Hepatitis Rapid Testing Market
Hepatitis Rapid Testing Market By Product Type (Test Strip, Cassette), By Test Type (Rapid Diagnostic Tests/ Point-of-care Tests, Immunoassay Tests, Molecular assay Tests), By Usage (Clinical Diagnostics, Academic Research Centers), By End-Users & By Region - Market Analysis Report till 2025 to 2035
Analysis of Hepatitis Rapid Testing market covering 30+ countries including analysis of US, Canada, UK, Germany, France, Nordics, GCC countries, Japan, Korea and many more
Hepatitis Rapid Testing Market Outlook 2025 to 2035
The Hepatitis Rapid Testing Market is expected to reach USD 5,481.1 Million by 2035, up from USD 3,145.6 Million in 2025, growing at a CAGR of 5.7%. With rising awareness and screening initiatives, demand for test strips and cassettes is rising across point-of-care settings globally.
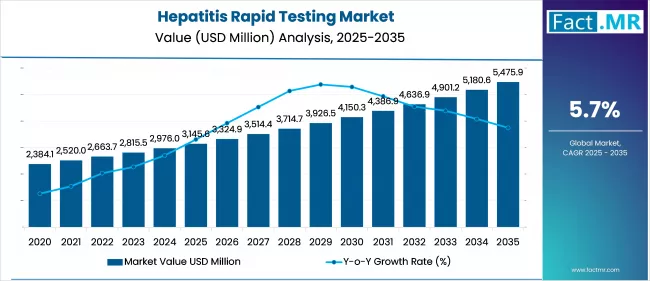
| Metric | Value |
|---|---|
| Hepatitis Rapid Testing Market Estimated Value in (2025 E) | USD 3145.6 million |
| Hepatitis Rapid Testing Market Forecast Value in (2035 F) | USD 5475.9 million |
| Forecast CAGR (2025 to 2035) | 5.7% |
According to latest research by Fact MR, hepatitis rapid testing market will experience substantial growth with CAGR of XX% during the forecast period 2021-2031. Increased demand for hepatitis rapid testing due to the high prevalence of hepatitis infections and increasing awareness about treatment and procedures available can give boost to this market.
| Countries | CAGR |
|---|---|
| China | 6.2% |
| United States | 5.9% |
| India | 5.4% |
| Brazil | 5.0% |
What is Driving Demand for Hepatitis Rapid Testing?
Hepatitis meaning inflammation or damage to the liver affects the functioning of liver associated with nutrient processing, filtering the blood and fighting against infection. Often caused by one of 5 viruses, hepatitis A is common in many countries and can be cured.
Whereas, hepatitis B (HBV) and hepatitis C (HCV) are majorly associated with liver transplants and liver cancer. If not diagnosed early, HBV and HCV can result in serious health issues including liver cirrhosis, liver failure, cancer or even death. Patients may not be able to recover from such viral infections, especially the immune-compromised people if left untreated.
Since medication and treatment are available for hepatitis A and B, regular testing has been recommend for diagnosing hepatitis C infections both acute and chronic cases. This will allow for preventive measures followed by lifesaving treatment to be undertaken by individuals infected with hepatitis C virus. This will also help to determine the degree of damage to the liver along with other health problems resulting from infection, thus preventing virus transmission to others.
As per World Health Organization (WHO) updates 2021, around 58 Mn people are suffering from hepatitis C virus infection around the world, with almost 1.5 Mn new cases of infection occurring every year.
The growing R&D facilities in the world, advances in rapid detection technology procedures, serological testing for identifying infected virus, increased access to test and care are driving the demand for hepatitis rapid testing during the forecast period from 2021 to 2031.
Technological Advancements opportunities for Hepatitis rapid Testing Market
With the help of technology and extensive studies on virus causing hepatitis especially HCV, establishment of various effective antiviral drugs and diagnostics devices have been carried out to treat the infections of HCV.
Improved therapeutic options for infection management and the development of new technologies to measure the viral load in the infected patients have significantly unlocked the market potential for hepatitis rapid testing.
Rapid diagnostics test are making their way in the field of hepatitis rapid testing, as these allow for diagnosing acute infections and confirming chronic cases of hepatitis allowing prompt action to be taken for treatment of the infected cases.
The technological innovations expected to provide more affordable yet simplified testing methods to test and monitor hepatitis infection is anticipated to make future growth of hepatitis rapid testing market.
US and Canada Hepatitis Rapid Testing Outlook
U.S. and Canada are among the largest market for Hepatitis Rapid Testing due to high prevalence of hepatitis cases.
As per Centers for Disease Control and Prevention (CDC) 2018 report, around 24,900 cases of hepatitis A are detected in USA almost every year. While 22600 cases of hepatitis B and 50,200 cases of hepatitis C were detected in year 2018. It was recorded that nearly 2 out 3 people having hepatitis B and 50% of those with hepatitis C were unaware of them being infected with respective viral infections.
Viral Hepatitis Surveillance report, 2019 stated that a total of 18846 cases of hepatitis A in USA were reported to CDC in 2019. Total cases of hepatitis B includes around 3192 acute cases and 13, 859 chronic cases while hepatitis C cases includes 4136 acute and 123,312 newly reported chronic cases during 2019.
Regular testing recommended by government for early diagnosis, various programs undertaken on national level to eliminate the viral hepatitis, public health programs initiated to prevent and control the hepatitis infection and recent development for the treatment of hepatitis C infection, all of these are expected to drive the demand for hepatitis rapid testing market in US and Canada.
Europe Demand Outlook for Hepatitis Rapid Testing
In Europe the prevalence of hepatitis infection cases are in rise due to transmission of virus through injecting drugs, sharing used and contaminated needles and from mother to child perinatally.
For Instances, as per reported data of European Centre for Disease Prevention and Control in 2019, 37,733 cases were reported for hepatitis C in Europe, of which 6% were acute, 22% chronic and 69% unknown. Most common mode of transmission was through injecting drug use.
On the other hand, 29,669 cases of hepatitis B virus infection were reported in 2019, with 6% as acute, 48% as chronic and rest either unknown or unclassified.
In order to reduce these numbers, it is important to test and diagnose the viral infection at the earliest to prevent further health deterioration. Thus creating demand for Hepatitis Rapid Testing Market in the forecast period.
Who are the Key Manufacturers and Suppliers of Hepatitis Rapid Testing?
Some of the leading manufacturers operating in the Hepatitis Rapid Testing include
- Abbott
- Standard Diagnostics Inc
- DiaSorin S.p.A
- SD Biosensor
- Kehua-Bio-Engineering Co. Ltd.
- bioMerieux SA
- Biokit S.A.
- RPC Diagnostics System
- Dia Pro- Diagnostic Bioprobes s.r.l.
- Creative Diagnostics
- Asan Pharm Co. Ltd.
- Fujirebio
- Orasure Technologies
- InTec Products Inc.
- Bio-Rad Laboratories Inc
- Sysmex Asia Pacific Pte Ltd.
- F. Hoffmann- La Roche Ltd.
Many leading manufacturers in the Hepatitis Rapid Testing are focusing on launching new products, collaboration, exclusive distribution and marketing agreements to enhance their market position.
Hepatitis Rapid Testing Market Report Highlights
- Detailed overview of parent market
- Changing market dynamics in the industry
- In-depth market segmentation
- Historical, current and projected market size in terms of volume and value
- Recent industry trends and developments
- Competitive landscape
- Strategies of key players and products offered
- Potential and niche segments, geographical regions exhibiting promising growth
- A neutral perspective on market performance
- Must-have information for market players to sustain and enhance their market footprint
Key Segments
-
By Product Type
- Test Strip
- Cassette
-
By Test Type
- Rapid Diagnostic Tests/ Point-of-care Tests
- Immunoassay Tests
- ELISA Kits
- Enzyme Immunoassay kit
- Molecular assay Tests
- Nucleic Acid Test/ PCR test
-
By Usage
- Clinical Diagnostics
- Academic Research Centers
-
By End Users
- Diagnostic Centers
- Pathology Laboratories
- Research Centers
- Specialty Clinics
- Hospitals
-
By Region
- North America
- Latin America
- Europe
- South Asia
- East Asia
- Oceania
- Middle East and Africa
The report is a compilation of first-hand information, qualitative and quantitative assessment by industry analysts, inputs from industry experts and industry participants across the value chain.
The report provides in-depth analysis of parent market trends, macro-economic indicators and governing factors along with market attractiveness as per segments. The report also maps the qualitative impact of various market factors on market segments and geographies.
NOTE: Although care has been taken to maintain the highest levels of accuracy in reports, recent market/vendor-specific changes may take time to reflect in the analysis.
Table of Content
- Executive Summary
- Global Market Outlook
- Demand-side Trends
- Supply-side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Inclusion and Exclusion
- Value Added Insights
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Supply Side Participants and their Roles
- Producers
- Mid-Level Participants (Traders/ Agents/ Brokers)
- Wholesalers and Distributors
- Value Added and Value Created at Node in the Supply Chain
- List of Raw Material Suppliers
- List of Existing and Potential Buyers
- Supply Side Participants and their Roles
- Pricing Analysis of Market
- Pricing Analysis By Region
- Pricing Analysis By Country
- Pricing Analysis By End Use
- Pricing Analysis By Distributor
- Pricing Analysis By Application
- Value Chain Analysis
- Profit Margin Analysis
- Wholesalers and Distributors
- Retailers
- Product Portfolio Analysis
- Regulatory Landscape
- By Key Regions
- By Key Countries
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Investment Feasibility Matrix
- PESTLE Analysis
- Political Factors
- Government stability and change
- Taxation policy and incentives
- Trade tariffs and import/export regulations
- Political risk and corruption levels
- Economic Factors
- GDP growth and economic cycles
- Inflation and interest rates
- Exchange rate fluctuations
- Unemployment and consumer spending power
- Social Factors
- Demographic trends (age, population growth)
- Cultural attitudes and lifestyle changes
- Education levels and workforce skills
- Health consciousness and social values
- Technological Factors
- Rate of technological innovation
- R&D activity and automation trends
- Digital infrastructure and connectivity
- Intellectual property protection
- Legal Factors
- Employment and labor laws
- Health & safety regulations
- Consumer protection laws
- Data privacy and antitrust legislation
- Environmental Factors
- Environmental regulations and compliance
- Climate change impacts and carbon footprint
- Resource availability and sustainability practices
- Waste management and recycling initiatives
- Political Factors
- Market Background and Dynamics
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- SWOT Analysis of Global Market
- Strengths
- Weaknesses
- Opportunities
- Threats
- Market Dynamics
- Global Market Analysis 2020-2024 and Forecast, 2025-2035
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Projections, 2025-2035
- Y-o-Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Revenue Forecast Scenario, 2020-2035
- Conservative Scenario
- Likely Scenario
- Optimistic Scenario
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis By Application, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By Application, 2025-2035
- Y-o-Y Growth Trend Analysis By Application, 2020-2024
- Absolute $ Opportunity Analysis By Application, 2025-2035
- Market Attractiveness Analysis By Application, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis By End Use, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By End Use, 2025-2035
- Y-o-Y Growth Trend Analysis By End Use, 2020-2024
- Absolute $ Opportunity Analysis By End Use, 2025-2035
- Market Attractiveness Analysis By End Use, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Region
- Introduction
- Historical Market Size Value (USD Mn) & Volume (Units) Analysis By Region, 2020-2024
- Current Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By Region, 2025-2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific & Pacific
- MEA
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020-2024 and Forecast 2025-2035
- Introduction
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Current and Future Market Size Value (USD Mn) & Volume (Units) Analysis and Forecast By Market Taxonomy, 2025-2035
- By Country
- USA
- Canada
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- USA Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Canada Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Mexico Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- USA Market
- Latin America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Brazil
- Argentina
- Chile
- Rest of Latin America
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Brazil Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Argentina Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Chile Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of Latin America Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Brazil Market
- Western Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Germany Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- UK Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Italy Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Spain Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- France Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Nordic Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- BENELUX Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of Western Europe Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Germany Market
- Eastern Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Russia Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Poland Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Hungary Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Balkan & Baltic Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of Eastern Europe Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Russia Market
- East Asia Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- China
- Japan
- South Korea
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- China Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Japan Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- South Korea Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- China Market
- South Asia and Pacific Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- India Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- ASEAN Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Australia & New Zealand Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of South Asia and Pacific Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- India Market
- MEA Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Mn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Mn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Application
- By End Use
- Market Attractiveness Analysis
- By Country
- By Application
- By End Use
- Key Takeaways
- Market Trends
- Key Market Participants - Intensity Mapping
- Drivers and Restraints - Impact Analysis
- Country Level Analysis & Forecast
- Kingdom of Saudi Arabia Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Other GCC Countries Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Turkiye Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- South Africa Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- South Africa Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of MEA Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Rest of MEA Market
- Introduction
- Market Analysis and Forecast by Market Taxonomy
- By Application
- By End Use
- Kingdom of Saudi Arabia Market
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Region
- By Application
- By End Use
- Competition Analysis
- Competition Deep Dive
- Global Key Players
- Key Players by Region
- Key Players by Country
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (US$ Mn) Forecast by Region, 2020 to 2035
- Table 2: Global Market Volume (Units) Forecast by Region, 2020 to 2035
- Table 3: Global Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 4: Global Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 5: Global Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 6: Global Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 7: North America Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 8: North America Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 9: North America Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 10: North America Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 11: North America Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 12: North America Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 13: Latin America Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 14: Latin America Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 15: Latin America Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 16: Latin America Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 17: Latin America Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 18: Latin America Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 19: Europe Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 20: Europe Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 21: Europe Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 22: Europe Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 23: Europe Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 24: Europe Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 25: East Asia Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 26: East Asia Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 27: East Asia Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 28: East Asia Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 29: East Asia Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 30: East Asia Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 31: South Asia Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 32: South Asia Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 33: South Asia Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 34: South Asia Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 35: South Asia Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 36: South Asia Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 37: Oceania Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 38: Oceania Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 39: Oceania Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 40: Oceania Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 41: Oceania Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 42: Oceania Market Volume (Units) Forecast by End Use, 2020 to 2035
- Table 43: MEA Market Value (US$ Mn) Forecast by Country, 2020 to 2035
- Table 44: MEA Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 45: MEA Market Value (US$ Mn) Forecast by Application, 2020 to 2035
- Table 46: MEA Market Volume (Units) Forecast by Application, 2020 to 2035
- Table 47: MEA Market Value (US$ Mn) Forecast by End Use, 2020 to 2035
- Table 48: MEA Market Volume (Units) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 2: Global Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 3: Global Market Value (US$ Mn) by Region, 2020 to 2035
- Figure 4: Global Market Value (US$ Mn) Analysis by Region, 2020 to 2035
- Figure 5: Global Market Volume (Units) Analysis by Region, 2020 to 2035
- Figure 6: Global Market Value Share (%) and BPS Analysis by Region, 2020 to 2035
- Figure 7: Global Market Y-o-Y Growth (%) Projections by Region, 2020 to 2035
- Figure 8: Global Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 9: Global Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 10: Global Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 11: Global Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 12: Global Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 13: Global Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 14: Global Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 15: Global Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 16: Global Market Attractiveness by Application, 2020 to 2035
- Figure 17: Global Market Attractiveness by End Use, 2020 to 2035
- Figure 18: Global Market Attractiveness by Region, 2020 to 2035
- Figure 19: North America Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 20: North America Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 21: North America Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 22: North America Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 23: North America Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 24: North America Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 25: North America Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 26: North America Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 27: North America Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 28: North America Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 29: North America Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 30: North America Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 31: North America Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 32: North America Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 33: North America Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 34: North America Market Attractiveness by Application, 2020 to 2035
- Figure 35: North America Market Attractiveness by End Use, 2020 to 2035
- Figure 36: North America Market Attractiveness by Country, 2020 to 2035
- Figure 37: Latin America Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 38: Latin America Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 39: Latin America Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 40: Latin America Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 41: Latin America Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 42: Latin America Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 43: Latin America Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 44: Latin America Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 45: Latin America Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 46: Latin America Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 47: Latin America Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 48: Latin America Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 49: Latin America Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 50: Latin America Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 51: Latin America Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 52: Latin America Market Attractiveness by Application, 2020 to 2035
- Figure 53: Latin America Market Attractiveness by End Use, 2020 to 2035
- Figure 54: Latin America Market Attractiveness by Country, 2020 to 2035
- Figure 55: Europe Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 56: Europe Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 57: Europe Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 58: Europe Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 59: Europe Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 60: Europe Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 61: Europe Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 62: Europe Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 63: Europe Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 64: Europe Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 65: Europe Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 66: Europe Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 67: Europe Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 68: Europe Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 69: Europe Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 70: Europe Market Attractiveness by Application, 2020 to 2035
- Figure 71: Europe Market Attractiveness by End Use, 2020 to 2035
- Figure 72: Europe Market Attractiveness by Country, 2020 to 2035
- Figure 73: East Asia Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 74: East Asia Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 75: East Asia Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 76: East Asia Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 77: East Asia Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 78: East Asia Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 79: East Asia Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 80: East Asia Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 81: East Asia Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 82: East Asia Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 83: East Asia Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 84: East Asia Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 85: East Asia Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 86: East Asia Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 87: East Asia Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 88: East Asia Market Attractiveness by Application, 2020 to 2035
- Figure 89: East Asia Market Attractiveness by End Use, 2020 to 2035
- Figure 90: East Asia Market Attractiveness by Country, 2020 to 2035
- Figure 91: South Asia Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 92: South Asia Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 93: South Asia Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 94: South Asia Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 95: South Asia Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 96: South Asia Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 97: South Asia Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 98: South Asia Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 99: South Asia Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 100: South Asia Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 101: South Asia Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 102: South Asia Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 103: South Asia Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 104: South Asia Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 105: South Asia Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 106: South Asia Market Attractiveness by Application, 2020 to 2035
- Figure 107: South Asia Market Attractiveness by End Use, 2020 to 2035
- Figure 108: South Asia Market Attractiveness by Country, 2020 to 2035
- Figure 109: Oceania Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 110: Oceania Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 111: Oceania Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 112: Oceania Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 113: Oceania Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 114: Oceania Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 115: Oceania Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 116: Oceania Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 117: Oceania Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 118: Oceania Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 119: Oceania Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 120: Oceania Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 121: Oceania Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 122: Oceania Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 123: Oceania Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 124: Oceania Market Attractiveness by Application, 2020 to 2035
- Figure 125: Oceania Market Attractiveness by End Use, 2020 to 2035
- Figure 126: Oceania Market Attractiveness by Country, 2020 to 2035
- Figure 127: MEA Market Value (US$ Mn) by Application, 2020 to 2035
- Figure 128: MEA Market Value (US$ Mn) by End Use, 2020 to 2035
- Figure 129: MEA Market Value (US$ Mn) by Country, 2020 to 2035
- Figure 130: MEA Market Value (US$ Mn) Analysis by Country, 2020 to 2035
- Figure 131: MEA Market Volume (Units) Analysis by Country, 2020 to 2035
- Figure 132: MEA Market Value Share (%) and BPS Analysis by Country, 2020 to 2035
- Figure 133: MEA Market Y-o-Y Growth (%) Projections by Country, 2020 to 2035
- Figure 134: MEA Market Value (US$ Mn) Analysis by Application, 2020 to 2035
- Figure 135: MEA Market Volume (Units) Analysis by Application, 2020 to 2035
- Figure 136: MEA Market Value Share (%) and BPS Analysis by Application, 2020 to 2035
- Figure 137: MEA Market Y-o-Y Growth (%) Projections by Application, 2020 to 2035
- Figure 138: MEA Market Value (US$ Mn) Analysis by End Use, 2020 to 2035
- Figure 139: MEA Market Volume (Units) Analysis by End Use, 2020 to 2035
- Figure 140: MEA Market Value Share (%) and BPS Analysis by End Use, 2020 to 2035
- Figure 141: MEA Market Y-o-Y Growth (%) Projections by End Use, 2020 to 2035
- Figure 142: MEA Market Attractiveness by Application, 2020 to 2035
- Figure 143: MEA Market Attractiveness by End Use, 2020 to 2035
- Figure 144: MEA Market Attractiveness by Country, 2020 to 2035