Arbovirus Testing Market
Arbovirus Testing Market Size and Share Forecast Outlook 2025 to 2035
Arbovirus testing market is projected to grow from USD 1.2 billion in 2025 to USD 1.8 billion by 2035, at a CAGR of 4.6%. ELISA-based Tests will dominate with a 4870.0% market share, while diagnostic laboratories will lead the end use segment with a 5250.0% share.
Arbovirus Testing Market Forecast and Outlook 2025 to 2035
The global arbovirus testing market is projected to grow from USD 1.15 billion in 2025 to approximately USD 1.80 billion by 2035, recording an absolute increase of USD 0.65 billion over the forecast period. This represents total growth of 56.5%, with the market expected to expand at a CAGR of around 4.6% between 2025 and 2035.
The market trajectory reflects the growing emphasis on vector-borne disease diagnostics, improved outbreak surveillance, and strengthened public health infrastructure, particularly in tropical and subtropical regions where arboviral infections remain endemic. The expansion of molecular and immunodiagnostic technologies continues to enhance clinical detection accuracy and epidemiological monitoring capabilities across both developed and emerging healthcare systems.
Quick Stats for Arbovirus Testing Market
- Arbovirus Testing Market Value (2025): USD 1.15 billion
- Arbovirus Testing Market Forecast Value (2035): USD 1.80 billion
- Arbovirus Testing Market Forecast CAGR: 4.6%
- Leading Test Type in Arbovirus Testing Market: ELISA-based Tests
- Key Growth Regions in Arbovirus Testing Market: North America, Europe, and Asia Pacific
- Top Players in Arbovirus Testing Market: Abbott Laboratories, Thermo Fisher Scientific Inc., Merck KGaA, F. Hoffmann-La Roche Ltd, Siemens Healthineers AG

Between 2025 and 2030, the arbovirus testing market is projected to rise from USD 1.15 billion to USD 1.43 billion, adding USD 0.28 billion in value and accounting for approximately 43% of total forecast growth. This phase will be characterized by increasing deployment of real-time polymerase chain reaction (RT-PCR) systems, driven by the growing need for high-sensitivity detection of viruses such as dengue, Zika, chikungunya, and West Nile.
Public health agencies and diagnostic laboratories are adopting multiplex PCR platforms capable of simultaneous detection of multiple viral strains, improving testing efficiency during outbreak conditions. Continuous technological innovation, including automated sample preparation and miniaturized thermal cycling systems, is enhancing diagnostic throughput and turnaround times. Regional governments in Asia, Africa, and Latin America are expanding laboratory networks and investing in molecular diagnostic infrastructure to strengthen early warning systems for arboviral outbreaks.
From 2030 to 2035, the market is expected to expand further, from USD 1.43 billion to USD 1.80 billion. This period will be marked by the mainstream adoption of point-of-care (POC) testing platforms and integration of rapid antigen and antibody-based diagnostics into community-level healthcare systems. The emergence of connected testing devices and data integration tools will enable real-time case reporting and cross-border disease tracking, aligning with global efforts to improve epidemic preparedness.
Diagnostic innovation will focus on high-specificity assays and portable biosensor systems, supporting decentralized testing in remote and resource-limited areas. Increasing partnerships between public health agencies, research institutions, and diagnostic manufacturers will help scale capacity for surveillance and field diagnostics, particularly during vector-borne disease outbreaks linked to climate and environmental change.
Between 2020 and 2025, the market experienced consistent progress as diagnostic technologies evolved beyond conventional serological assays toward molecular-based systems offering improved accuracy and specificity. The rise of multiplexed immunoassays and nucleic acid amplification tests strengthened laboratory capabilities for differentiating co-circulating arboviruses, an essential requirement for regions with overlapping transmission patterns. Research initiatives during this period focused on assay standardization, biosafety improvements, and cost-efficient detection kits for low-resource settings.
Where revenue comes from - Now Vs Next (industry-level view)
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | ELISA-based diagnostic tests | 48.7% | Reliable, affordable, broad implementation |
| RT-PCR molecular tests | 37.5% | High sensitivity, RNA detection focus | |
| Other testing technologies | 13.8% | Rapid tests, culture methods | |
| Diagnostic laboratory services | 52.5% | Large-scale screening capacity | |
| Research center applications | 18.2% | Epidemic surveillance, vaccine R&D | |
| Hospital and clinical testing | 29.3% | Patient care, outbreak response | |
| Future (3-5 yrs) | Advanced ELISA platforms | 47-50% | Automated systems, multiplex panels |
| Next-gen RT-PCR systems | 39-42% | Fastest growth, point-of-care integration | |
| Rapid diagnostic tests | 14-17% | Field deployment, emergency response | |
| Integrated lab networks | 53-57% | Digital connectivity, quality assurance | |
| Surveillance programs | 20-24% | Epidemic intelligence, predictive analytics | |
| Decentralized testing | 8-12% | Community health, remote diagnostics |
Arbovirus Testing Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 1.15 billion |
| Market Forecast (2035) ↑ | USD 1.80 billion |
| Growth Rate ★ | 4.6% CAGR |
| Leading Test Type → | ELISA-based Tests |
| Primary End Use → | Diagnostic Laboratories |
The market demonstrates strong fundamentals with ELISA-based tests capturing a dominant share through proven reliability and affordability advantages. Diagnostic laboratories drive primary demand, supported by increasing vector-borne disease surveillance requirements and epidemic response development.
Geographic expansion remains concentrated in North America with established diagnostic infrastructure, while emerging markets show accelerating adoption rates driven by tropical disease control initiatives and rising public health standards.
Imperatives for Stakeholders in Arbovirus Testing Market
Design for rapid detection, not just diagnostic accuracy
- Offer complete testing packages: assay supply + quality controls + training services + technical specialists + performance validation.
- Preconfigured protocols: specimen collection procedures, testing workflows, quality assurance standards, and digital reporting on diagnostic performance.
Technology readiness for surveillance networks
- Real-time epidemic monitoring analytics, outbreak prediction capabilities, and health system integration (digital reporting, case management systems).
Accuracy-by-design approach
- High sensitivity diagnostic systems, real-time quality monitoring, automated result interpretation, and digital performance documentation.
Value-based service models
- Clear base reagent price + transparent service tiers (training support, quality programs, technical guarantees); subscriptions for digital surveillance and analytics.
Segmental Analysis
The market segments by type into ELISA-based tests and RT-PCR-based tests variants, representing the evolution from immunological detection methods to molecular diagnostic solutions for precise viral identification and surveillance optimization. The end-use segmentation covers diagnostic laboratories (52.5%), research centers (18.2%), and hospital and clinical testing (29.3%), demonstrating varied testing requirements and operational capacity standards.
The segmentation structure reveals technology progression from standard serological methods toward comprehensive molecular diagnostics with enhanced sensitivity consistency and detection capabilities, while application diversity spans from routine screening to epidemic surveillance requiring precise arboviral identification solutions.
Why do ELISA-based Tests Command Market Leadership?

ELISA-based tests command the leading position in the arbovirus testing market with 48.7% market share through proven reliability features, including superior cost-effectiveness characteristics, operational simplicity, and diagnostic laboratory optimization that enable facilities to achieve optimal screening outcomes across diverse arboviral diseases and endemic applications.
The segment benefits from laboratory preference for established immunoassay systems that provide consistent performance characteristics, reduced equipment complexity, and testing optimization without requiring specialized molecular biology infrastructure. Advanced assay features enable antibody detection, antigen identification, and integration with existing laboratory workflows, where reliability performance and affordability represent critical diagnostic requirements.
ELISA-based Tests differentiate through proven operational reliability, consistent performance characteristics, and compatibility with standard laboratory equipment that enhance diagnostic effectiveness while maintaining optimal cost standards for diverse surveillance and clinical applications.
Key market characteristics:
- Advanced immunoassay designs with optimized antibody panels and detection sensitivity
- Enhanced diagnostic effectiveness, enabling large-scale screening with reliable seroprevalence assessment
- Laboratory workflow compatibility, including automation integration, quality validation, and process standardization for epidemiological surveillance
Why do RT-PCR Tests Achieve Fastest Growth?
RT-PCR-based Tests maintain 37.5% market position in the arbovirus testing market with fastest CAGR of 4.9% due to superior sensitivity properties and early detection characteristics. These systems appeal to laboratories requiring definitive viral identification with premium positioning for outbreak response applications. Market growth is driven by molecular diagnostic requirements, emphasizing RNA detection capabilities and viral load quantification through optimized amplification designs.
RT-PCR technology demonstrates fastest expansion through critical applications in outbreak identification, where high sensitivity enables early-stage infection detection and viral strain characterization essential for epidemic control and public health intervention strategies.
Which End-Use Category Leads in the Arbovirus Testing Domain?

Diagnostic laboratories demonstrate market leadership in the arbovirus testing market with 52.5% share due to widespread adoption of vector-borne disease screening programs and increasing focus on large-scale testing optimization, quality assurance, and epidemiological surveillance applications that maximize diagnostic capacity while maintaining accuracy standards.
Laboratory managers prioritize testing throughput, quality consistency, and integration with existing diagnostic infrastructure that enables coordinated screening operations across multiple specimen types. The segment benefits from substantial public health investment and disease control programs that emphasize the acquisition of reliable testing systems for surveillance optimization and outbreak response applications.
Public health programs incorporate arbovirus testing as standard procedures for endemic disease monitoring, while outbreak preparedness initiatives increase demand for diagnostic capabilities that comply with quality standards and enable rapid case identification.
Application dynamics include:
- Strong growth in reference laboratories and public health facilities requiring high-throughput testing capabilities
- Increasing adoption in regional diagnostic networks and syndromic surveillance applications for disease monitoring
- Rising integration with digital health information systems for case reporting and epidemic intelligence
How do Research Centers Drive Surveillance Innovation?
Research centers capture 18.2% market share with fastest CAGR of 5.2% through comprehensive surveillance requirements in epidemic intelligence, vaccine development, and vector control research.
These facilities demand advanced molecular testing systems capable of supporting viral characterization while providing effective epidemiological monitoring and intervention evaluation capabilities, particularly driven by expanding surveillance programs and research funding initiatives.
What positions Hospital & Clinical Testing for Patient Care?
Hospital and clinical testing maintains a 29.3% market share through direct patient care requirements in fever evaluation, imported infection diagnosis, and clinical management. These facilities demand rapid diagnostic systems capable of supporting treatment decisions while providing effective differential diagnosis and quality documentation capabilities.
What are the Drivers, Restraints, and Key Trends of the Arbovirus Testing Market?
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Climate change & vector expansion (mosquito habitat expansion, geographic spread) | ★★★★★ | Global disease distribution requires expanding, reliable diagnostic solutions with consistent performance and detection optimization across emerging endemic regions. |
| Driver | Outbreak frequency & epidemic preparedness (dengue resurgence, Zika emergence, West Nile spread) | ★★★★★ | Drives demand for sensitive testing systems and rapid diagnostic capabilities; suppliers providing accurate detection equipment gain competitive advantage. |
| Driver | Public health surveillance & disease control programs (WHO initiatives, national programs) | ★★★★☆ | Health systems need comprehensive, coordinated testing solutions; demand for surveillance diagnostics expanding addressable market segments. |
| Restraint | Test cost sensitivity & healthcare budgets | ★★★★☆ | Resource-limited settings face budget constraints; increases cost sensitivity and affects adoption in low-income endemic regions. |
| Restraint | Infrastructure limitations & technical capacity | ★★★☆☆ | Quality-focused testing faces challenges with equipment availability and trained personnel, limiting adoption in underserved areas. |
| Trend | Multiplex diagnostics & syndromic panels (combined pathogen detection, differential diagnosis) | ★★★★★ | Growing demand for comprehensive testing equipment; multi-pathogen detection becomes core value proposition in fever diagnostic segments. |
| Trend | Point-of-care testing & decentralized diagnostics | ★★★★☆ | Remote area healthcare drives demand for portable solutions; field-deployable diagnostics enable community-level surveillance and early detection. |
Analysis of the Arbovirus Testing Market by Key Country
The arbovirus testing market demonstrates varied regional dynamics with growth leaders including China (5.3% growth rate) and India (5.1% growth rate) driving expansion through vector control initiatives and diagnostic infrastructure development.
Strong performers encompass Brazil (4.9% growth rate), USA (4.6% growth rate), and Germany (4.4% growth rate), benefiting from established surveillance systems and advanced diagnostic adoption. Mature markets feature UK (4.3% growth rate) and Japan (4.1% growth rate), where public health advancement and testing standardization requirements support consistent growth patterns.
Regional synthesis reveals Asian markets leading adoption through endemic disease management and diagnostic capacity development, while Western countries maintain strong expansion supported by surveillance activity and outbreak preparedness requirements. Emerging markets show robust growth driven by tropical disease applications and vector control program trends.
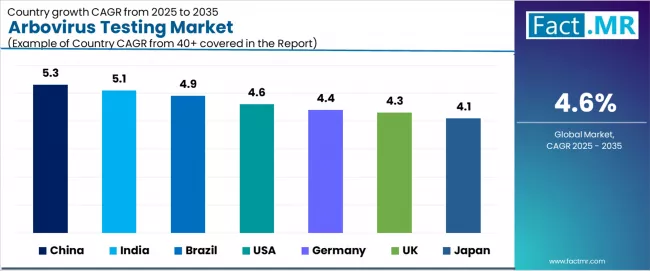
| Region/Country | 2025-2035 Growth | How to win | What to watch out |
|---|---|---|---|
| China | 5.3% | Focus on rapid diagnostic kits | Distribution challenges; price sensitivity |
| India | 5.1% | Offer subsidized test programs | Infrastructure gaps; quality variations |
| Brazil | 4.9% | Provide outbreak response systems | Economic volatility; funding fluctuations |
| USA | 4.6% | Lead with telehealth integration | Market maturity; reimbursement complexity |
| Germany | 4.4% | Push standardization programs | Regulatory requirements; coordination complexity |
| UK | 4.3% | NHS laboratory networks | Budget constraints; centralization challenges |
| Japan | 4.1% | Advanced containment systems | Limited endemic presence; import reliance |
How does China Drive Vector Control Diagnostic Expansion in the Arbovirus Testing Domain?
China establishes fastest market growth through aggressive public health programs and comprehensive vector control initiatives, integrating arbovirus testing as standard components in disease surveillance networks and epidemic response systems. The country's 5.3% growth rate reflects government initiatives promoting infectious disease monitoring and rapid diagnostic deployment that encourage the use of efficient testing systems in public health facilities.
Growth concentrates in major urban centers, including Guangdong, Yunnan, and Hainan, where tropical disease surveillance showcases integrated diagnostic systems that appeal to health authorities seeking reliable detection capabilities and outbreak response applications.
Chinese manufacturers are developing cost-effective diagnostic solutions that combine domestic production advantages with reliable detection features, including rapid test formats and automated platforms. Distribution channels through CDC networks and public health laboratories expand market access, while government support for epidemic preparedness supports adoption across diverse surveillance segments.
What Positions India as Emerging Endemic Disease Testing Market?
In Karnataka, Maharashtra, and Tamil Nadu regions, diagnostic laboratories and public health facilities are implementing arbovirus testing as standard equipment for dengue surveillance and chikungunya monitoring enhancement, driven by increasing national disease control programs and diagnostic infrastructure initiatives that emphasize the importance of systematic testing capabilities.
The market holds a 5.1% growth rate, supported by government health initiatives and test kit subsidy programs that promote accessible diagnostic systems for vector-borne applications. Indian operators are adopting testing systems that provide consistent diagnostic performance and cost-effective features, particularly appealing in endemic regions where disease burden and healthcare affordability represent critical public health requirements.
Market expansion benefits from growing healthcare investment capabilities and national control program integration that enables widespread deployment of subsidized testing systems for surveillance applications. Technology adoption follows patterns established in infectious disease diagnostics, where affordability and accessibility drive procurement decisions and system deployment.
How does Brazil Demonstrate Outbreak-Driven Testing Expansion?
Brazil establishes outbreak-driven testing expansion through endemic disease management and neglected tropical disease (NTD) control activity, integrating arbovirus testing across public health laboratories and clinical facilities. The country's 4.9% growth rate reflects growing epidemic preparedness and increasing adoption of diagnostic technology that supports expanding testing deployment in Brazilian health systems. Growth concentrates in major endemic regions, including São Paulo, Rio de Janeiro, and Amazonas, where tropical disease infrastructure showcases integrated testing systems that appeal to health managers seeking reliable solutions with operational compatibility.
Brazilian health authorities focus on balancing accessibility with diagnostic performance, creating demand for systems that combine detection capabilities with cost advantages. The market benefits from growing public health infrastructure and expanding vector control programs that support testing adoption while maintaining affordability standards important to Brazilian healthcare applications.
What drives USA Telehealth-Integrated Diagnostic Leadership?

The USA establishes telehealth-integrated diagnostic leadership through comprehensive surveillance programs and established public health infrastructure, integrating arbovirus testing across state laboratories and clinical diagnostic facilities. The country's 4.6% growth rate reflects mature healthcare system relationships and telehealth platform adoption that supports widespread use of molecular diagnostics in surveillance operations and early detection applications. Growth concentrates in major endemic states, including Florida, Texas, and California, where vector-borne disease monitoring showcases mature testing deployment that appeals to public health officials seeking proven detection capabilities and digital reporting applications.
American manufacturers leverage established regulatory networks and comprehensive technical capabilities, including CDC coordination programs and laboratory response networks that create surveillance relationships and operational advantages. The market benefits from mature public health frameworks and outbreak preparedness requirements that encourage system upgrades while supporting technology advancement and early warning optimization.
How does Germany Build Diagnostic Standardization Programs?
Germany's advanced public health system demonstrates sophisticated arbovirus testing deployment with documented operational effectiveness in imported disease detection and surveillance applications through integration with existing laboratory quality systems and healthcare infrastructure. The country leverages expertise in diagnostic standardization and quality assurance methodology to maintain a 4.4% growth rate. Public health centers, including North Rhine-Westphalia, Bavaria, and Baden-Württemberg, showcase premium installations where testing systems integrate with comprehensive surveillance platforms and notification systems to optimize disease detection operations and response effectiveness.
German health authorities prioritize system standardization and quality compliance in testing development, creating demand for certified systems with advanced features, including proficiency testing and quality documentation. The market benefits from established public health infrastructure and willingness to invest in standardized technologies that provide long-term surveillance benefits and compliance with international diagnostic standards.
What Positions UK for Centralized Laboratory Networks in the Arbovirus Testing Landscape?
The UK's public health system demonstrates sophisticated testing integration with documented operational effectiveness in reference laboratory applications and imported fever diagnosis through integration with NHS systems and surveillance infrastructure. The country maintains a 4.3% growth rate, leveraging established public health frameworks and laboratory network integration through centralized diagnostic programs. Reference centers, including London, Birmingham, and Manchester, showcase integrated installations where testing systems coordinate with clinical management platforms and epidemic intelligence systems to optimize disease detection operations and maintain surveillance profiles.
British health authorities prioritize network coordination and diagnostic consistency in testing development, creating demand for systems with advanced features, including digital reporting and quality assurance. The market benefits from established NHS infrastructure and commitment to surveillance quality standards that provide long-term public health benefits and compliance with diagnostic regulations.
How does Japan Emphasize Advanced Containment Technology?
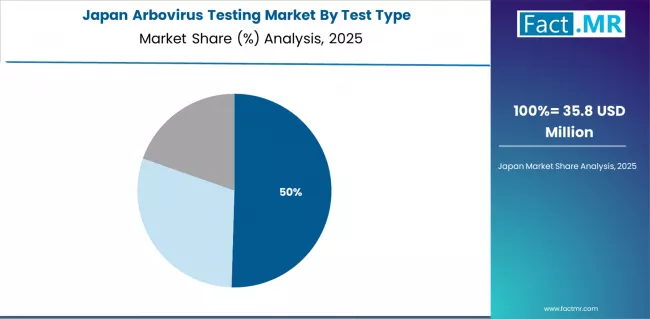
Japan's advanced healthcare technology market demonstrates sophisticated testing integration with documented operational effectiveness in imported case detection and regional surveillance applications through integration with existing infectious disease systems and public health infrastructure.
The country maintains a 4.1% growth rate, leveraging traditional quality expertise and precision diagnostic integration in vector-borne disease technology. Healthcare centers, including Tokyo, Osaka, and Fukuoka, showcase premium installations where testing systems integrate with quarantine platforms and surveillance management systems to optimize disease detection operations and maintain containment profiles.
Japanese health authorities prioritize diagnostic precision and system reliability in testing development, creating demand for premium systems with advanced features, including automated workflows and quality verification. The market benefits from established public health infrastructure and commitment to containment excellence standards that provide long-term epidemic prevention benefits and compliance with international health regulations.
Europe Market Split by Country
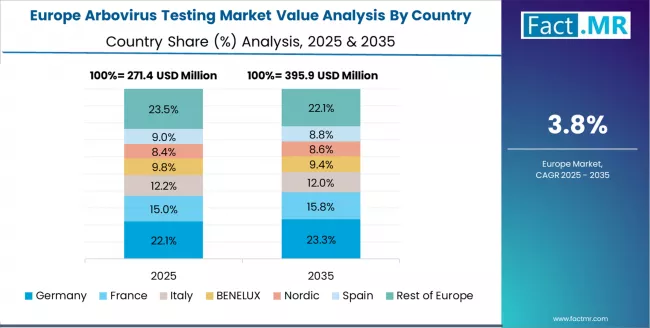
The arbovirus testing market in Europe is projected to grow from USD 315 million in 2025 to USD 469 million by 2035, registering a CAGR of 4.1% over the forecast period. Germany is expected to maintain its leadership position with a 28.6% market share in 2025, declining slightly to 27.8% by 2035, supported by its advanced public health infrastructure and major reference laboratories including Berlin, Hamburg, and Munich.
France follows with a 21.4% share in 2025, projected to reach 21.9% by 2035, driven by comprehensive tropical medicine programs at Institut Pasteur and other major surveillance facilities. The UK holds a 19.2% share in 2025, expected to increase to 19.7% by 2035 due to centralized NHS laboratory network initiatives.
Italy commands a 14.8% share, while Spain accounts for 10.5% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 5.5% to 7.2% by 2035, attributed to increasing arbovirus testing adoption in Mediterranean countries and emerging Central European public health laboratories implementing surveillance modernization programs.
Competitive Landscape of the Arbovirus Testing Market
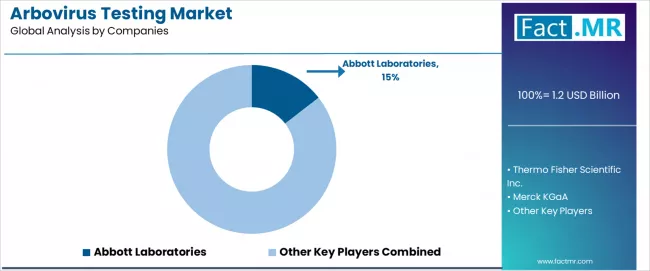
The arbovirus testing market exhibits a moderately consolidated competitive structure with approximately 25-35 active players operating across global and regional markets. The top 3-4 manufacturers collectively command roughly 39-42% of total market revenue, with Abbott Laboratories maintaining market leadership at approximately 14.5% share.
This competitive landscape reflects the diverse nature of arboviral diagnostic requirements across surveillance and clinical segments, where disease complexity, testing specifications, and regulatory standards create opportunities for both multinational corporations and specialized diagnostic providers to capture meaningful market positions.
Market leadership is maintained through several critical competitive advantages that extend beyond assay manufacturing capabilities. Global distribution networks enable leading players to reach diverse geographic markets and serve customers across multiple continents with consistent product availability and technical support. Regulatory expertise capabilities provide competitive advantages and compliance leadership that allow market leaders to navigate complex approval pathways while maintaining quality standards.
Comprehensive technical services represent a crucial differentiator, as arbovirus testing systems require validation protocols, quality controls, troubleshooting support, and responsive technical assistance throughout their operational lifecycle. The combination of multi-pathogen versatility, validated clinical performance, and robust quality assurance programs creates customer loyalty and long-term diagnostic partnership patterns that reinforce market position over time.
The market demonstrates clear commoditization trends in standard single-target ELISA kits and basic serological tests, where product differentiation has diminished and price competition intensifies. These entry-level products face margin pressure as manufacturing capabilities spread globally and technological barriers decrease.
Significant margin opportunities persist in advanced diagnostic categories and value-added services. Multiplex molecular panels command premium pricing through their simultaneous pathogen detection and differential diagnosis capabilities, appealing to customers seeking comprehensive fever evaluation and outbreak investigation performance benefits.
Automated platform integration features connect diagnostic assays with laboratory information systems, robotic workflows, and quality management platforms, creating differentiation through operational efficiency, result reliability, and regulatory compliance functions.
Comprehensive service programs, including professional training services, external quality assessment schemes, technical consultation, and assay customization support, generate recurring revenue streams and strengthen customer relationships beyond initial reagent sales.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Global diagnostic platforms | Manufacturing networks, broad assay portfolios, regulatory infrastructure | Proven reliability, multi-region approvals, comprehensive validation | Endemic region customization; point-of-care innovation |
| Molecular specialists | R&D capabilities; RT-PCR systems; sequencing integration | Latest technology first; attractive sensitivity on premium platforms | Cost accessibility; field deployment limitations |
| Regional manufacturers | Local production, fast delivery, nearby technical support | Cost-effective solutions; regional disease focus; responsive service | Technology gaps; international certification recognition |
| Surveillance providers | Public health expertise, epidemic intelligence, network coordination | Population-level insights; outbreak detection; data analytics | Individual diagnostic markets; commercial scalability |
| Point-of-care innovators | Rapid test development, portable platforms, field validation | Accessibility advantages; decentralized testing; emergency response | Sensitivity limitations; laboratory network integration |
Key Players in the Arbovirus Testing Market
- Abbott Laboratories
- Thermo Fisher Scientific Inc.
- Merck KGaA
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers AG
- QIAGEN
- EUROIMMUN AG
- Agilent Technologies, Inc.
- NovaTec Immundiagnostica GmbH
- Bio-Rad Laboratories, Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Value (USD Million)s (2025) | USD 1.15 billion |
| Test Type | ELISA-based Tests, RT-PCR-based Tests, Other Testing Technologies |
| End Use | Diagnostic Laboratories, Research Centers, Hospital & Clinical Testing |
| Diseases Covered | Dengue, Zika, Chikungunya, West Nile, Yellow Fever, Other Arboviruses |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | China, India, Brazil, USA, Germany, UK, Japan, and 25+ additional countries |
| Key Companies Profiled | Abbott Laboratories, Thermo Fisher Scientific Inc., Merck KGaA, F. Hoffmann-La Roche Ltd, Siemens Healthineers AG, QIAGEN |
| Additional Attributes | Dollar sales by test type and end-use categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with diagnostic manufacturers and surveillance providers, healthcare preferences for diagnostic accuracy and cost effectiveness, integration with laboratory information systems and public health platforms, innovations in molecular technology and assay enhancement, and development of advanced diagnostic solutions with enhanced sensitivity and operational optimization capabilities. |
Arbovirus Testing Market by Segments
-
Test Type :
- ELISA-based Tests
- RT-PCR-based Tests
- Other Testing Technologies
-
End Use :
- Diagnostic Laboratories
- Research Centers
- Hospital & Clinical Testing
-
Disease Type :
- Dengue
- Zika
- Chikungunya
- West Nile
- Yellow Fever
- Other Arboviruses
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Test Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Test Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Test Type, 2025 to 2035
- ELISA-based Tests
- RT-PCR-based Tests
- Other Testing Technologies
- Y to o to Y Growth Trend Analysis By Test Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Test Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Diagnostic Laboratories
- Research Centers
- Hospital & Clinical Testing
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Test Type
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Test Type
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Test Type
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Test Type
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Test Type
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Test Type
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Test Type
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Test Type
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Test Type
- By End Use
- Competition Analysis
- Competition Deep Dive
- Abbott Laboratories
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Thermo Fisher Scientific Inc.
- Merck KGaA
- F. Hoffmann-La Roche Ltd
- Siemens Healthineers AG
- QIAGEN
- EUROIMMUN AG
- Agilent Technologies, Inc.
- NovaTec Immundiagnostica GmbH
- Bio-Rad Laboratories, Inc.
- Abbott Laboratories
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Test Type
- Figure 6: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by End Use
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 22: North America Market Attractiveness Analysis by Test Type
- Figure 23: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by End Use
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 29: Latin America Market Attractiveness Analysis by Test Type
- Figure 30: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 32: Latin America Market Attractiveness Analysis by End Use
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 36: Western Europe Market Attractiveness Analysis by Test Type
- Figure 37: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 39: Western Europe Market Attractiveness Analysis by End Use
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Test Type
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 50: East Asia Market Attractiveness Analysis by Test Type
- Figure 51: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 53: East Asia Market Attractiveness Analysis by End Use
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Test Type
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Test Type
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the arbovirus testing market in 2025?
The global arbovirus testing market is estimated to be valued at USD 1.2 billion in 2025.
What will be the size of arbovirus testing market in 2035?
The market size for the arbovirus testing market is projected to reach USD 1.8 billion by 2035.
How much will be the arbovirus testing market growth between 2025 and 2035?
The arbovirus testing market is expected to grow at a 4.6% CAGR between 2025 and 2035.
What are the key product types in the arbovirus testing market?
The key product types in arbovirus testing market are elisa-based tests, rt-pcr-based tests and other testing technologies.
Which end use segment to contribute significant share in the arbovirus testing market in 2025?
In terms of end use, diagnostic laboratories segment to command 5250.0% share in the arbovirus testing market in 2025.