Hernia Mesh Devices Market
Hernia Mesh Devices Market Analysis- Size, Share, and Forecast Outlook 2025 to 2035
The global hernia mesh devices market is forecast to reach USD 7.5 billion by 2035, up from USD 5.1 billion in 2025. During the forecast period, the industry is projected to register at a CAGR of 4.0%.
Hernia Mesh Devices Market Outlook (2025 to 2035)
The global hernia mesh devices market is forecast to reach USD 7.5 billion by 2035, up from USD 5.1 billion in 2025. During the forecast period, the industry is projected to register at a CAGR of 4.0%, driven by the rising global prevalence of hernias due to aging and obesity, the adoption of minimally invasive surgeries for faster recovery, and advancements in mesh materials for better outcomes. Healthcare investments and awareness in emerging economies create significant opportunities for expansion.
Quick Stats of Hernia Mesh Devices Market
- Hernia Mesh Devices Market Size (2025): USD 5.1 billion.
- Projected Hernia Mesh Devices Market Size (2035): USD 7.5 billion
- Forecast CAGR of Hernia Mesh Devices Market (2025 to 2035): 4.0%
- Leading Hernia Type Segment of Hernia Mesh Devices Market: Inguinal Hernia
- Key Growth Regions of Hernia Mesh Devices Market: United States, Germany, Japan
- Prominent Players in the Hernia Mesh Devices Market: Aspide Medical, Atrium, B. Braun Melsungen AG, Baxter International Inc., Others
2025-to-2035.webp)
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 5.1 billion |
| Industry Size (2035F) | USD 7.5 billion |
| CAGR (2025-2035) | 4.0% |
The market for hernia mesh devices is expected to experience steady growth, with projections indicating it will reach USD 5.30 billion by 2026, USD 5.73 billion by 2028, and USD 6.68 billion by 2032. This shows a consistent annual growth rate of around 3.93% from 2025 to 2035.
The growing incidence of hernias, influenced by an aging demographic, escalating obesity levels, and complications following surgery, is maintaining a strong demand for mesh implants. The increase in elective surgeries in both developed and developing markets highlights the demand for mesh-based repair solutions.
Manufacturers are developing advanced lightweight, high-strength meshes aimed at minimizing post-operative complications and enhancing recovery times. The integration of biologic and biosynthetic meshes, which provide enhanced biocompatibility and lower infection risks, is shaping surgical decisions. Minimally invasive techniques like laparoscopic and robotic-assisted repairs are driving market expansion, as they necessitate specialized mesh products.
North America and Europe are poised to uphold robust market standings, driven by cutting-edge healthcare systems and the swift adoption of innovative surgical technologies. Asia-Pacific, Latin America, and the Middle East are rising growth areas, bolstered by enhanced healthcare access, increasing surgical capabilities, and medical tourism centers like India, Thailand, and Turkey.
Top Hernia Mesh Devices Market Dynamics
Hernia prevalence, improvements in mesh materials, and minimally invasive and robotic-assisted surgery are driving the hernia mesh devices industry. Innovative products include lightweight, biocompatible, and partially absorbable meshes that improve patient recovery and clinical results, making manufacturers more competitive.
Strict regulations constrain the market, product recalls, and patient concerns about risks. High product costs and little reimbursement in developing nations hinder adoption. Despite these challenges, the rising economies' healthcare infrastructure and medical tourism offer economic prospects.
Rising Prevalence of Hernia Cases Drives Device Demand
The global incidence of hernias continues to climb, providing a steady demand for repair devices and consumables. Hernias develop when an organ or fatty tissue protrudes through the surrounding muscle, necessitating surgical intervention to relocate and stabilize the tissue.
According to PLOS Biology (April 2022), inguinal hernias alone affect a sizable proportion of the global population, causing over 40,000 deaths and requiring an estimated 20 billion repairs each year. This expanding patient base translates into ongoing market prospects for manufacturers and suppliers of sophisticated mesh technologies.
Technological Advancements Improve Surgical Outcomes
Continuous improvement in mesh design is improving patient recovery and surgical efficiency. Lightweight, composite, and absorbable materials are being developed to improve biocompatibility, reduce postoperative pain, and correspond with increasing surgical preferences.
Manufacturers who include these sophisticated materials in their product portfolios are better positioned to gain market share by providing solutions that produce improved clinical outcomes.
Surge in Minimally Invasive and Robotic-Assisted Hernia Surgery
The usage of mesh-based repairs is increasing as laparoscopic and robotic-assisted procedures become more common. These minimally invasive methods enable speedier patient recovery, lower complication rates, and higher satisfaction levels, making them increasingly popular among surgeons.
With healthcare facilities investing in robotic-assisted capabilities, demand for high-performance hernia mesh devices is expected to rise, providing significant revenue opportunities for market participants.
Frequent Product Recalls and Lawsuits May Impede Growth
The increasing number of product recalls and lawsuits related to complications associated with mesh devices complicates the dynamics. Reports of chronic pain, infection, mesh migration, and adhesion have alarmed patients and healthcare providers. For instance, the FDA has received thousands of adverse event reports involving hernia mesh, prompting recalls by several manufacturers.
Patient skepticism and increased awareness of these complications have also contributed to hesitancy in choosing mesh-based repairs. Some patients prefer non-mesh alternatives due to concerns about side effects, which limits the market's potential in certain regions. This reluctance affects procedure volume, resulting in a preference for traditional suturing methods in some healthcare settings.
Tightening Regulatory Frameworks Delaying Product Approvals
Stringent regulatory frameworks and lengthy product approval processes in key markets such as the United States and the European Union further limit market growth. To obtain regulatory clearance, manufacturers must invest heavily in clinical trials and documentation, which can cause product launch delays.
Additionally, the high cost of advanced mesh products and limited reimbursement in developing countries hinder adoption. Many healthcare facilities in low-income areas lack access to minimally invasive surgical tools and premium mesh devices, limiting market penetration.
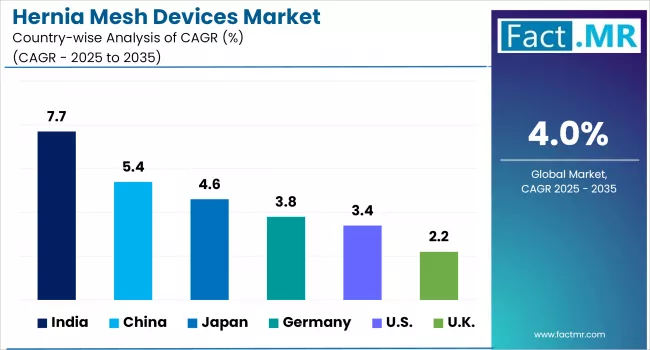
Top Countries in the Global Hernia Mesh Devices Market
The United States continues to be the leading market for hernia mesh devices, propelled by significant surgical volumes, swift integration of minimally invasive and robotic-assisted methods, and a sophisticated healthcare infrastructure. Strict FDA regulations uphold rigorous product safety standards, yet they also establish considerable entry barriers that benefit established manufacturers.
Germany’s market thrives on data-driven surgical protocols, comprehensive clinical registries, and a high level of adherence to EU MDR standards, fostering confidence in certified devices. The nation is experiencing an increase in the use of biologic and partially absorbable meshes, especially for intricate or recurring hernias.
Japan’s universal healthcare framework guarantees consistent demand for hernia repair devices, with the uniform application of synthetic meshes in both public and private hospitals.
The initial embrace of robotic-assisted and laparoscopic methods in urban centers is generating prospects for lightweight, anatomically tailored meshes. The PMDA’s stringent regulatory framework maintains elevated safety and efficacy standards, although it restricts market entry for firms with significant clinical evidence.
Country-Wise Outlook
| Countries | CAGR (2025 to 2035) |
|---|---|
| United States | 2.8% |
| Germany | 5.6% |
| Japan | 3.2% |
United States is the Dominant Market with High Surgical Volume
2025-to-2035.webp)
The United States dominates the global hernia mesh devices market, bolstered by a substantial volume of annual repair surgeries and a sophisticated healthcare infrastructure. Inguinal hernias constitute around 800,000 surgical procedures annually. The swift implementation of laparoscopic and robotic-assisted methods is elevating the demand for specialized, high-performance meshes.
Rigorous FDA regulation guarantees product safety and efficacy, however, it imposes significant entry hurdles due to expensive and protracted clinical trials. The recall of specific mesh types has intensified regulatory oversight, compelling producers to allocate resources towards safety data and innovation.
- Market propelled by elevated procedural volume and sophisticated facilities.
- Robust acceptance of minimally invasive and robotic-assisted repairs.
- Stringent regulatory standards increase barriers while augmenting product trust.
Evidence-Based Strategy Enhances Adoption in Germany
The German market is bolstered by defined surgical methods and clinical registries, particularly via the Herniamed database of the German Hernia Society. Mesh-based methodologies are extensively employed, particularly for inguinal hernias, with an increasing utilization of biological and partially absorbable meshes for intricate instances.
Compliance with the EU Medical Device Regulation (MDR) necessitates comprehensive clinical data, prioritizing superior products from reputable manufacturers. Hospitals are increasingly prioritizing value-based solutions that alleviate chronic pain and mitigate foreign body responses, particularly in geriatric patients.
- Evidence-based protocols and registries promote quality and uniformity.
- MDR compliance benefits are established, and thoroughly documented product portfolios.
- Biologic and partially absorbable meshes are increasingly utilized for intricate repairs.
Japan’s Comprehensive Healthcare helps Pioneer Technology Adoption
Japan's universal healthcare system guarantees extensive access to hernia treatments, with the utilization of synthetic mesh standardized throughout both public and commercial hospitals. Inguinal hernias persist as a prevalent surgical problem, sustaining consistent procedural volumes.
The nation is an early adopter of robotic-assisted surgery, driving demand for laparoscopic repair. Urban hospitals are allocating resources on lightweight, anatomically designed meshes appropriate for less invasive surgeries. The PMDA's rigorous regulatory framework guarantees product safety while restricting market access to firms possessing robust clinical evidence.
- Universal healthcare maintains stable treatment rates.
- Significant prevalence of laparoscopic and robotic-assisted hernia repairs.
- Stringent PMDA requirements guarantee safety while limiting market access to validated items.
Analyzing Hernia Mesh Devices Market by Leading Segments
Inguinal hernias represent the majority of surgical procedures, whereas incisional hernias are on the rise due to higher surgical volumes and increasing obesity rates.
Synthetic meshes dominate the material segment, recognized for their durability and low recurrence rates, whereas biologic meshes are increasingly popular due to their excellent tissue integration and lower complication risks.
Open surgery remains relevant for addressing intricate cases, yet minimally invasive techniques are increasingly gaining traction thanks to quicker recovery periods and less patient discomfort.
Hospitals stand out as the leading end-users because of their significant patient volume and advanced surgical capabilities. Ambulatory Surgical Centers (ASCs) represent a rapidly expanding sector, driven by cost efficiency, patient inclination towards outpatient services.
Inguinal and Incisional Hernias- Key Factors Influencing Device Demand
Inguinal hernias continue to be the most prevalent, accounting for the majority of hernia repair procedures. Their significant prevalence among men and increasing patient awareness about timely treatment drive a steady need for innovative repair solutions.
Incisional hernias, frequently arising from abdominal surgeries, are becoming more common due to increased surgical procedures and growing obesity rates. The demand for efficient repair solutions—especially mesh and fixation devices—is increasing due to these considerations.
- Inguinal hernias are prevalent worldwide, leading to significant surgical intervention rates.
- Incisional hernia cases are increasing in tandem with the growth of surgical procedures worldwide and the prevalence of obesity.
- Cutting-edge mesh-based solutions are essential for the growth of both segments.
Synthetic Mesh the Leading Choice, Biological Mesh Gaining Traction
Synthetic meshes dominate the market thanks to their established durability, minimal recurrence rates, and strong preference among surgeons. Research indicates that recurrence rates can be as low as 1-3% for inguinal repairs utilizing synthetic mesh, whereas non-mesh repairs may have rates as high as 30%.
Biological meshes, though more expensive, are increasingly being adopted due to their enhanced biocompatibility and reduced risk of complications, especially in challenging or high-risk scenarios. Ongoing research and development in biologics is broadening their application in surgical settings.
- Synthetic meshes dominate the market, propelled by their proven clinical effectiveness and economic advantages.
- Biological meshes represent a rapidly expanding area, preferred for their ability to minimize chronic pain and enhance tissue integration.
- Continuous advancements in materials are influencing the landscape of competition.
Open Surgery Prevails, Less Invasive Techniques Gaining Popularity
Traditional open surgery remains significant for addressing large or complex hernias, even though it may involve extended recovery times. The ongoing utilization is supported by established results and the familiarity of surgeons.
Laparoscopic techniques are increasingly becoming popular because they offer shorter recovery times, less pain, and improved cosmetic outcomes. Healthcare facilities and surgical centers are enhancing their minimally invasive offerings, leading to a growing need for specialized mesh products.
- Open surgery continues to be a fundamental approach for intricate hernia cases.
- Laparoscopic repair is on the rise as both patients and surgeons increasingly favor minimally invasive techniques.
- Specialized meshes for minimally invasive surgeries represent an emerging opportunity for increased revenue.
Hospitals at the Forefront, ASCs Growing Quickly
Healthcare facilities lead the sector thanks to substantial surgical activity and sophisticated infrastructure. Their role is further strengthened by the presence of dedicated hernia repair facilities.
Ambulatory Surgical Centers (ASCs) represent a rapidly expanding segment, propelled by cost efficiency, patient inclination towards outpatient services, and the growing practicality of conducting minimally invasive hernia repairs in non-hospital environments.
- Healthcare facilities continue to be the main setting for hernia repair procedures, accommodating a significant number of patients.
- ASCs are experiencing swift growth driven by their cost-effectiveness and convenience benefits.
- The increase in outpatient minimally invasive procedures drives the adoption of ASCs.
Competitive Analysis
The hernia mesh devices market is characterized by fierce competition, which is driven by technological advancements, rising surgical volumes, and the growing prevalence of hernia cases worldwide. Mesh implants are the preferred solution for hernia repairs because they can reduce recurrence rates, improve surgical outcomes, and shorten patient recovery time. Companies in this space are constantly competing to improve product biocompatibility, durability, and ease of use for both open and laparoscopic procedures.
The type of mesh used is a key market differentiator. Synthetic meshes, particularly polypropylene and composite variants, are preferred due to their demonstrated clinical performance and cost-effectiveness. However, the emergence of biologic meshes made from human or animal tissue complicates the competitive landscape. This biologic meshes are gaining popularity in high-risk patients due to lower infection rates and the ability to integrate more naturally with human tissue, but their high cost remains a barrier to widespread adoption.
Continuous product innovations, such as self-fixating meshes, 3D anatomical meshes, and meshes with antimicrobial coatings, add to the competitive intensity. These innovations aim to address clinical challenges such as chronic pain, infection, and adhesion formation, giving businesses a competitive advantage. Companies are also focusing on minimally invasive surgical solutions, which are gaining popularity among both patients and surgeons due to their faster recovery time and lower risk of postoperative complications.
Key players in the hernia mesh devices industry are Aspide Medical, Atrium, B. Braun Melsungen AG, Baxter International Inc., C. R. Bard Inc., Cook Medical, Cousin Biotech, Covidien (Part of Medtronic), Dipromed, Ethicon Inc., Feg Textiltechnik MBH, Herniamesh S.r.l, LifeCell Corporation, W. L. Gore & Associates and other players.
Recent Developments
- In May 2024,Medtronic began additional clinical studies on its Hugo robotic-assisted surgery system in order to broaden its indications to include hernias and gynecology.
- In April 2025, Becton, Dickinson and Company, a medical technology company, received 510(k) clearance from the U.S. Food and Drug Administration (FDA) and commercialized the Phasix™ ST Umbilical Hernia Patch. It was the first and only fully absorbable hernia patch created specifically for umbilical hernias.
- In April 2024, TELA Bio, a medical technology company, has launched the OviTex IHR (Inguinal Hernia Repair) Reinforced Tissue Matrix in the United States. This product was created specifically for laparoscopic and robotic-assisted procedures. This addressed the need for more natural repair options in the nation's over 665,000 inguinal hernia surgeries each year.
Segmentation of Hernia Mesh Devices Market
-
By Hernia Type :
- Inguinal
- Incisional
- Femoral
-
By Mesh Type :
- Synthetic
- Biological
-
By Procedure :
- Open Surgeries
- Laparoscopic Surgeries
- Robotic Surgeries
-
By End-User :
- Hospitals
- Ambulatory Surgical Centers
- Clinics
-
By Region :
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia & Pacific
- Middle East & Africa
Table of Content
- Executive Summary
- Global Market Outlook
- Demand-side Trends
- Supply-side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Supply Side Participants and their Roles
- Producers
- Mid-Level Participants (Traders/ Agents/ Brokers)
- Wholesalers and Distributors
- Value Added and Value Created at Node in the Supply Chain
- List of Component Suppliers
- List of Existing and Potential Buyers
- Supply Side Participants and their Roles
- Investment Feasibility Matrix
- Value Chain Analysis
- Profit Margin Analysis
- Wholesalers and Distributors
- Retailers
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- By Key Regions
- By Key Countries
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020-2024 and Forecast, 2025-2035
- Historical Market Size Value (USD Bn) & Volume (Units) Analysis, 2020-2024
- Current and Future Market Size Value (USD Bn) & Volume (Units) Projections, 2025-2035
- Y-o-Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020-2024 and Forecast 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Hernia Type
- Introduction / Key Findings
- Historical Market Size Value (USD Bn) & Volume (Units) Analysis By Hernia Type, 2020-2024
- Current and Future Market Size Value (USD Bn) & Volume (Units) Analysis and Forecast By Hernia Type, 2025-2035
- Inguinal
- Incisional
- Femoral
- Y-o-Y Growth Trend Analysis By Hernia Type, 2020-2024
- Absolute $ Opportunity Analysis By Hernia Type, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Mesh Type
- Introduction / Key Findings
- Historical Market Size Value (USD Bn) & Volume (Units) Analysis By Mesh Type, 2020-2024
- Current and Future Market Size Value (USD Bn) & Volume (Units) Analysis and Forecast By Mesh Type, 2025-2035
- Synthetic
- Biological
- Y-o-Y Growth Trend Analysis By Mesh Type, 2020-2024
- Absolute $ Opportunity Analysis By Mesh Type, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Procedure
- Introduction / Key Findings
- Historical Market Size Value (USD Bn) & Volume (Units) Analysis By Procedure, 2020-2024
- Current and Future Market Size Value (USD Bn) & Volume (Units) Analysis and Forecast By Procedure, 2025-2035
- Open Surgeries
- Laparoscopic Surgeries
- Robotic Surgeries
- Y-o-Y Growth Trend Analysis By Procedure, 2020-2024
- Absolute $ Opportunity Analysis By Procedure, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By End-User
- Introduction / Key Findings
- Historical Market Size Value (USD Bn) & Volume (Units) Analysis By End-User, 2020-2024
- Current and Future Market Size Value (USD Bn) & Volume (Units) Analysis and Forecast By End-User, 2025-2035
- Hospitals
- Ambulatory Surgical Centers
- Clinics
- Y-o-Y Growth Trend Analysis By End-User, 2020-2024
- Absolute $ Opportunity Analysis By End-User, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Region
- Introduction
- Historical Market Size Value (USD Bn) & Volume (Units) Analysis By Region, 2020-2024
- Current Market Size Value (USD Bn) & Volume (Units) Analysis and Forecast By Region, 2025-2035
- North America
- Latin America
- Western Europe
- East Asia
- South Asia Pacific
- Eastern Europe
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Bn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Bn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- U.S.
- Canada
- Mexico
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- By Country
- Market Attractiveness Analysis
- By Country
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Key Takeaways
- Latin America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Bn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Bn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- By Country
- Market Attractiveness Analysis
- By Country
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Key Takeaways
- Western Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Bn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Bn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Germany
- Italy
- France
- U.K.
- Spain
- Russia
- BENELUX
- Rest of Europe
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- By Country
- Market Attractiveness Analysis
- By Country
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Key Takeaways
- East Asia Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Bn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Bn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- China
- Japan
- South Korea
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- By Country
- Market Attractiveness Analysis
- By Country
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Key Takeaways
- South Asia Pacific Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Bn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Bn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- India
- ASEAN Countries
- Australia & New Zealand
- Rest of South Asia Pacific
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- By Country
- Market Attractiveness Analysis
- By Country
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Key Takeaways
- Eastern Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Bn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Bn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltics
- Rest of Eastern Europe
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- By Country
- Market Attractiveness Analysis
- By Country
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Key Takeaways
- Middle East & Africa Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Bn) & Volume (Units) Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Bn) & Volume (Units) Forecast By Market Taxonomy, 2025-2035
- By Country
- KSA
- Other GCC Countries
- Turkiye
- South Africa
- Rest of MEA
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- By Country
- Market Attractiveness Analysis
- By Country
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Key Takeaways
- Key Countries Market Analysis
- Value (USD Bn) & Volume (Units)ed States
- Pricing Analysis
- Market Share Analysis, 2024
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Value (USD Bn) & Volume (Units)ed Kingdom
- Pricing Analysis
- Market Share Analysis, 2024
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Value (USD Bn) & Volume (Units)ed States
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Hernia Type
- By Mesh Type
- By Procedure
- By End-User
- Competition Analysis
- Competition Deep Dive
- Ethicon, Inc
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Feg Textiltechnik MBH
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Herniamesh S.r.l
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- LifeCell Corporation
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- W. L. Gore & Associates
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Ethicon, Inc
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Bn) Forecast by Region, 2020 to 2035
- Table 2: Global Market Volume (Units) Forecast by Region, 2020 to 2035
- Table 3: Global Market Value (USD Bn) Forecast by Hernia Type, 2020 to 2035
- Table 4: Global Market Volume (Units) Forecast by Hernia Type, 2020 to 2035
- Table 5: Global Market Value (USD Bn) Forecast by Mesh Type, 2020 to 2035
- Table 6: Global Market Volume (Units) Forecast by Mesh Type, 2020 to 2035
- Table 7: Global Market Value (USD Bn) Forecast by Procedure, 2020 to 2035
- Table 8: Global Market Volume (Units) Forecast by Procedure, 2020 to 2035
- Table 9: Global Market Value (USD Bn) Forecast by End-User, 2020 to 2035
- Table 10: Global Market Volume (Units) Forecast by End-User, 2020 to 2035
- Table 11: North America Market Value (USD Bn) Forecast by Country, 2020 to 2035
- Table 12: North America Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 13: North America Market Value (USD Bn) Forecast by Hernia Type, 2020 to 2035
- Table 14: North America Market Volume (Units) Forecast by Hernia Type, 2020 to 2035
- Table 15: North America Market Value (USD Bn) Forecast by Mesh Type, 2020 to 2035
- Table 16: North America Market Volume (Units) Forecast by Mesh Type, 2020 to 2035
- Table 17: North America Market Value (USD Bn) Forecast by Procedure, 2020 to 2035
- Table 18: North America Market Volume (Units) Forecast by Procedure, 2020 to 2035
- Table 19: North America Market Value (USD Bn) Forecast by End-User, 2020 to 2035
- Table 20: North America Market Volume (Units) Forecast by End-User, 2020 to 2035
- Table 21: Latin America Market Value (USD Bn) Forecast by Country, 2020 to 2035
- Table 22: Latin America Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 23: Latin America Market Value (USD Bn) Forecast by Hernia Type, 2020 to 2035
- Table 24: Latin America Market Volume (Units) Forecast by Hernia Type, 2020 to 2035
- Table 25: Latin America Market Value (USD Bn) Forecast by Mesh Type, 2020 to 2035
- Table 26: Latin America Market Volume (Units) Forecast by Mesh Type, 2020 to 2035
- Table 27: Latin America Market Value (USD Bn) Forecast by Procedure, 2020 to 2035
- Table 28: Latin America Market Volume (Units) Forecast by Procedure, 2020 to 2035
- Table 29: Latin America Market Value (USD Bn) Forecast by End-User, 2020 to 2035
- Table 30: Latin America Market Volume (Units) Forecast by End-User, 2020 to 2035
- Table 31: Western Europe Market Value (USD Bn) Forecast by Country, 2020 to 2035
- Table 32: Western Europe Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 33: Western Europe Market Value (USD Bn) Forecast by Hernia Type, 2020 to 2035
- Table 34: Western Europe Market Volume (Units) Forecast by Hernia Type, 2020 to 2035
- Table 35: Western Europe Market Value (USD Bn) Forecast by Mesh Type, 2020 to 2035
- Table 36: Western Europe Market Volume (Units) Forecast by Mesh Type, 2020 to 2035
- Table 37: Western Europe Market Value (USD Bn) Forecast by Procedure, 2020 to 2035
- Table 38: Western Europe Market Volume (Units) Forecast by Procedure, 2020 to 2035
- Table 39: Western Europe Market Value (USD Bn) Forecast by End-User, 2020 to 2035
- Table 40: Western Europe Market Volume (Units) Forecast by End-User, 2020 to 2035
- Table 41: East Asia Market Value (USD Bn) Forecast by Country, 2020 to 2035
- Table 42: East Asia Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 43: East Asia Market Value (USD Bn) Forecast by Hernia Type, 2020 to 2035
- Table 44: East Asia Market Volume (Units) Forecast by Hernia Type, 2020 to 2035
- Table 45: East Asia Market Value (USD Bn) Forecast by Mesh Type, 2020 to 2035
- Table 46: East Asia Market Volume (Units) Forecast by Mesh Type, 2020 to 2035
- Table 47: East Asia Market Value (USD Bn) Forecast by Procedure, 2020 to 2035
- Table 48: East Asia Market Volume (Units) Forecast by Procedure, 2020 to 2035
- Table 49: East Asia Market Value (USD Bn) Forecast by End-User, 2020 to 2035
- Table 50: East Asia Market Volume (Units) Forecast by End-User, 2020 to 2035
- Table 51: South Asia Pacific Market Value (USD Bn) Forecast by Country, 2020 to 2035
- Table 52: South Asia Pacific Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 53: South Asia Pacific Market Value (USD Bn) Forecast by Hernia Type, 2020 to 2035
- Table 54: South Asia Pacific Market Volume (Units) Forecast by Hernia Type, 2020 to 2035
- Table 55: South Asia Pacific Market Value (USD Bn) Forecast by Mesh Type, 2020 to 2035
- Table 56: South Asia Pacific Market Volume (Units) Forecast by Mesh Type, 2020 to 2035
- Table 57: South Asia Pacific Market Value (USD Bn) Forecast by Procedure, 2020 to 2035
- Table 58: South Asia Pacific Market Volume (Units) Forecast by Procedure, 2020 to 2035
- Table 59: South Asia Pacific Market Value (USD Bn) Forecast by End-User, 2020 to 2035
- Table 60: South Asia Pacific Market Volume (Units) Forecast by End-User, 2020 to 2035
- Table 61: Eastern Europe Market Value (USD Bn) Forecast by Country, 2020 to 2035
- Table 62: Eastern Europe Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 63: Eastern Europe Market Value (USD Bn) Forecast by Hernia Type, 2020 to 2035
- Table 64: Eastern Europe Market Volume (Units) Forecast by Hernia Type, 2020 to 2035
- Table 65: Eastern Europe Market Value (USD Bn) Forecast by Mesh Type, 2020 to 2035
- Table 66: Eastern Europe Market Volume (Units) Forecast by Mesh Type, 2020 to 2035
- Table 67: Eastern Europe Market Value (USD Bn) Forecast by Procedure, 2020 to 2035
- Table 68: Eastern Europe Market Volume (Units) Forecast by Procedure, 2020 to 2035
- Table 69: Eastern Europe Market Value (USD Bn) Forecast by End-User, 2020 to 2035
- Table 70: Eastern Europe Market Volume (Units) Forecast by End-User, 2020 to 2035
- Table 71: Middle East & Africa Market Value (USD Bn) Forecast by Country, 2020 to 2035
- Table 72: Middle East & Africa Market Volume (Units) Forecast by Country, 2020 to 2035
- Table 73: Middle East & Africa Market Value (USD Bn) Forecast by Hernia Type, 2020 to 2035
- Table 74: Middle East & Africa Market Volume (Units) Forecast by Hernia Type, 2020 to 2035
- Table 75: Middle East & Africa Market Value (USD Bn) Forecast by Mesh Type, 2020 to 2035
- Table 76: Middle East & Africa Market Volume (Units) Forecast by Mesh Type, 2020 to 2035
- Table 77: Middle East & Africa Market Value (USD Bn) Forecast by Procedure, 2020 to 2035
- Table 78: Middle East & Africa Market Volume (Units) Forecast by Procedure, 2020 to 2035
- Table 79: Middle East & Africa Market Value (USD Bn) Forecast by End-User, 2020 to 2035
- Table 80: Middle East & Africa Market Volume (Units) Forecast by End-User, 2020 to 2035
List Of Figures
- Figure 1: Global Market Volume (Units) Forecast 2020 to 2035
- Figure 2: Global Market Pricing Analysis
- Figure 3: Global Market Value (USD Bn) Forecast 2020 to 2035
- Figure 4: Global Market Value Share and BPS Analysis by Hernia Type, 2025 and 2035
- Figure 5: Global Market Y-o-Y Growth Comparison by Hernia Type, 2025 to 2035
- Figure 6: Global Market Attractiveness Analysis by Hernia Type
- Figure 7: Global Market Value Share and BPS Analysis by Mesh Type, 2025 and 2035
- Figure 8: Global Market Y-o-Y Growth Comparison by Mesh Type, 2025 to 2035
- Figure 9: Global Market Attractiveness Analysis by Mesh Type
- Figure 10: Global Market Value Share and BPS Analysis by Procedure, 2025 and 2035
- Figure 11: Global Market Y-o-Y Growth Comparison by Procedure, 2025 to 2035
- Figure 12: Global Market Attractiveness Analysis by Procedure
- Figure 13: Global Market Value Share and BPS Analysis by End-User, 2025 and 2035
- Figure 14: Global Market Y-o-Y Growth Comparison by End-User, 2025 to 2035
- Figure 15: Global Market Attractiveness Analysis by End-User
- Figure 16: Global Market Value (USD Bn) Share and BPS Analysis by Region, 2025 and 2035
- Figure 17: Global Market Y-o-Y Growth Comparison by Region, 2025 to 2035
- Figure 18: Global Market Attractiveness Analysis by Region
- Figure 19: North America Market Incremental $ Opportunity, 2025 to 2035
- Figure 20: Latin America Market Incremental $ Opportunity, 2025 to 2035
- Figure 21: Western Europe Market Incremental $ Opportunity, 2025 to 2035
- Figure 22: East Asia Market Incremental $ Opportunity, 2025 to 2035
- Figure 23: South Asia Pacific Market Incremental $ Opportunity, 2025 to 2035
- Figure 24: Eastern Europe Market Incremental $ Opportunity, 2025 to 2035
- Figure 25: Middle East & Africa Market Incremental $ Opportunity, 2025 to 2035
- Figure 26: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: North America Market Value Share and BPS Analysis by Hernia Type, 2025 and 2035
- Figure 28: North America Market Y-o-Y Growth Comparison by Hernia Type, 2025 to 2035
- Figure 29: North America Market Attractiveness Analysis by Hernia Type
- Figure 30: North America Market Value Share and BPS Analysis by Mesh Type, 2025 and 2035
- Figure 31: North America Market Y-o-Y Growth Comparison by Mesh Type, 2025 to 2035
- Figure 32: North America Market Attractiveness Analysis by Mesh Type
- Figure 33: North America Market Value Share and BPS Analysis by Procedure, 2025 and 2035
- Figure 34: North America Market Y-o-Y Growth Comparison by Procedure, 2025 to 2035
- Figure 35: North America Market Attractiveness Analysis by Procedure
- Figure 36: North America Market Value Share and BPS Analysis by End-User, 2025 and 2035
- Figure 37: North America Market Y-o-Y Growth Comparison by End-User, 2025 to 2035
- Figure 38: North America Market Attractiveness Analysis by End-User
- Figure 39: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 40: Latin America Market Value Share and BPS Analysis by Hernia Type, 2025 and 2035
- Figure 41: Latin America Market Y-o-Y Growth Comparison by Hernia Type, 2025 to 2035
- Figure 42: Latin America Market Attractiveness Analysis by Hernia Type
- Figure 43: Latin America Market Value Share and BPS Analysis by Mesh Type, 2025 and 2035
- Figure 44: Latin America Market Y-o-Y Growth Comparison by Mesh Type, 2025 to 2035
- Figure 45: Latin America Market Attractiveness Analysis by Mesh Type
- Figure 46: Latin America Market Value Share and BPS Analysis by Procedure, 2025 and 2035
- Figure 47: Latin America Market Y-o-Y Growth Comparison by Procedure, 2025 to 2035
- Figure 48: Latin America Market Attractiveness Analysis by Procedure
- Figure 49: Latin America Market Value Share and BPS Analysis by End-User, 2025 and 2035
- Figure 50: Latin America Market Y-o-Y Growth Comparison by End-User, 2025 to 2035
- Figure 51: Latin America Market Attractiveness Analysis by End-User
- Figure 52: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Western Europe Market Value Share and BPS Analysis by Hernia Type, 2025 and 2035
- Figure 54: Western Europe Market Y-o-Y Growth Comparison by Hernia Type, 2025 to 2035
- Figure 55: Western Europe Market Attractiveness Analysis by Hernia Type
- Figure 56: Western Europe Market Value Share and BPS Analysis by Mesh Type, 2025 and 2035
- Figure 57: Western Europe Market Y-o-Y Growth Comparison by Mesh Type, 2025 to 2035
- Figure 58: Western Europe Market Attractiveness Analysis by Mesh Type
- Figure 59: Western Europe Market Value Share and BPS Analysis by Procedure, 2025 and 2035
- Figure 60: Western Europe Market Y-o-Y Growth Comparison by Procedure, 2025 to 2035
- Figure 61: Western Europe Market Attractiveness Analysis by Procedure
- Figure 62: Western Europe Market Value Share and BPS Analysis by End-User, 2025 and 2035
- Figure 63: Western Europe Market Y-o-Y Growth Comparison by End-User, 2025 to 2035
- Figure 64: Western Europe Market Attractiveness Analysis by End-User
- Figure 65: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 66: East Asia Market Value Share and BPS Analysis by Hernia Type, 2025 and 2035
- Figure 67: East Asia Market Y-o-Y Growth Comparison by Hernia Type, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Hernia Type
- Figure 69: East Asia Market Value Share and BPS Analysis by Mesh Type, 2025 and 2035
- Figure 70: East Asia Market Y-o-Y Growth Comparison by Mesh Type, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by Mesh Type
- Figure 72: East Asia Market Value Share and BPS Analysis by Procedure, 2025 and 2035
- Figure 73: East Asia Market Y-o-Y Growth Comparison by Procedure, 2025 to 2035
- Figure 74: East Asia Market Attractiveness Analysis by Procedure
- Figure 75: East Asia Market Value Share and BPS Analysis by End-User, 2025 and 2035
- Figure 76: East Asia Market Y-o-Y Growth Comparison by End-User, 2025 to 2035
- Figure 77: East Asia Market Attractiveness Analysis by End-User
- Figure 78: South Asia Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 79: South Asia Pacific Market Value Share and BPS Analysis by Hernia Type, 2025 and 2035
- Figure 80: South Asia Pacific Market Y-o-Y Growth Comparison by Hernia Type, 2025 to 2035
- Figure 81: South Asia Pacific Market Attractiveness Analysis by Hernia Type
- Figure 82: South Asia Pacific Market Value Share and BPS Analysis by Mesh Type, 2025 and 2035
- Figure 83: South Asia Pacific Market Y-o-Y Growth Comparison by Mesh Type, 2025 to 2035
- Figure 84: South Asia Pacific Market Attractiveness Analysis by Mesh Type
- Figure 85: South Asia Pacific Market Value Share and BPS Analysis by Procedure, 2025 and 2035
- Figure 86: South Asia Pacific Market Y-o-Y Growth Comparison by Procedure, 2025 to 2035
- Figure 87: South Asia Pacific Market Attractiveness Analysis by Procedure
- Figure 88: South Asia Pacific Market Value Share and BPS Analysis by End-User, 2025 and 2035
- Figure 89: South Asia Pacific Market Y-o-Y Growth Comparison by End-User, 2025 to 2035
- Figure 90: South Asia Pacific Market Attractiveness Analysis by End-User
- Figure 91: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 92: Eastern Europe Market Value Share and BPS Analysis by Hernia Type, 2025 and 2035
- Figure 93: Eastern Europe Market Y-o-Y Growth Comparison by Hernia Type, 2025 to 2035
- Figure 94: Eastern Europe Market Attractiveness Analysis by Hernia Type
- Figure 95: Eastern Europe Market Value Share and BPS Analysis by Mesh Type, 2025 and 2035
- Figure 96: Eastern Europe Market Y-o-Y Growth Comparison by Mesh Type, 2025 to 2035
- Figure 97: Eastern Europe Market Attractiveness Analysis by Mesh Type
- Figure 98: Eastern Europe Market Value Share and BPS Analysis by Procedure, 2025 and 2035
- Figure 99: Eastern Europe Market Y-o-Y Growth Comparison by Procedure, 2025 to 2035
- Figure 100: Eastern Europe Market Attractiveness Analysis by Procedure
- Figure 101: Eastern Europe Market Value Share and BPS Analysis by End-User, 2025 and 2035
- Figure 102: Eastern Europe Market Y-o-Y Growth Comparison by End-User, 2025 to 2035
- Figure 103: Eastern Europe Market Attractiveness Analysis by End-User
- Figure 104: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 105: Middle East & Africa Market Value Share and BPS Analysis by Hernia Type, 2025 and 2035
- Figure 106: Middle East & Africa Market Y-o-Y Growth Comparison by Hernia Type, 2025 to 2035
- Figure 107: Middle East & Africa Market Attractiveness Analysis by Hernia Type
- Figure 108: Middle East & Africa Market Value Share and BPS Analysis by Mesh Type, 2025 and 2035
- Figure 109: Middle East & Africa Market Y-o-Y Growth Comparison by Mesh Type, 2025 to 2035
- Figure 110: Middle East & Africa Market Attractiveness Analysis by Mesh Type
- Figure 111: Middle East & Africa Market Value Share and BPS Analysis by Procedure, 2025 and 2035
- Figure 112: Middle East & Africa Market Y-o-Y Growth Comparison by Procedure, 2025 to 2035
- Figure 113: Middle East & Africa Market Attractiveness Analysis by Procedure
- Figure 114: Middle East & Africa Market Value Share and BPS Analysis by End-User, 2025 and 2035
- Figure 115: Middle East & Africa Market Y-o-Y Growth Comparison by End-User, 2025 to 2035
- Figure 116: Middle East & Africa Market Attractiveness Analysis by End-User
- Figure 117: Global Market - Tier Structure Analysis
- Figure 118: Global Market - Company Share Analysis
- FAQs -
What is the Global Hernia Mesh Devices Market size in 2025?
The hernia mesh devices market is valued at USD 5.1 billion in 2025.
Who are the Major Players Operating in the Hernia Mesh Devices Market?
Prominent players in the market include Ethicon, Inc., Feg Textiltechnik MBH, Herniamesh S.r.l, LifeCell Corporation, W. L. Gore & Associates.
What is the Estimated Valuation of the Hernia Mesh Devices Market by 2035?
The market is expected to reach a valuation of USD 7.5 billion by 2035.
At what CAGR is the Hernia Mesh Devices Market slated to grow during the study period?
The growth rate of the Hernia Mesh Devices market is 4.0% from 2025-2035.