cosmetic formulation applications across global beauty operations. Cosmetic manufacturers are increasingly focused on allantoin products that can improve skin texture, enhance wound healing, and optimize moisture retention while meeting stringent dermatological safety requirements.
The proven efficacy of allantoin in various skincare applications makes it an essential component of comprehensive cosmetic formulation strategies and dermatological product development programs. The growing emphasis on ingredient transparency standards and efficacy-driven formulation integration is driving demand for scientifically validated allantoin solutions that meet stringent performance specifications and regulatory requirements for cosmetic applications.
Cosmetic manufacturers' preference for reliable, high-performance active ingredients that can ensure consistent dermatological outcomes is creating opportunities for innovative synthesis techniques and customized formulation solutions. The rising influence of clean beauty trends and natural ingredient preferences is also contributing to increased adoption of premium-grade allantoin across different product categories and formulation systems requiring dermatologically tested active ingredients.
Opportunity Pathways - Allantoin Market
The allantoin market represents a transformative growth opportunity, expanding from USD 0.7 billion in 2025 to USD 1.3 billion by 2035 at a 6.4% CAGR. As cosmetic formulators prioritize skin-health optimization, dermatological efficacy validation, and formulation innovation in complex personal care environments, allantoin has evolved from a conventional skin-soothing agent to an essential cosmetic active enabling precise skin regeneration, comprehensive irritation management strategies, and multi-benefit product development operations across premium skincare platforms and pharmaceutical applications.
The convergence of premium beauty consumption acceleration, increasing dermatological ingredient demand penetration, advanced synthesis technology integration, and stringent cosmetic safety mandates creates momentum in demand. High-purity pharmaceutical-grade allantoin offering superior clinical efficacy, cost-effective cosmetic-grade solutions balancing functionality with economics, and specialized formulations for cosmetics applications will capture market premiums, while geographic expansion into high-growth Asian beauty manufacturing markets and emerging K-beauty ecosystems will drive volume leadership. Cosmetic manufacturer emphasis on ingredient innovation and dermatological reliability provides structural support.
- Pathway A - Cosmetics Application Dominance: Leading with 42.8% market share, cosmetics applications drive primary demand through diverse skincare formulation workflows requiring comprehensive skin-soothing functionality for product efficacy optimization. Advanced allantoin grades enabling improved skin healing, enhanced moisture retention, and superior anti-irritant properties command premium pricing from cosmetic manufacturers requiring stringent dermatological specifications and safety compliance. Expected revenue pool: USD 0.3-0.5 billion.
- Pathway B - Pharmaceuticals Application Leadership: Commanding 31.4% market share through optimal balance of therapeutic necessity and clinical validation requirements, pharmaceutical applications serve critical dermatological treatment requirements while meeting stringent regulatory demands. This application category addresses both topical medication needs and wound care expectations, making it the preferred segment for pharmaceutical manufacturers and healthcare product operations seeking comprehensive therapeutic capabilities. Opportunity: USD 0.2-0.4 billion.
- Pathway C - Asian Market Acceleration: India (7.4% CAGR) and South Korea (7.2% CAGR) lead global growth through pharmaceutical ingredient production expansion, K-beauty export infrastructure development, and cosmetic manufacturing capability advancement. Strategic partnerships with local beauty brands, ingredient localization expertise, and formulation optimization enable expansion of allantoin deployment in major cosmetics production and pharmaceutical ingredient hubs. Geographic expansion upside: USD 0.3-0.5 billion.
- Pathway D - Asia Pacific Regional Leadership: Asia Pacific with 37.2% market share serves critical cosmetic manufacturing and pharmaceutical production requirements for diverse product categories. Optimized supply chains supporting multiple beauty markets, ingredient production infrastructure, and proven manufacturing effectiveness maintain significant volumes from cosmetic producers and pharmaceutical ingredient facilities. Revenue potential: USD 0.2-0.5 billion.
- Pathway E - Advanced Synthesis Technologies & Biotechnology Innovation: Companies investing in sophisticated biotechnology-based production, green chemistry synthesis, and advanced purification systems gain competitive advantages through consistent ingredient quality delivery and formulation success. Advanced capabilities enabling customized purity specifications and rapid cosmetic formulation development capture premium beauty brand partnerships. Technology premium: USD 0.1-0.2 billion.
- Pathway F - Supply Chain Optimization & Quality Assurance: Specialized ingredient sourcing networks, strategic quality certification integration, and reliable pharmaceutical-grade production systems create competitive differentiation in cosmetic markets requiring consistent allantoin availability. Companies offering guaranteed ingredient purity, comprehensive technical support services, and regulatory documentation support gain preferred supplier status with quality-focused cosmetic manufacturing operations. Service network value: USD 0.1-0.2 billion.
- Pathway G - Emerging Applications & Market Development: Beyond traditional cosmetic applications, allantoin in veterinary dermatology, plant growth stimulation, and specialized pharmaceutical excipients represent growth opportunities. Companies developing new formulation technologies, supporting application innovation initiatives, and expanding into adjacent healthcare and agricultural markets capture incremental demand while diversifying revenue streams. Emerging opportunity: USD 0.08-0.1 billion.
Segmental Analysis
The market is segmented by application and region. By application, the market is divided into cosmetics, pharmaceuticals, oral hygiene, and others. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and MEA.
Which Application Area Dominates in the Global Allantoin Market?
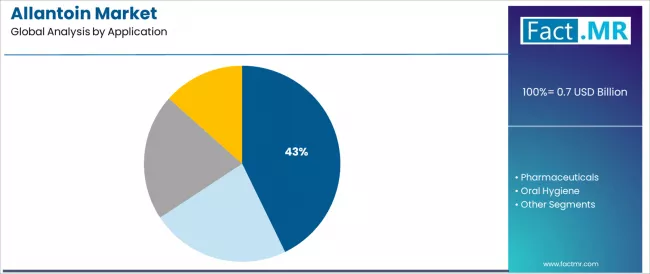
The cosmetics application segment is projected to account for 42.8% of the allantoin market in 2025, reaffirming its position as the category's dominant end-use specification. Cosmetic formulators increasingly recognize the optimal balance of skin-soothing efficacy and formulation versatility offered by allantoin for skincare product applications, particularly in anti-aging formulations and sensitive skin care product environments.
This application category addresses both dermatological effectiveness requirements and consumer appeal demands while providing reliable performance outcomes across diverse cosmetic manufacturing operations. This segment forms the foundation of most premium skincare protocols for active ingredient utilization and product differentiation, as it represents the most extensively researched and commercially established application category in the allantoin industry.
Clinical validation standards and extensive dermatological testing continue to strengthen confidence in allantoin-containing cosmetics among beauty brands and product development professionals. With increasing recognition of active ingredient impact on product efficacy and consumer satisfaction requirements, allantoin-based cosmetics align with both current beauty industry practices and clean formulation evolution goals, making them the central growth driver of comprehensive skincare strategies across multiple product platforms.
Cosmetics applications demonstrate broad functionality across diverse product types, providing skin cell regeneration promotion, keratolytic properties, and anti-inflammatory benefits that enhance product performance. The ingredient's compatibility with natural beauty positioning and dermatologically tested product claims further strengthens market position, as consumers increasingly demand scientifically validated active ingredients in premium skincare formulations.
Why do Pharmaceutical Applications Command a Significant Share?
Pharmaceutical applications are projected to represent 31.4% of allantoin demand in 2025, underscoring its role as a critical therapeutic application category driving clinical product adoption and pharmaceutical formulation deployment. Pharmaceutical manufacturers recognize that therapeutic requirements, including wound healing acceleration, ulcer treatment protocols, and comprehensive dermatological intervention needs, provide substantial addressable market demand that consumer cosmetics cannot match in clinical validation and therapeutic efficacy.
Allantoin utilized in pharmaceutical production offers enhanced healing capabilities and proven therapeutic compatibility essential for serving topical medication formulation and dermatological treatment requirements. The segment is supported by the expanding nature of global dermatological pharmaceutical consumption, requiring active ingredients capable of addressing skin regeneration promotion, anti-inflammatory intervention, and wound healing enhancement applications, and the increasing recognition that clinically validated compounds can improve patient outcomes and therapeutic success.
Manufacturers are increasingly adopting evidence-based formulation strategies that mandate pharmaceutical-grade allantoin for optimal therapeutic response and regulatory compliance. As understanding of dermatological pharmacology advances and therapeutic standards become more sophisticated, clinically validated allantoin ingredients will continue to play a crucial role in comprehensive pharmaceutical product differentiation strategies.
Pharmaceutical applications benefit from premium pricing structures and specialized regulatory frameworks associated with therapeutic products, where allantoin serves as a foundational active ingredient for achieving desired clinical outcomes. The segment's strength reflects both the essential therapeutic role of skin-healing compounds in modern dermatology and the rigorous quality standards that pharmaceutical-grade allantoin must meet across diverse therapeutic applications.
What are the Prospects for the Allantoin Market in the Asia Pacific?

Asia Pacific is projected to account for 37.2% of the allantoin market share in 2025, establishing its position as the leading geographic market segment. Regional cosmetic manufacturers increasingly recognize that Asia Pacific markets, encompassing diverse beauty industry ecosystems affecting multiple product categories, represent the most economically significant and commercially dynamic region requiring specialized ingredient supply due to manufacturing concentration and consumer demand growth. This regional category addresses both local production requirements and export market demands while delivering critical supply chain outcomes across varied cosmetic manufacturing systems.
Growth is supported by the continuing nature of beauty product consumption expansion in both developed and emerging Asian markets, driven by K-beauty innovation leadership, Japanese skincare excellence, and Chinese cosmetic manufacturing scale enabling comprehensive ingredient demand. Regional manufacturers are increasingly focusing on advanced ingredient sourcing protocols that enhance product quality and formulation effectiveness while maintaining competitive cost structures.
As cosmetic manufacturing expertise expands and beauty market sophistication grows, Asia Pacific-based allantoin production and consumption will continue to serve a crucial role in ensuring global ingredient supply and market development within the international cosmetic ingredient market. Asia Pacific regional leadership provides manufacturing efficiency advantages and proximity to rapidly growing beauty consumer markets that differentiate this region from Western markets. The geographic concentration reflects both the extensive cosmetic production capacity located in Asian countries and the dynamic consumer demand for skincare products incorporating proven active ingredients across diverse Asian beauty markets.
What are the Drivers, Restraints, and Key Trends of the Allantoin Market?
The allantoin market is advancing rapidly due to increasing recognition of dermatological active ingredient importance and growing demand for scientifically validated cosmetic compounds across the beauty and pharmaceutical sectors.
The market faces challenges, including raw material price volatility, competition from alternative skin-soothing ingredients, and formulation complexity in natural beauty products requiring multiple active ingredient combinations. Innovation in biotechnology synthesis and advanced encapsulation technologies continues to influence ingredient development and market expansion patterns.
Proliferation of Advanced Biotechnology Production and Green Chemistry
The accelerating adoption of sophisticated biotechnology platforms is enabling the development of more sustainable allantoin production processes and ingredient specifications that can meet stringent environmental and quality requirements.
Cosmetic manufacturers demand comprehensive sustainability credentials for active ingredients, including renewable production methods and biodegradable formulation characteristics that are particularly important for achieving clean beauty positioning requirements in premium cosmetic applications.
Advanced biotechnology production provides access to high purity allantoin that can optimize environmental sustainability strategies and enhance ingredient quality outcomes while maintaining cost-effectiveness for diverse cosmetic manufacturing environments.
Integration of Clinical Validation Platforms and Dermatological Testing
Modern cosmetic organizations are incorporating advanced technologies such as in-vitro testing systems, clinical efficacy evaluation capabilities, and dermatological safety assessment interfaces to enhance allantoin credibility and product performance claims.
These systems improve ingredient validation, enable seamless conventional cosmetics-dermatological product transitions, and provide better integration between cosmetic chemists and dermatological experts throughout the formulation development and commercialization experience.
Advanced clinical testing capabilities also enable substantiated marketing claims and early identification of formulation optimization opportunities or safety concerns, supporting proactive product development management and improved consumer confidence outcomes.
Analysis of the Allantoin Market by Key Countries

| Country | CAGR (2025-2035) |
|---|---|
| India | 7.4% |
| South Korea | 7.2% |
| China | 7.0% |
| Japan | 6.7% |
| Brazil | 6.5% |
| USA | 6.2% |
| Germany | 6.1% |
The allantoin market continues to expand across major economies, supported by variable demand patterns linked to pharmaceutical intermediates, cosmetic formulation requirements, and evolving personal care manufacturing ecosystems. India’s 7.4% CAGR through 2035 reflects rising capacity in active ingredient production, broader formulation diversification, and a steady increase in domestic personal care consumption across industrial hubs. South Korea’s 7.2% CAGR is shaped by sustained K-beauty export momentum, advanced skincare R&D capabilities, and modernization of cosmetic ingredient platforms.
China’s 7.0% growth outlook is underpinned by large-scale cosmetics manufacturing infrastructure, high-volume ingredient synthesis, and broader supply chain consolidation. Japan, growing at 6.7%, maintains strong traction through premium skincare development, dermatology-focused innovation, and a stable consumer base favouring functional cosmetic ingredients. Brazil’s 6.5% CAGR reflects expanding oral hygiene segment activity and a maturing personal care sector with increasing formulation sophistication.
The USA, at 6.2% growth, benefits from extensive cosmetic R&D leadership, regulatory-aligned product development, and formulary advancement across skincare and haircare applications. Germany’s 6.1% CAGR is driven by dermatology-led product adoption, pharmaceutical-grade ingredient validation, and a consistent emphasis on high-quality cosmetic formulations.
How does India Demonstrate Exceptional Market Potential with Pharmaceutical Production Expansion?
The allantoin market in India is projected to grow at a 7.4% CAGR through 2035, supported by rapid expansion in pharmaceutical ingredient manufacturing and stronger demand for functional actives in therapeutic formulations. The country’s evolving pharmaceutical ingredient ecosystem, combined with rising reliance on APIs for large-scale generic production, is strengthening the need for consistent, high-purity allantoin across topical, dermatological, and wound-care applications. Growing domestic consumption in personal care, skincare, and OTC categories further supports ingredient uptake across major industrial regions.
India is also witnessing accelerated investment from international ingredient suppliers and domestic chemical producers, who are establishing integrated facilities to serve pharmaceutical manufacturers, cosmetic formulators, and personal care brands. These facilities focus on enhanced synthesis technologies, purity consistency, and formulation support systems to meet the needs of expanding production hubs across Gujarat, Maharashtra, Telangana, and Tamil Nadu. As formulation diversification expands, allantoin is increasingly deployed in emollient-based skincare, medicated creams, and high-volume personal care products.
Government policies centered on strengthening pharmaceutical manufacturing, promoting self-reliance in APIs, and upgrading cosmetic product quality standards continue to shape the market environment. Supportive frameworks are improving quality assurance infrastructures, enabling advanced process optimization, and incentivizing manufacturers to scale pharmaceutical-grade allantoin production. Combined with the country’s strong generic drug export base and rising premium skincare consumption, these dynamics provide fertile conditions for sustained market expansion.
Strategic Considerations:
- Government-led pharmaceutical and cosmetic manufacturing initiatives strengthen active ingredient demand
- Capacity expansion and improved chemical synthesis enable broader allantoin utilization nationwide
- Ingredient importance in formulation science drives new pharmaceutical and cosmetic opportunities
- Export-driven pharmaceutical investment and dermatological active awareness accelerate adoption
What Makes South Korea Demonstrate Market Leadership with K-Beauty Ecosystem?

The allantoin market in South Korea is expanding at a 7.2% CAGR, supported by the country’s dominant K-beauty export ecosystem, strong formulation science culture, and rapid adoption of dermatological actives across premium and mass-market skincare ranges. The market benefits from global recognition of K-beauty efficacy standards, which continue to drive demand for clinically proven ingredients with functional performance characteristics such as exfoliation support, barrier repair, and irritation reduction. As beauty exports grow, allantoin increasingly supports multi-step skincare systems and new product innovations.
South Korea’s cosmetic manufacturers operate within a highly advanced formulation ecosystem, leveraging R&D excellence, strong ingredient testing protocols, and a high degree of consumer sophistication. These factors enable efficient integration of allantoin into serums, moisturizers, functional cleansers, and sensitive-skin formulations. Partnerships between domestic cosmetic houses and international active-ingredient suppliers further support expansion, enabling alignment between global ingredient trends and domestic product innovation cycles.
Additionally, investments in cosmetic science education, dermatological research, and new clinical validation standards are reinforcing the country’s ability to bring technically advanced skincare solutions to market. As consumers increasingly prioritize science-backed formulations, manufacturers are incorporating allantoin to strengthen efficacy claims, optimize product performance, and differentiate within competitive personal care categories. These developments ensure that allantoin remains integral to South Korea’s position as a global beauty innovation leader.
Strategic Considerations:
- Rising ingredient efficacy standards enhance demand for high-quality allantoin
- Expanding export volumes strengthen deployment of dermatological actives
- Cosmetic science development drives innovative applications in premium skincare
- Advanced formulation capabilities reinforce global K-beauty competitiveness
Why does China Maintain Cosmetic Manufacturing Leadership?
China’s allantoin market is projected to grow at a 7.0% CAGR through 2035, driven by the country’s large-scale cosmetics manufacturing capacity, high-volume ingredient production, and rising domestic consumption of skincare and personal care products. As China consolidates its role as a global manufacturing centre, demand for active ingredients that support moisturizing, soothing, and skin-repair benefits continues to rise. Allantoin plays a key functional role in both mass-market and mid-range cosmetic segments, where cost efficiency and consistent performance are essential.
The country’s extensive industrial infrastructure enables optimized ingredient sourcing, large-batch production consistency, and streamlined integration of actives into large-volume product lines. This structure supports competitive pricing for cosmetics manufacturers across regions such as Guangdong, Zhejiang, Jiangsu, and Shanghai. Growing awareness of ingredient safety, product quality, and regulatory compliance is also influencing manufacturers to prioritize reliable domestic supply chains, including pharmaceutical grade allantoin for specialized applications.
China’s investment in cosmetic science, chemical synthesis technologies, and quality control systems is gradually shifting the market towards higher-value formulations. As the government strengthens regulatory requirements for cosmetic ingredients—particularly under the Cosmetic Supervision and Administration Regulation (CSAR)—manufacturers are adopting more refined quality specifications. These improvements create opportunities for premium allantoin grades and reinforce China’s competitive positioning in the broader Asian active ingredient market.
Strategic Considerations:
- Mass-market cosmetics and pharmaceutical segments dominate demand
- Expanding quality standards are diversifying allantoin portfolios
- Chemical synthesis expertise enhances competitive advantage
- Strengthening regulations guide ingredient specifications and technology adoption
How Does Japan Maintain Premium Skincare Leadership?

Japan’s allantoin market maintains a 6.7% CAGR through 2035, supported by the country’s premium skincare focus, advanced formulation science, and longstanding dermatological research culture. The Japanese beauty sector relies heavily on clinically validated, high-purity ingredients, making allantoin a valuable component in formulations designed for sensitive skin, anti-inflammatory benefits, and skin-repair outcomes. This aligns with Japan’s tradition of prioritizing gentleness, safety, and long-term skin health.
Manufacturers in Japan emphasize pharmaceutical-grade purity, consistent ingredient performance, and integration with extensive testing protocols. Allantoin is increasingly utilized in specialized dermatological lines, cosmeceuticals, and high-end moisturization and repair products. Tokyo, Osaka, and Kanagawa host a concentration of premium formulation laboratories, where active ingredients undergo rigorous evaluation to meet strict domestic quality standards and internationally recognized certification requirements.
Japan’s market also benefits from mature beauty infrastructure, clinical validation pathways, and consumer expectations centred on trust, efficacy, and ingredient transparency. Investments in dermatological research, controlled clinical trials, and formulation optimization continue to reinforce the role of allantoin in premium skincare. This scientific orientation enables manufacturers to innovate while maintaining Japan’s reputation for high-performance beauty technologies.
Strategic Considerations:
- Premium skincare leadership drives demand for high-purity, clinically validated allantoin
- Quality expectations foster movement from cosmetic-grade to pharmaceutical-grade platforms
- Dermatological research capabilities support active ingredient innovation
- Rigorous standards ensure ingredient purity and superior formulation outcomes
What drives Brazil’s Market Growth with Oral Hygiene Sector Expansion?
Brazil’s allantoin market is expanding at a 6.5% CAGR through 2035, supported by rapid growth in oral hygiene, rising personal care consumption, and ongoing formalization of cosmetic formulation practices. Demand for allantoin is increasing in toothpaste, oral gels, mouth ulcer treatments, and general skincare products due to its soothing and epithelial-supporting properties. These attributes align well with Brazil’s rising focus on functional hygiene products and broader wellness-driven consumer behaviour.
Brazilian manufacturers are investing in multifunctional ingredients that offer both performance and affordability, a key criteria for the country’s diverse consumer base. Allantoin’s compatibility with large-volume production, stability across formulations, and value-aligned benefits support its adoption across personal care and oral hygiene brands. Growth in mid-range skincare offerings and expansion of local manufacturing capacity also enhance ingredient demand.
The country’s improving cosmetic science expertise, regulatory structuring under ANVISA, and growth of domestic personal care brands are helping standardize ingredient selection and formulation quality. Manufacturers seeking reliable, efficacy-driven actives are incorporating allantoin into broader product portfolios, supporting category expansion across drugstores, supermarkets, and professional care channels.
Strategic Considerations:
- Oral hygiene and personal care lead demand for multi-functional ingredients
- Consumer needs drive diversification into skincare and oral care formulations
- Growing manufacturing expertise strengthens sourcing and formulation efficiency
- Preference for proven efficacy supports broader allantoin adoption
How does the USA Maintain Cosmetic R&D Leadership?

The USA’s allantoin market maintains a 6.2% CAGR through 2035, driven by advanced cosmetic R&D programs, strong dermatological research infrastructure, and high consumer expectations for clinically validated products. The country’s beauty and pharmaceutical industries rely on actives with documented safety and efficacy, making allantoin a consistent component in repair creams, dermocosmetics, OTC treatments, and specialized skincare products.
U.S. manufacturers benefit from deep scientific expertise, robust regulatory frameworks, and well-established formulation research hubs across California, New York, New Jersey, and Massachusetts. Allantoin is integrated into formulations supported by stability testing, dermatological assessments, and alignment with FDA and OTC monographs. These processes reinforce the use of high purity allantoin and ensure that ingredient performance aligns with market and regulatory expectations.
Strong investment in formulation innovation, biotechnology, clinical testing, and dermatological science continues to drive new applications for functional ingredients. As manufacturers expand into barrier repair, eczema-care, sensitive-skin products, and multipurpose skincare, allantoin remains a preferred active due to its safety profile, compatibility with modern carriers, and clinical documentation supporting skin-soothing efficacy.
Strategic Considerations:
- Premium cosmetic and therapeutic segments prioritise clinically validated actives
- Consumer expectations drive adoption of higher-grade, performance-oriented ingredients
- R&D leadership supports advanced formulation of allantoin-based solutions
- Regulatory demand ensures consistent quality and product reliability
What drives Market Growth in Germany with Dermatological Product Excellence?
Germany’s allantoin market grows at a 6.1% CAGR through 2035, supported by the country’s strong pharmaceutical manufacturing tradition, dermatological research excellence, and rigorous product quality frameworks. Allantoin is a key ingredient in therapeutic skincare, OTC dermatology products, wound-care formulations, and regulated cosmetic applications, where safety and efficacy remain paramount. German manufacturers continue to prioritize pharmaceutical-grade actives to maintain alignment with stringent European quality and safety regulations.
The country benefits from a mature healthcare and pharmaceutical ecosystem, enabling efficient integration of allantoin into therapeutic dermocosmetic formulations. Manufacturers across regions such as Baden-Württemberg, North Rhine-Westphalia, and Bavaria operate with advanced analytical capabilities, enabling consistent ingredient validation and compliance with EMA and EU cosmetic standards. This contributes to high adoption of allantoin in formulations requiring clinically supported outcomes.
Germany’s focus on research-driven skincare development and expanding interest in dermatological wellness continue to shape ingredient demand. As consumers prioritize scientifically supported products, manufacturers enhance formulation complexity, adopting ingredients like allantoin to expand therapeutic performance, moisture-barrier protection, and skin regeneration outcomes. These dynamics reinforce Germany’s position as a key European hub for dermatology-aligned ingredient innovation.
Strategic Considerations:
- Dermatological and pharmaceutical applications drive clinical-grade ingredient demand
- Regulatory frameworks enforce high purity and therapeutic performance standards
- Chemical and pharmaceutical manufacturing expertise sustains innovation
- Manufacturer priorities focus on therapeutic reliability and regulatory validation
Europe Market Split by Country
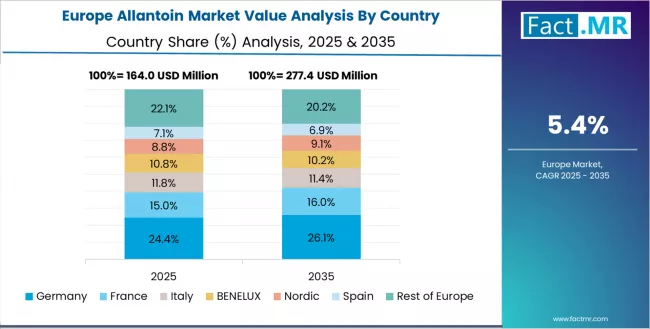
The allantoin market in Europe is projected to expand from USD 0.1 billion in 2025 to USD 0.3 billion by 2035, registering a CAGR of 6.4% over the forecast period. Growth reflects the region’s strong pharmaceutical manufacturing base, advancements in dermatology-focused ingredient development, and sustained demand for cosmetic actives within established personal care markets. Structural investments in formulation science, regulatory compliance, and ingredient quality assurance continue to reinforce Europe’s position as a significant contributor to global allantoin consumption.
Germany is expected to maintain its leading position with a 32.8% share in 2025, increasing to 34.2% by 2035. This dominance is supported by the country’s advanced pharmaceutical production infrastructure, well-developed dermatological research networks, and high standards for cosmetic ingredient quality. These factors enable strong integration of allantoin into therapeutic skincare, pharmaceutical formulations, and premium cosmetic applications across major industrial regions.
France follows with a 26.4% share in 2025, rising to 27.1% by 2035, driven by the strength of its luxury cosmetic industry, strong pharmaceutical ingredient capabilities, and growing sophistication in premium beauty formulations serving domestic and wider European markets. The UK is projected to increase its share from 19.3% in 2025 to 20.1% by 2035, supported by comprehensive pharmaceutical manufacturing capacities, established personal care production clusters, and sustained interest in dermatology-oriented skincare.
Italy holds a 12.7% share in 2025, expected to moderate to 11.4% by 2035, reflecting steady but comparatively slower expansion within its cosmetic and pharmaceutical ingredient sectors. Spain accounts for 6.8% in 2025, moving to 6.1% by 2035, influenced by moderate growth in personal care manufacturing and increasing competition from larger European production hubs.
The rest of Europe, including Nordic countries with high pharmaceutical standards, Eastern European markets developing cosmetic production capabilities, and smaller Western European centres specializing in select ingredient categories, is projected to hold 2.0% of the market in 2025, declining slightly to 1.1% by 2035. This shift reflects continued consolidation toward larger, mature markets with established pharmaceutical and cosmetic ingredient infrastructure.
Competitive Landscape of the Allantoin Market

The allantoin market is characterized by intense competition among established chemical synthesis companies, specialized cosmetic ingredient manufacturers, and comprehensive active ingredient organizations focused on delivering high-quality, reliable, and dermatologically effective allantoin solutions.
Companies are investing in production technology programs, advanced purification development, strategic cosmetic brand partnerships, and comprehensive technical support initiatives to deliver effective, efficient, and reliable allantoin that meets stringent pharmaceutical and cosmetic standards and customer quality expectations. Product standardization, purity optimization, and regulatory compliance strategies are central to strengthening product portfolios and market presence.
Ashland Global Holdings Inc. leads the market with a 17.1% market share, offering comprehensive allantoin solutions with a focus on pharmaceutical-grade ingredient expertise and advanced production capabilities for cosmetic and therapeutic applications. Clariant AG provides specialized chemical ingredient platforms with emphasis on cosmetic formulation support and comprehensive application development across international beauty markets. Arkema SA focuses on active ingredient technologies and comprehensive cosmetic compound solutions serving personal care industries. Luotian Guanghui delivers cost-effective allantoin production with strong Asian market presence and manufacturing integration.
Lubon Industry operates with a focus on bringing large-scale chemical synthesis to ingredient markets and industrial applications. Huanghua Suntime provides specialized allantoin manufacturing emphasizing production efficiency and comprehensive supply capabilities. Rita Corp specializes in cosmetic active ingredient distribution and formulation support with emphasis on technical service delivery. EMD Performance Materials delivers specialty chemical technologies to enhance cosmetic applications and provide comprehensive ingredient solutions through dedicated production expertise strategies focused on pharmaceutical and cosmetic grade allantoin manufacturing excellence.
Key Players in the Allantoin Market
- Ashland Global Holdings Inc.
- Clariant International Ltd.
- AArkema S.A.
- Luotian Guanghui
- Lubon Industry
- Huanghua Suntime
- Rita Corp
- EMD Performance Materials (Merck KGaA)
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 0.7 Billion |
| Application | Cosmetics, Pharmaceuticals, Oral Hygiene, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, MEA |
| Countries Covered | USA, Germany, UK, Japan, India, China, Brazil, South Korea and 40+ countries |
| Key Companies Profiled | Ashland Global Holdings Inc., Clariant, Akema, Luotian Guanghui, Lubon Industry, Huanghua Suntime, Rita Corp, EMD Performance Materials |
| Additional Attributes | Dollar sales by application, regional demand trends, competitive landscape, cosmetic manufacturer preferences for specific allantoin grades, integration with comprehensive formulation systems, innovations in synthesis technology development, purification capability advancement, and dermatological efficacy optimization capabilities |
Allantoin Market by Segments
-
Application :
- Cosmetics
- Pharmaceuticals
- Oral Hygiene
- Others
-
Region :
-
Asia Pacific
- China
- India
- Japan
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
-
Europe
- Germany
- France
- UK
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
-
North America
- USA
- Canada
- Mexico
-
Latin America
- Brazil
- Argentina
- Chile
- Rest of Latin America
-
MEA
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Countries
- Rest of MEA
-
Table of Content
- Executive Summary
- USA Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- USA Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- USA Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- Cosmetics
- Pharmaceuticals
- Oral Hygiene
- Others
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- Asia Pacific
- North America
- Europe
- Latin America
- MEA
- Y to o to Y Growth Trend Analysis By Region, 2020 to 2024
- Absolute $ Opportunity Analysis By Region, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- USA
- Market Attractiveness Analysis By Region
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- By Application
- By Region
- Market Attractiveness Analysis
- By Country
- By Application
- By Region
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Application
- By Region
- Competition Analysis
- Competition Deep Dive
- Ashland Global Holdings Inc.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Clariant International Ltd.
- AArkema S.A.
- Luotian Guanghui
- Lubon Industry
- Huanghua Suntime
- Rita Corp
- EMD Performance Materials (Merck KGaA)
- Ashland Global Holdings Inc.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 3: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 4: USA Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 6: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
List Of Figures
- Figure 1: USA Market Pricing Analysis
- Figure 2: USA Market Value (USD Million) Forecast 2020-2035
- Figure 3: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 4: USA Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 5: USA Market Attractiveness Analysis by Application
- Figure 6: USA Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 7: USA Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 8: USA Market Attractiveness Analysis by Region
- Figure 9: USA Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: USA Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 11: USA Market Attractiveness Analysis by Region
- Figure 12: USA Market Incremental Dollar Opportunity, 2025-2035
- Figure 13: USA Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 14: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 15: USA Market Y to o to Y Growth Comparison by Application, 2025-2035
- Figure 16: USA Market Attractiveness Analysis by Application
- Figure 17: USA Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 18: USA Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 19: USA Market Attractiveness Analysis by Region
- Figure 20: USA Market - Tier Structure Analysis
- Figure 21: USA Market - Company Share Analysis
- FAQs -
How big is the allantoin market in 2025?
The global allantoin market is estimated to be valued at USD 0.7 billion in 2025.
What will be the size of allantoin market in 2035?
The market size for the allantoin market is projected to reach USD 1.3 billion by 2035.
How much will be the allantoin market growth between 2025 and 2035?
The allantoin market is expected to grow at a 6.4% CAGR between 2025 and 2035.
What are the key product types in the allantoin market?
The key product types in allantoin market are cosmetics, pharmaceuticals, oral hygiene and others.
Which region segment to contribute significant share in the allantoin market in 2025?
In terms of region, asia pacific segment to command 37.2% share in the allantoin market in 2025.