Pyrogen Testing Market
Pyrogen Testing Market Size and Share Forecast Outlook 2025 to 2035
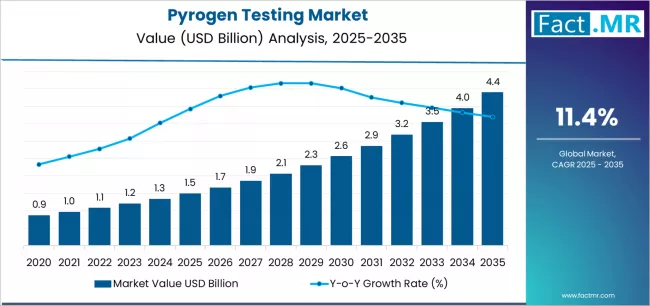
Pyrogen testing market is projected to grow from USD 1.5 billion in 2025 to USD 4.4 billion by 2035, at a CAGR of 11.4%. Consumables will dominate with a 43.6% market share, while lal test will lead the test type segment with a 63.2% share.
Pyrogen Testing Market Forecast and Outlook 2025 to 2035
The global pyrogen testing market is set to grow from USD 1.5 billion in 2025 to USD 4.4 billion by 2035, adding USD 2.9 billion in new revenue and advancing at a CAGR of 11.4%. Growth is driven by escalating demand for pharmaceutical quality control validation, expanding biologics manufacturing infrastructure across regulated markets, and accelerating regulatory compliance requirements among pharmaceutical and biotechnology organizations seeking contamination-free production solutions.
Pyrogen testing technologies are increasingly recognized as essential tools for pharmaceutical quality assurance practitioners, offering precise endotoxin detection capabilities, regulatory compliance assurance, and comprehensive safety validation characteristics compared to traditional bacterial contamination assessment approaches.
Quick Stats for Pyrogen Testing Market
- Pyrogen Testing Market Value (2025): USD 1.5 billion
- Pyrogen Testing Market Forecast Value (2035): USD 4.4 billion
- Pyrogen Testing Market Forecast CAGR: 11.4%
- Leading Test Type in Pyrogen Testing Market: LAL Test (63.2%)
- Key Growth Regions in Pyrogen Testing Market: Asia Pacific, North America, and Europe
- Top Players in Pyrogen Testing Market: Charles River Laboratories, Novo Nordisk, Merck KGaA, GenScript, bioMérieux SA
LAL Test formulations dominate the market, favored in pharmaceutical and biotechnology environments for their established validation properties, providing sensitive detection mechanisms, rapid turnaround capabilities, and regulatory acceptance across diverse product safety applications and manufacturing demographics.
Consumables remain fundamental in laboratory testing protocols where routine endotoxin screening and batch release testing match operational requirements and quality assurance confidence standards. Pharmaceutical and biotechnology companies are advancing among end-use categories as specialized manufacturing facility networks expand and regulatory compliance infrastructure increases accessibility in production-convenient locations with stringent safety structures.
Geographic concentration demonstrates dynamic growth patterns with India and China leading expansion, supported by rising pharmaceutical manufacturing capacity, biosafety consciousness expansion among quality control populations, and testing laboratory establishment programs in production centers.
USA, Germany, UK, France, and Canada demonstrate robust development through established pharmaceutical quality ecosystems, regulatory framework maturity for injectable products, and standardized acceptance of contamination screening procedures. Competitive advantage is consolidating around detection sensitivity profiles, regulatory compliance documentation, laboratory automation compatibility, and integrated safety testing portfolios rather than standalone assay formulations alone.
The first half of the decade will witness the market climbing from USD 1.5 billion to approximately USD 2.6 billion, adding USD 1.1 billion in value, which constitutes 38% of the total forecast growth period. This phase will be characterized by the continued dominance of LAL test methodologies in pharmaceutical testing settings, combined with accelerating adoption of recombinant factor C technologies in biologics manufacturing applications where regulatory validation and animal-free testing create favorable quality compliance outcomes.
The latter half will witness sustained expansion from USD 2.6 billion to USD 4.4 billion, representing an addition of USD 1.8 billion or 62% of the decade's growth, defined by broadening acceptance of alternative pyrogen testing protocols and integration of automated detection platforms across mainstream pharmaceutical manufacturing facilities.
Where revenue comes from - Now Vs Next (industry-level view)
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | LAL Test | 63.2% | Regulatory dominance |
| Consumables | 43.6% | Primary product category | |
| Pharmaceutical & Biotechnology | 62.2% | Leading user segment | |
| Chromogenic LAL | 24-28% | Rapid detection | |
| Medical Device Testing | 26-30% | Safety validation | |
| Future (3-5 yrs) | Recombinant Factor C Testing | 38-44% | Animal-free advancement |
| Automated Testing Platforms | 32-38% | Laboratory efficiency | |
| MAT Technologies | 18-24% | Alternative methods | |
| Point-of-Use Testing | 22-28% | Decentralized screening | |
| Biologics Testing | 42-48% | Biosimilar expansion | |
| Digital Integration | 28-34% | Data connectivity | |
| Emerging Markets | 35-41% | Manufacturing expansion |
Pyrogen Testing Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 1.5 billion |
| Market Forecast (2035) ↑ | USD 4.4 billion |
| Growth Rate ★ | 11.4% CAGR |
| Leading Test Type → | LAL Test |
| Primary Product → | Consumables |
The market demonstrates exceptional fundamentals with LAL Test capturing a commanding 63.2% share through superior regulatory acceptance characteristics, established validation advantages, and proven sensitivity profiles across pharmaceutical testing applications. Consumables drive primary product demand at 43.6% share, supported by established quality control infrastructure and routine testing requirements that maintain operational compliance across diverse manufacturing segments.
Geographic concentration remains anchored in Asia Pacific and North America with emerging market leadership through pharmaceutical manufacturing expansion and testing laboratory infrastructure development, while developed markets show accelerated adoption rates driven by biologics production demographics and regulatory validation procedure preferences.
Imperatives for Stakeholders in Pyrogen Testing Market
Design for sensitivity and compliance, not just endotoxin detection
- Offer complete quality assurance solutions: advanced testing reagents + laboratory automation integration + regulatory documentation support + method validation systems + compliance tracking platforms.
- Preconfigured testing packages: pharmaceutical batch release specifications, biologics screening configurations, medical device validation programs, and combination testing protocols for diverse manufacturing requirements.
Regulatory readiness for pharmaceutical applications
- Comprehensive validation documentation, regulatory compliance systems, and quality infrastructure (batch traceability, stability assurance, cold chain management protocols).
Affordability-by-design approach
- Cost-optimized product portfolios, flexible testing pricing models, laboratory loyalty programs, and transparent total testing cost documentation.
Laboratory training-focused market penetration
- Established method validation workshops + comprehensive certification programs (assay performance, regulatory compliance, troubleshooting management); direct laboratory engagement for relationship development and testing confidence building.
Segmental Analysis
The market segments by product into consumables, instruments, and services, representing the evolution from basic endotoxin detection toward sophisticated quality validation with automated capabilities, comprehensive documentation, and integrated laboratory characteristics.
The test type segmentation divides the market into LAL Test (63.2%) with turbidimetric, chromogenic, and gel clot methodologies, in vitro pyrogen test, recombinant factor C testing, monocyte activation test, and rabbit test.
The end-use segmentation shows Pharmaceutical and Biotechnology Companies' commanding 62.2% position, followed by Medical Device Companies and others, demonstrating varied manufacturing specialization levels and quality control infrastructure concentrations.
What Makes LAL Test Command the Largest Share in the Pyrogen Testing Market?
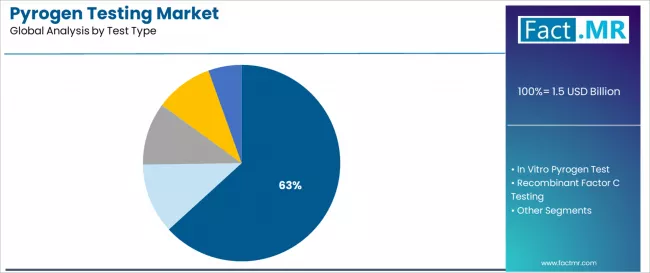
LAL test commands the leading position in the pyrogen testing market with a 63.2% share. This is due to its superior regulatory acceptance characteristics, including established pharmacopeia presence, extensive validation documentation, and standardized detection pathways that enable laboratories to achieve predictable quality outcomes across varied pharmaceutical product categories and diverse manufacturing demographics.
The segment benefits from sensitivity advantages through enzymatic cascade amplification, rapid turnaround results without lengthy incubation periods, and established method validation documentation without requiring extensive requalification procedures. Advanced formulation technology enables chromogenic endpoint optimization, turbidimetric kinetic variation, and gel clot simplicity customization, where detection precision and result reliability represent critical quality assurance requirements.
Chromogenic LAL formulations hold significant share within the test type segment, appealing to laboratories seeking quantitative measurement capabilities for precise endotoxin determination. LAL test products differentiate through proven regulatory acceptance profiles, laboratory familiarity advantages, and integration with established quality control protocols that enhance testing confidence while maintaining compliant quality outcomes for diverse pharmaceutical screening applications.
Key market characteristics:
- Advanced sensitivity properties with low detection limits and reproducible performance for trace endotoxin quantification
- Superior validation documentation, enabling method transfer and regulatory inspection readiness for pharmaceutical applications
- Comprehensive regulatory acceptance, including USP, EP, and JP monograph inclusion for global market approval applications
Why do In Vitro Pyrogen Test Methods Represent a Specialized Validation Segment?
In vitro pyrogen test methods maintain specialized market position through alternative validation characteristics and human-relevant response capabilities. These products appeal to laboratories and manufacturers seeking broader pyrogenic substance detection with comprehensive contamination screening, offering immune-based detection patterns and complementary assessment approaches through cell-based systems. Market adoption is driven by biologics testing applications, emphasizing complete pyrogenic response evaluation and regulatory acceptance progression through innovative detection mechanisms.
Which Category will Dominate the Pyrogen Testing Market by Product Type?
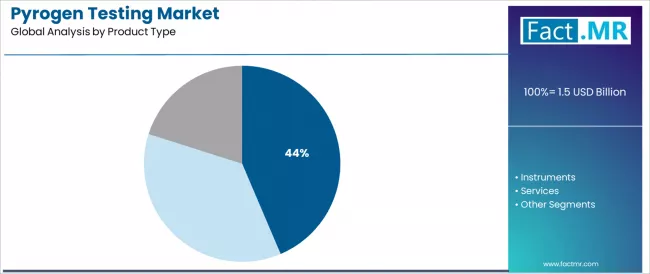
Consumables demonstrate product leadership in the pyrogen testing market with a 43.6% share due to widespread routine testing requirements and established focus on reagent replenishment, assay kit utilization, and laboratory supply consumption that maximizes operational continuity while maintaining consistent quality validation characteristics.
Laboratories prioritize consumable products for batch release testing frequency, regulatory compliance maintenance, and integration with established quality control workflows that enables coordinated validation experiences across multiple testing protocols. The segment benefits from substantial method standardization and product performance documentation that emphasizes reagent-based approaches for endotoxin and pyrogen screening across diverse pharmaceutical manufacturing demographics.
LAL reagent cartridges capture significant share within the consumables segment, demonstrating laboratory preference for convenient testing formats. Pharmaceutical manufacturing expansion incorporates consumables as essential operational components for quality assurance programs, while automated testing platform adoption increases demand for reagent integration with instrument systems for comprehensive laboratory productivity outcomes.
What drives Instruments Adoption in Laboratory Automation Applications?
Instruments capture substantial product share through comprehensive requirements in automated detection systems, kinetic measurement capabilities, and result documentation optimization. These products demand sophisticated validation protocols capable of achieving regulatory compliance standards while providing consistent performance reliability and laboratory workflow integration, appealing to manufacturers and quality control teams seeking evidence-based efficiency advantages beyond manual testing approaches.
Who are the Primary End Users of Pyrogen Testing Products?
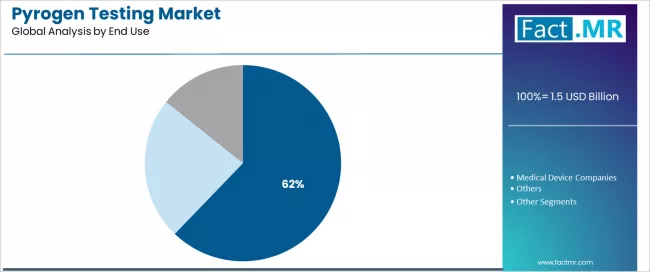
Pharmaceutical and biotechnology companies are the primary end users of pyrogen testing products. This segment commands a 62.2% share due to comprehensive regulatory compliance requirements and sustained focus on injectable product safety, sterile manufacturing validation, and batch release quality that maximizes patient protection while maintaining appropriate regulatory oversight standards.
Manufacturers and quality teams prioritize pharmaceutical testing environments for mandatory endotoxin screening, comprehensive safety validation services, and integration with Good Manufacturing Practice requirements that enables coordinated compliance experiences across multiple production categories. The sector benefits from substantial regulatory infrastructure maturity and pharmacopeia guideline campaigns that emphasize pharmaceutical-based testing delivery for critical quality assurance applications.
Biologics manufacturing expansion incorporates pharmaceutical testing as standard safety protocols for injectable products, while biosimilar development increases facility validation that meets complexity requirements and ensures contamination detection capabilities.
End-use dynamics include:
- Strong growth in contract manufacturing operations requiring validated testing methods and consistent quality documentation arrangements
- Increasing adoption in biotechnology startups for regulatory submission and investor confidence positioning
- Rising integration with cell and gene therapy platforms for advanced therapeutic product development
What are the Drivers, Restraints, and Key Trends of the Pyrogen Testing Market?
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | Rising biologics production & injectable drug development (pharmaceutical expansion, biosimilar growth) | ★★★★★ | Regulatory requirements enable pyrogen testing demand for parenteral product validation; increasing biologics manufacturing drivesscreening adoption across pharmaceutical markets and diverse therapeutic segments. |
| Driver | Growth in pharmaceutical manufacturing facilities and quality control infrastructure (capacity expansion, compliance investment) | ★★★★★ | Drives demand for reliable contamination detection systems and standardized testing protocols; laboratories providing consistent validation outcomes gain competitive advantage in regulatory-focused pharmaceutical segments. |
| Driver | Regulatory stringency and pharmacopeia requirements (USP standards, FDA guidelines) | ★★★★☆ | Manufacturers demand validated screening methods and documented compliance systems; regulatory framework visibility expanding addressable segments beyond traditional pharmaceutical demographics and research laboratory clientele. |
| Restraint | High testing costs & capital equipment investment (instrument expenses, reagent pricing) | ★★★★☆ | Cost-conscious manufacturers face budget limitations and testing frequency constraints, restricting method adoption and affecting technology penetration in resource-limited organizations and developing market laboratories. |
| Restraint | Technical complexity & method validation requirements (training needs, expertise gaps) | ★★★☆☆ | Laboratories face capability concerns and personnel limitations; increases implementation barriers and affects adoption penetration in smaller pharmaceutical facilities and emerging market manufacturing operations. |
| Trend | Alternative method development & animal-free testing technologies (rFC assays, MAT systems) | ★★★★★ | Growing regulatory acceptance for non-animal approaches and ethical testing preferences beyond traditional LAL rabbit-based validation; advanced methodologies become core differentiation strategy for progressive laboratory positioning. |
| Trend | Laboratory automation & digital integration (robotic systems, data connectivity) | ★★★★☆ | Quality control evolving beyond manual processing toward automated testing protocols; technology positioning drives enhanced efficiency and result reliability in sophisticated pharmaceutical manufacturing environments. |
Analysis of the Pyrogen Testing Market by Key Countries
The pyrogen testing market demonstrates robust regional growth dynamics with emerging leaders including India (13.4% CAGR) and China (12.8% CAGR) driving expansion through pharmaceutical manufacturing programs and testing laboratory infrastructure development. Strong performers encompass the USA (10.8% CAGR), Canada (9.9% CAGR), and Germany (8.7% CAGR), benefiting from established pharmaceutical quality infrastructure and biologics production demographics. Developed markets feature UK (8.2% CAGR) and France (7.6% CAGR), where regulatory compliance normalization and laboratory expertise support consistent growth patterns.
Regional synthesis reveals Asian markets leading adoption through comprehensive manufacturing capacity positioning and pharmaceutical investment expansion, while Western countries demonstrate measured growth potential supported by biologics development preferences and regulatory validation influence. North American markets show solid development driven by quality assurance culture integration and advanced testing infrastructure.
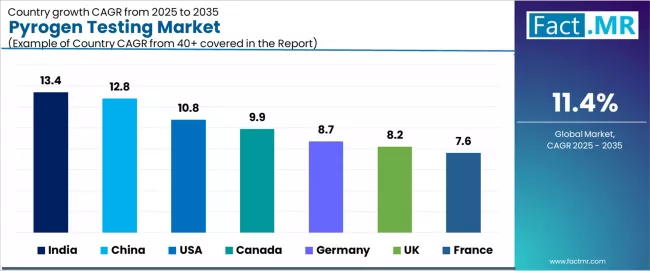
| Region/Country | 2025-2035 Growth | How to win | What to watch out |
|---|---|---|---|
| India | 13.4% | Focus on affordable testing portfolios | Regulatory evolution; laboratory training gaps |
| China | 12.8% | Lead with automated system positioning | Quality standard variations; price pressures |
| USA | 10.8% | Provide advanced validation technologies | Market maturity; compliance complexity |
| Canada | 9.9% | Offer regulatory support strategies | Economic constraints; facility concentration |
| Germany | 8.7% | Maintain quality-focused positioning | Conservative adoption patterns; documentation requirements |
| UK | 8.2% | Deliver compliance-ready solutions | Post-Brexit adjustments; market fragmentation |
| France | 7.6% | Push validation expertise programs | Budget limitations; centralized procurement |
India Drives Fastest Market Growth
India establishes fastest market growth through progressive pharmaceutical manufacturing expansion and comprehensive testing laboratory infrastructure development, positioning pyrogen testing technologies as essential quality solutions in pharmaceutical production centers and emerging contract manufacturing facilities.
The country's 13.4% growth rate reflects rising pharmaceutical investment levels supporting quality control spending and growing biosimilar manufacturing segments that encourage the deployment of cost-optimized testing products in diverse validation settings.
Growth concentrates in major pharmaceutical clusters, including Hyderabad, Ahmedabad, and Bangalore, where manufacturing facilities showcase increasing capacity for international quality standard adoption that appeal to export-focused producers demanding validated contamination screening and regulatory compliance outcomes.
Indian pharmaceutical manufacturers are developing standardized testing protocols that combine imported validation reagents with domestic laboratory partnerships, including contract testing expansion and quality control laboratory growth.
Distribution channels through pharmaceutical testing distributors and laboratory equipment suppliers expand market access, while technician certification initiatives support adoption across diverse facility types and manufacturing specialization levels.
China Emerges as Manufacturing Quality Leader
In Shanghai, Beijing, and Jiangsu regions, pharmaceutical manufacturing facilities and biologics production plants are adopting advanced pyrogen testing technologies as essential quality tools for injectable product operations, driven by increasing regulatory alignment with international standards and elevation of pharmaceutical quality expectations that emphasize the importance of validated contamination detection.
The market holds a 12.8% growth rate, supported by pharmaceutical park development and quality infrastructure investment that promote testing adoption for export-oriented applications. Chinese manufacturers are favoring automated testing platforms that provide comprehensive validation documentation and regulatory compliance evidence, particularly appealing in pharmaceutical clusters where method reliability and inspection readiness represent critical operational factors.
Market expansion benefits from substantial pharmaceutical facility investment and quality control laboratory establishment that enable widespread adoption of evidence-based testing methodologies for diverse injectable product applications. Industry adoption follows patterns established in pharmaceutical manufacturing excellence, where quality system advantages and regulatory compliance documentation drive manufacturer confidence and facility certification achievement.
USA Shows Commercial Quality Leadership
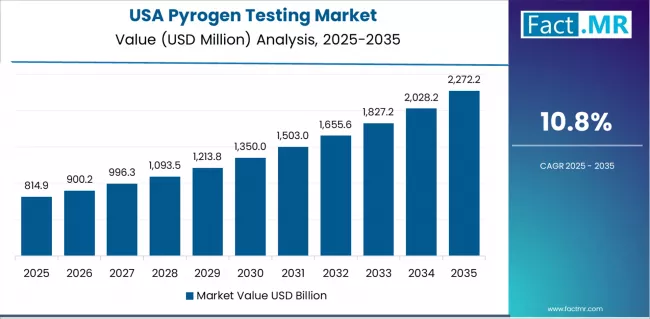
USA establishes commercial quality leadership through comprehensive pharmaceutical manufacturing infrastructure and established regulatory compliance ecosystem, integrating pyrogen testing technologies across biologics facilities, pharmaceutical plants, and contract manufacturing organizations. The country's 10.8% growth rate reflects established FDA framework maturity and sophisticated quality assurance levels that support widespread deployment of validated testing products in commercial and research applications.
Growth concentrates in established pharmaceutical regions, including New Jersey, Massachusetts, and California, where manufacturers showcase advanced quality control adoption that appeals to regulatory-focused organizations seeking predictable validation outcomes and comprehensive method documentation.
American pharmaceutical providers leverage established supplier relationships and comprehensive quality system frameworks, including regulatory inspection readiness and validation documentation programs that create testing confidence and compliance assurance. The market benefits from substantial biosimilar development and advanced therapeutic manufacturing that encourage premium product purchases while supporting continuous innovation investments and method validation funding.
Canada Shows Regulatory Compliance Integration
Canada's progressive pharmaceutical market demonstrates established pyrogen testing adoption with documented quality emphasis in method implementation and validation protocol execution through specialized biopharmaceutical manufacturers and established quality control laboratories. The country maintains a 9.9% growth rate, leveraging harmonized regulatory frameworks with USA standards and quality-focused manufacturing cultures in pharmaceutical production.
Major pharmaceutical hubs, including Quebec, Ontario, and British Columbia regions, showcase compliance-driven testing priorities where pyrogen testing technologies integrate with established Good Manufacturing Practice environments and thorough documentation practices to optimize regulatory submission success and maintain international market access under stringent health authority requirements.
Canadian manufacturers prioritize regulatory alignment strategies and comprehensive quality system integration in testing program implementation, creating demand for validated products with extensive regulatory support, including method transfer assistance, audit preparation services, and comparative validation studies. The market benefits from established biosimilar development clusters and specialized pharmaceutical manufacturing segments that provide premium positioning opportunities and maintain alignment with Health Canada regulatory expectations.
Germany Shows Quality-Focused Testing Integration
Germany's advanced pharmaceutical market demonstrates sophisticated pyrogen testing integration with documented quality emphasis in method selection and validation protocol precision through specialized pharmaceutical manufacturers and established quality control networks. The country leverages rigorous quality system principles and evidence-based validation approaches to maintain an 8.7% growth rate.
Premium pharmaceutical centers, including Bavaria, North Rhine-Westphalia, and Hesse, showcase regulatory compliance priorities where pyrogen testing technologies integrate with established pharmaceutical cultures and thorough quality practices to optimize manufacturing confidence and ensure appropriate contamination assessment.
German manufacturers prioritize method validation requirements and comprehensive quality documentation in testing implementation, creating demand for regulatory-compliant products with extensive validation characteristics, including long-term stability data, comparative method studies, and comprehensive technical documentation. The market benefits from established pharmaceutical specialty segments and quality assurance maturity that provide differentiation opportunities and compliance with strict German pharmaceutical regulations.
UK Demonstrates Regulatory Alignment Preferences
UK's mature pharmaceutical market demonstrates established pyrogen testing integration with documented regulatory compliance focus in method application and quality assurance protocols through comprehensive pharmaceutical manufacturing facilities and specialized contract testing organizations. The country maintains an 8.2% growth rate, leveraging MHRA regulatory framework alignment with international standards and established pharmaceutical manufacturing expertise in injectable production.
Key pharmaceutical regions, including England and Scotland, showcase quality-driven testing approaches where pyrogen testing technologies integrate with established validation cultures and comprehensive documentation systems to optimize regulatory inspection readiness and maintain market authorization compliance under evolving pharmaceutical quality requirements.
The manufacturers prioritize comprehensive validation documentation and regulatory compliance assurance in testing program development, creating demand for established products with extensive regulatory acceptance, including pharmacopeia compliance certification, validation package completeness, and audit trail documentation. The market benefits from established contract development and manufacturing organization sectors and specialized biologics production facilities that provide quality positioning opportunities and maintain alignment with stringent UK pharmaceutical device regulations.
France Shows Pharmaceutical Excellence Integration
France's sophisticated pharmaceutical market demonstrates progressive pyrogen testing adoption with documented quality standards in validation methodology and compliance protocol execution through established pharmaceutical manufacturers and specialized quality control facilities. The country maintains a 7.6% growth rate, leveraging established pharmaceutical industry heritage and comprehensive regulatory oversight frameworks in injectable manufacturing.
Major pharmaceutical centers, including Ile-de-France and Rhone-Alpes regions, showcase traditional quality emphasis where pyrogen testing technologies integrate with established European Pharmacopoeia standards and meticulous validation practices to optimize manufacturing quality assurance and maintain regulatory compliance under comprehensive health authority supervision.
French manufacturers prioritize European regulatory alignment and comprehensive quality system integration in testing program implementation, creating demand for validated products with extensive documentation characteristics, including pharmacopeia monograph compliance, method equivalency studies, and comprehensive validation reports. The market benefits from established pharmaceutical manufacturing reputation and quality-focused production culture that provide premium positioning opportunities and comply with strict ANSM regulatory requirements.
Europe Market Split by Country
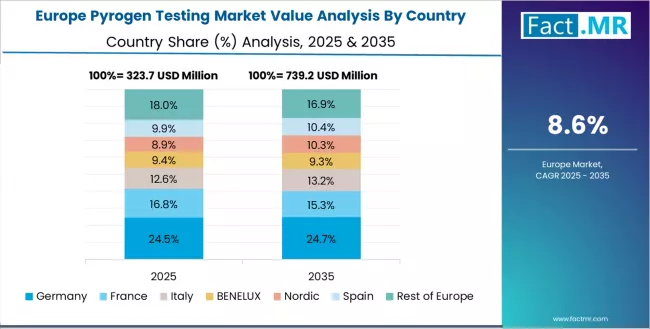
The European pyrogen testing market is projected to grow from USD 0.4 billion in 2025 to USD 1.0 billion by 2035, representing 26.7% of the global market in 2025 and expanding to 22.7% by 2035. Germany is expected to maintain its leadership position with USD 0.1 billion in 2025, accounting for 26.8% of the European market, supported by its advanced pharmaceutical manufacturing infrastructure and established quality control laboratory networks.
France follows with USD 0.1 billion, representing 18.9% of the European market in 2025, driven by comprehensive pharmaceutical production integration and Paris pharmaceutical center concentration. UK holds USD 0.1 billion with 17.3% market share through established regulatory compliance acceptance and manufacturing facility density.
Italy commands USD 0.1 billion representing 14.2% share, while Spain accounts for USD 0.0 billion or 11.6% in 2025. The rest of Europe region maintains USD 0.0 billion, representing 11.2% of the European market, attributed to increasing pharmaceutical testing adoption in Nordic countries and emerging Eastern European contract manufacturing sectors implementing quality control programs.
Competitive Landscape of the Pyrogen Testing Market
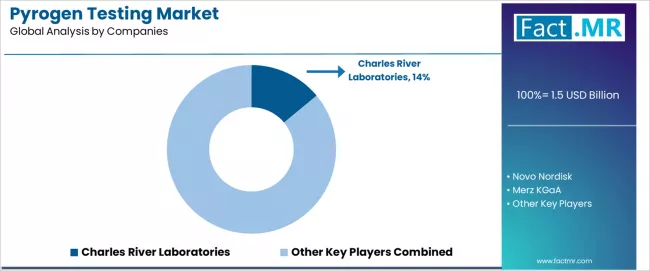
The pyrogen testing market exhibits a moderately consolidated competitive structure with approximately 40-60 active players operating across global pharmaceutical testing networks and regional laboratory product distribution portfolios. Charles River Laboratories maintains market leadership at a 14.0% share, reflecting strong product portfolio positioning across diverse testing methodologies with sophisticated global service strategies.
This competitive landscape demonstrates the maturation of pyrogen testing technology, where established players leverage brand recognition advantages, extensive validation evidence documentation, and laboratory relationship programs to maintain dominant positions, while emerging alternative method developers and regional reagent manufacturers create niche opportunities through specialized assay offerings and competitive pricing strategies.
Market leadership is maintained through several critical competitive advantages extending beyond manufacturing capabilities and product portfolios. Global distribution networks enable leading players to navigate diverse regulatory requirements and access varied laboratory segments including pharmaceutical quality control, contract testing organizations, and medical device manufacturers.
Technical support infrastructure and laboratory training program availability represent crucial differentiators in pyrogen testing categories, where decades of method validation expertise, troubleshooting protocols, and regulatory submission frameworks create purchasing preference among quality-focused laboratories.
Manufacturing efficiency in pharmaceutical-grade reagent production facilities, supply chain cold chain management, and raw material quality control separate major suppliers from smaller competitors, while comprehensive validation documentation addressing pharmacopeia compliance, method comparison studies, and stability data strengthen market position and laboratory confidence.
The market demonstrates emerging differentiation opportunities in recombinant factor C testing categories and monocyte activation technologies, where traditional LAL test methodologies face competition from innovation-focused entrants offering animal-free advantages.
Significant competitive advantages persist in established LAL test categories through comprehensive regulatory acceptance portfolios and laboratory relationship depth. Premium positioning strategies with automated platform integration and digital connectivity capabilities command margin premiums through superior efficiency and data management integration.
Specialized testing portfolios combining multiple detection methodologies with application-specific protocols create comprehensive positioning that justifies higher price points beyond commodity reagent competition. Integrated quality solution offerings emphasizing complementary service compatibility, unified technical support, and cross-methodology training programs generate brand loyalty and product line preferences beyond transactional testing purchases.
| Stakeholder | What they actually control | Typical strengths | Typical blind spots |
|---|---|---|---|
| Global pharmaceutical testing corporations | Comprehensive testing portfolios; global distribution; regulatory documentation | Brand recognition; validation evidence; laboratory relationships; service networks | Innovation speed; pricing flexibility; niche applications; emerging market customization |
| Specialized testing companies | Innovation expertise; testing technology; methodology optimization | Product differentiation; technical sophistication; regulatory training; laboratory loyalty | Market penetration; distribution infrastructure; price competitiveness; geographic coverage |
| Regional reagent manufacturers | Local production; cost optimization; regional distribution; laboratory proximity | Affordability positioning; delivery speed; local support; cultural understanding | Clinical validation; brand recognition; international expansion; advanced methodologies |
| Contract testing organizations | Service delivery; laboratory access; testing expertise; compliance documentation | Quality assurance; regulatory knowledge; method standardization; convenience positioning; repeat business | Product development; technical innovation; reagent manufacturing; premium positioning |
| Academic institutions | Research expertise; method development; laboratory education; validation studies | Scientific credibility; innovation influence; training programs; evidence generation | Commercial scaling; product distribution; marketing resources; customer awareness |
Key Players in the Pyrogen Testing Market
- Charles River Laboratories
- Novo Nordisk
- Merz KGaA
- GenScript
- bioMérieux SA
- Lonza Group
- Thermo Fisher Scientific, Inc.
- Seikagaku Biobusiness Corporation
- FUJIFILM Wako Chemicals USA Corporation (Pyrostar)
- MiCAN Technologies Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 1.5 billion |
| Product Type | Consumables, Instruments, Services |
| Test Type | LAL Test (Turbidimetric, Chromogenic, Gel Clot), In Vitro Pyrogen Test, Recombinant Factor C Testing, Monocyte Activation Test, Rabbit Test |
| End-Use | Pharmaceutical & Biotechnology Companies, Medical Device Companies, Others |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | USA, China, India, Germany, UK, France, Canada, and 15+ additional countries |
| Key Companies Profiled | Charles River Laboratories, Novo Nordisk, Merz KGaA, GenScript, bioMérieux SA, Lonza Group, Thermo Fisher Scientific, Inc. |
| Additional Attributes | Dollar sales by product and test type categories, regional adoption trends across Asia Pacific, North America, and Europe, competitive landscape with established pharmaceutical testing corporations and specialized laboratory companies, manufacturer preferences for LAL test methodologies and regulatory compliance, integration with pharmaceutical quality control facilities and contract testing organizations, innovations in alternative testing technologies and automated detection platforms, and development of sophisticated validation systems with enhanced sensitivity profiles and comprehensive regulatory documentation frameworks. |
Pyrogen Testing Market by Segments
-
Product Type :
- Consumables
- Instruments
- Services
-
Test Type :
- LAL Test
- Turbidimetric
- Chromogenic
- Gel Clot
- In Vitro Pyrogen Test
- Recombinant Factor C Testing
- Monocyte Activation Test
- Rabbit Test
- LAL Test
-
End-Use :
- Pharmaceutical & Biotechnology Companies
- Medical Device Companies
- Others
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- India
- China
- Japan
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product Type, 2025 to 2035
- Consumables
- Instruments
- Services
- Y to o to Y Growth Trend Analysis By Product Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Product Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Test Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Test Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Test Type, 2025 to 2035
- LAL Test
- In Vitro Pyrogen Test
- Recombinant Factor C Testing
- Monocyte Activation Test
- Rabbit Test
- Y to o to Y Growth Trend Analysis By Test Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Test Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Pharmaceutical & Biotechnology Companies
- Medical Device Companies
- Others
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Product Type
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Test Type
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Product Type
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Test Type
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Product Type
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Test Type
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Product Type
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Test Type
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Product Type
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Test Type
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Product Type
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Test Type
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Product Type
- By Test Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Test Type
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Product Type
- By Test Type
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product Type
- By Test Type
- By End Use
- Competition Analysis
- Competition Deep Dive
- Charles River Laboratories
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Novo Nordisk
- Merz KGaA
- GenScript
- bioMérieux SA
- Lonza Group
- Thermo Fisher Scientific, Inc.
- Seikagaku Biobusiness Corporation
- FUJIFILM Wako Chemicals USA Corporation (Pyrostar)
- MiCAN Technologies Inc.
- Charles River Laboratories
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Test Type, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Product Type
- Figure 6: Global Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Test Type
- Figure 9: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by End Use
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Product Type
- Figure 26: North America Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Test Type
- Figure 29: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by End Use
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Product Type
- Figure 36: Latin America Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Test Type
- Figure 39: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by End Use
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Product Type
- Figure 46: Western Europe Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Test Type
- Figure 49: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by End Use
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Product Type
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Test Type
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Product Type
- Figure 66: East Asia Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Test Type
- Figure 69: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by End Use
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Product Type
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Test Type
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Product Type
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Test Type, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Test Type, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Test Type
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the pyrogen testing market in 2025?
The global pyrogen testing market is estimated to be valued at USD 1.5 billion in 2025.
What will be the size of pyrogen testing market in 2035?
The market size for the pyrogen testing market is projected to reach USD 4.4 billion by 2035.
How much will be the pyrogen testing market growth between 2025 and 2035?
The pyrogen testing market is expected to grow at a 11.4% CAGR between 2025 and 2035.
What are the key product types in the pyrogen testing market?
The key product types in pyrogen testing market are consumables, instruments and services.
Which test type segment to contribute significant share in the pyrogen testing market in 2025?
In terms of test type, lal test segment to command 63.2% share in the pyrogen testing market in 2025.