Hospital Acquired Infections Therapeutics Market
Hospital Acquired Infections Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
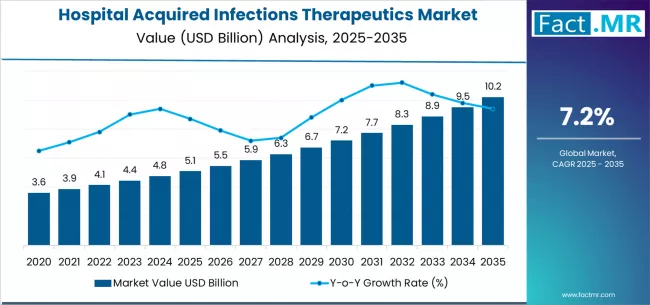
Hospital acquired infections therapeutics market is projected to grow from USD 5.1 billion in 2025 to USD 10.2 billion by 2035, at a CAGR of 7.2%. Antibacterial Drugs will dominate with a 87.2% market share, while surgical site infections (ssis) will lead the infection type segment with a 31.8% share.
Hospital Acquired Infections Therapeutics Market Forecast and Outlook 2025 to 2035
The global hospital acquired infections therapeutics market is projected to reach USD 10.2 billion by 2035, recording an absolute increase of USD 5.1 billion over the forecast period. The market is valued at USD 5.1 billion in 2025 and is set to rise at a CAGR of 7.2% during the assessment period.
The overall market size is expected to grow by nearly 2.0 times during the same period, supported by increasing prevalence of healthcare-associated infections in critical care settings and expanding antimicrobial resistance driving demand for novel therapeutic interventions worldwide, creating substantial opportunities for pharmaceutical manufacturers developing advanced infection control solutions and increasing investments in hospital infection prevention protocols and regulatory compliance initiatives globally.
Quick Stats for Hospital acquired infections therapeutics market
- Hospital acquired infections therapeutics market Value (2025): USD 5.1 billion
- Hospital acquired infections therapeutics market Forecast Value (2035): USD 10.2 billion
- Hospital acquired infections therapeutics market Forecast CAGR: 7.2%
- Leading Drug Class in Hospital acquired infections therapeutics market: Antibacterial Drugs
- Key Growth Regions in Hospital acquired infections therapeutics market: Europe, North America, and Asia Pacific
- Top Players in Hospital acquired infections therapeutics market: Merck & Co., Inc., Pfizer Inc., Bayer AG, GlaxoSmithKline Plc, Daiichi Sankyo Company, Limited, AbbVie Inc., F. Hoffmann-La Roche Ltd, Allergan Plc, Abbott Laboratories
The healthcare sector faces mounting pressure to reduce infection rates while maintaining patient safety standards, with modern HAI therapeutics providing documented clinical effectiveness of 60-75% compared to traditional antibiotic regimens in resistant strain management. Rising hospital admissions and expanding surgical procedures across emerging economies create substantial opportunities for pharmaceutical developers and healthcare service providers. However, high drug development costs and growing antimicrobial resistance challenges may pose obstacles to market expansion.
The antibacterial drugs segment dominates market activity with approximately 87.2% share in 2025, driven by the extensive prevalence of bacterial HAIs requiring immediate therapeutic intervention and regulatory compliance improvements across hospital facilities worldwide. Healthcare providers increasingly recognize the clinical benefits of advanced antibacterial formulations, with typical treatment success rates ranging from 70-85% through targeted antimicrobial therapy and reduced resistance development.
The antiviral drugs segment demonstrates moderate growth potential, supported by increasing viral HAI incidence and hospital immunocompromised patient populations. Cell wall synthesis inhibitors emerge as the leading antibacterial category with 48.0% share within antibacterials, reflecting pharmaceutical industry focus on proven mechanisms of action combined with established safety profiles. Surgical site infections represent the most significant infection type application with 31.8% share, driven by expanding surgical volumes and stringent post-operative infection prevention requirements in hospital environments.
Regional dynamics show Europe maintaining market leadership with 34.0% share in 2025, supported by advanced healthcare infrastructure and comprehensive infection control protocols across Germany, UK, and France. North America demonstrates strong therapeutic adoption driven by sophisticated hospital systems and stringent HAI reporting requirements, while Asia Pacific emphasizes rapid healthcare expansion and infection management capabilities. India leads country-level growth at 8.5% CAGR through extensive hospital development and growing infection awareness, followed by China at 8.0% supported by government-backed healthcare quality initiatives.
The competitive landscape features moderate concentration with Merck & Co., Inc. holding 17.0% market share, while established players including Pfizer Inc., Bayer AG, and GlaxoSmithKline Plc compete through comprehensive therapeutic portfolios and advanced antimicrobial development capabilities across diverse hospital-acquired infection applications.
Hospital acquired infections therapeutics market Year-over-Year Forecast (2025-2035)
Between 2025 and 2029, the hospital acquired infections therapeutics market is projected to expand from USD 5.1 billion to USD 6.7 billion, resulting in a value increase of USD 1.6 billion, which represents 31.4% of the total forecast growth for the period. This phase of development will be shaped by rising demand for novel antibacterial formulations addressing resistant pathogen strains, product innovation in combination therapy approaches and targeted antimicrobial interventions, as well as expanding integration with hospital infection surveillance systems and antimicrobial stewardship programs. Pharmaceutical companies are establishing competitive positions through investment in specialized drug development capabilities, advanced resistance mitigation technologies, and strategic market expansion across surgical, critical care, and intensive care applications.
From 2029 to 2035, the market is forecast to grow from USD 6.7 billion to USD 10.2 billion, adding another USD 3.5 billion, which constitutes 68.6% of the overall expansion. This period is expected to be characterized by the expansion of specialized therapeutic applications, including advanced antiviral agents and next-generation antifungal solutions tailored for specific infection profiles, strategic collaborations between pharmaceutical manufacturers and hospital networks, and an enhanced focus on antimicrobial resistance management and infection prevention protocols. The growing emphasis on surgical site infection prevention and bloodstream infection management will drive demand for comprehensive therapeutic solutions across diverse hospital-acquired infection applications.
Hospital acquired infections therapeutics market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 5.1 billion |
| Market Forecast Value (2035) | USD 10.2 billion |
| Forecast CAGR (2025-2035) | 7.2% |
Why is the Hospital acquired infections therapeutics market Growing?
The hospital acquired infections therapeutics market grows by enabling healthcare providers and hospital administrators to optimize infection management protocols while accessing advanced antimicrobial formulations without substantial in-house pharmaceutical development requirements. Hospital operators and infectious disease departments face mounting pressure to reduce HAI incidence rates and improve patient outcomes while managing complex antimicrobial resistance challenges, with modern HAI therapeutics typically providing 60-75% clinical success rates compared to conventional alternatives, making therapeutic advancement essential for competitive clinical positioning. The healthcare industry's need for regulatory compliance and infection-specific treatment capabilities creates demand for comprehensive therapeutic solutions that can provide superior efficacy, maintain consistent safety standards, and ensure reliable outcomes without compromising patient satisfaction or hospital quality metrics.
Government initiatives promoting infection prevention and antimicrobial stewardship programs drive adoption in surgical departments, intensive care units, and critical care applications, where therapeutic performance has a direct impact on patient mortality rates and hospital reimbursement compliance. However, drug resistance development constraints during prolonged antimicrobial exposure and the technical requirements for combination therapy protocols may limit accessibility among smaller healthcare facilities and developing regions with limited resources for sophisticated HAI therapeutic interventions.
Segmental Analysis
The market is segmented by drug class, infection type, and region. By drug class, the market is divided into antibacterial drugs, antiviral drugs, and antifungal drugs. Based on infection type, the market is categorized into surgical site infections, urinary tract infections, ventilator-associated pneumonia, bloodstream infections, and other HAIs. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
By Drug Class, Which Segment Accounts for a Dominant Market Share?
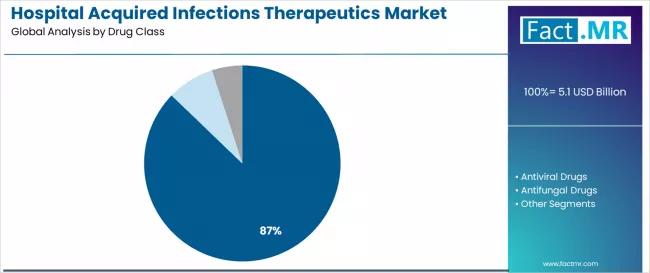
The antibacterial drugs segment represents the dominant force in the hospital acquired infections therapeutics market, capturing approximately 87.2% of total market share in 2025. This established drug category encompasses solutions featuring broad-spectrum antimicrobial activity and targeted pathogen elimination capabilities, including advanced beta-lactam formulations and specialized resistance-breaking agents that enable superior clinical outcomes and infection resolution across all hospital applications. The antibacterial drugs segment's market leadership stems from its superior therapeutic advantages, with formulations capable of addressing diverse bacterial strains while maintaining consistent efficacy standards and safety compliance across all hospital environments.
The antiviral drugs segment maintains a 7.0% market share, serving healthcare facilities that require specialized therapeutic interventions for viral HAI management in immunocompromised patient populations and critical care applications. These solutions offer targeted antiviral mechanisms for specific infection types while providing sufficient flexibility to meet contemporary resistance management demands. The antifungal drugs segment holds 5.7% market share, addressing opportunistic fungal infections in high-risk patient groups.
Key therapeutic advantages driving the antibacterial drugs segment include:
- Advanced resistance-breaking mechanisms with targeted bacterial cell wall disruption that enhance infection clearance and ensure consistent clinical performance
- Established safety profiles allowing streamlined treatment protocols across different patient populations without extensive toxicity monitoring
- Enhanced combination therapy compatibility enabling diverse antimicrobial configurations while maintaining treatment efficacy and patient safety
- Superior clinical outcomes providing optimal therapeutic performance for various hospital-acquired bacterial infection applications
By Infection Type, why do Surgical Site Infections account for the Largest Share?
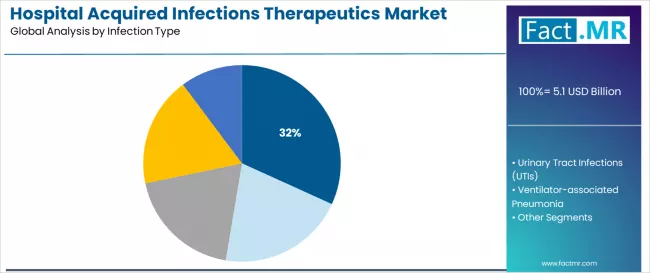
Surgical site infections dominate the hospital acquired infections therapeutics market with approximately 31.8% market share in 2025, reflecting the critical role of post-operative infection management in supporting patient recovery requirements and surgical quality outcomes worldwide. The surgical site infections segment's market leadership is reinforced by increasing surgical procedure volumes, complex wound management requirements, and rising needs for specialized antimicrobial prophylaxis capabilities in hospital applications across developed and emerging markets.
The urinary tract infections segment represents the second-largest infection category, demonstrating 27.0% market share through specialized requirements for catheter-associated infection management, antimicrobial treatment optimization, and direct integration with hospital urological care protocols. This segment benefits from growing catheterization procedures that require specific therapeutic interventions, treatment monitoring standards, and infection prevention protocols in hospital environments.
Ventilator-associated pneumonia holds 20.0% market share, serving intensive care units and critical care departments with specialized respiratory infection management needs. Bloodstream infections account for 13.0% share, addressing life-threatening septicemia in vulnerable patient populations.
Key market dynamics supporting infection type growth include:
- Surgical site infection expansion driven by rising surgical volumes and complex procedure requirements, demanding advanced antimicrobial prophylaxis solutions in expanding hospital markets
- Urinary tract infection management trends require flexible, targeted therapeutic systems for catheter-associated infection prevention and treatment optimization
- Integration of infection surveillance technologies enabling advanced hospital epidemiology capabilities and automated monitoring systems
- Growing emphasis on critical care infection management driving demand for specialized, validated therapeutic solutions without traditional resistance limitations
What are the Drivers, Restraints, and Key Trends of the Hospital acquired infections therapeutics market?
The market is driven by three concrete demand factors tied to patient care outcomes. First, increasing HAI prevalence rates and surgical procedure volumes create escalating demand for specialized antimicrobial therapeutics, with hospital-acquired infections affecting 5-10% of hospitalized patients globally, requiring comprehensive pharmaceutical interventions. Second, government initiatives promoting infection prevention protocols and antimicrobial stewardship programs drive increased adoption of advanced HAI therapeutics, with many countries implementing mandatory infection reporting frameworks and quality improvement requirements by 2030. Third, technological advancements in antimicrobial formulations and combination therapy approaches enable more effective and targeted infection management solutions that improve patient outcomes while reducing treatment duration and hospital readmission rates.
Market restraints include high drug development costs and regulatory approval requirements for novel antimicrobials that can challenge pharmaceutical companies in developing effective HAI therapeutics, particularly in regions where reimbursement structures for infection treatment remain uncertain. Growing antimicrobial resistance and therapeutic efficacy limitations pose another significant challenge, as HAI pathogens demonstrate increasing resistance profiles, potentially affecting treatment success rates and clinical outcomes. Healthcare budget constraints and formulary restrictions across different hospital systems create additional operational challenges for pharmaceutical manufacturers, demanding ongoing investment in clinical evidence generation and health economic outcome demonstrations.
Key trends indicate accelerated therapeutic adoption in Asia-Pacific markets, particularly India and China, where hospital expansion and healthcare quality improvement initiatives drive comprehensive HAI therapeutic utilization. Technology integration trends toward rapid diagnostic testing with targeted antimicrobial selection, advanced pharmacokinetic monitoring capabilities, and integrated infection management platforms enable efficient treatment approaches that optimize therapeutic outcomes and minimize resistance development risks. However, the market thesis could face disruption if significant advances in infection prevention technologies or major breakthroughs in alternative antimicrobial modalities reduce reliance on traditional HAI therapeutic interventions.
Analysis of the Hospital Acquired Infections Therapeutics Market by Key Countries
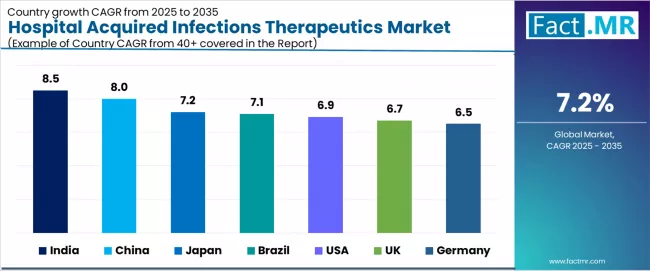
| Country | CAGR (2025-2035) |
|---|---|
| India | 8.5% |
| China | 8.0% |
| Japan | 7.2% |
| Brazil | 7.1% |
| USA | 6.9% |
| UK | 6.7% |
| Germany | 6.5% |
The global hospital acquired infections therapeutics market is expanding steadily, with India leading at an 8.5% CAGR through 2035, driven by rapid healthcare infrastructure expansion, growing hospital admission rates, and increasing infection awareness. China follows at 8.0%, supported by government-backed healthcare quality initiatives, expanding critical care capacity, and large-scale hospital modernization programs. Japan records 7.2%, reflecting an established healthcare landscape with advanced infection surveillance systems and comprehensive antimicrobial stewardship programs.
Brazil grows at 7.1%, anchored by expanding hospital networks and emerging infection prevention protocols. USA advances at 6.9%, leveraging sophisticated healthcare systems and stringent HAI reporting requirements. The UK posts 6.7%, focusing on NHS infection reduction targets, while Germany grows steadily at 6.5%, emphasizing regulatory compliance and evidence-based infection management.
What is the Forecast for the Hospital Acquired Infections Therapeutics Market in India?
India demonstrates the strongest growth potential in the hospital acquired infections therapeutics market with a CAGR of 8.5% through 2035. The country's leadership position stems from rapid hospital infrastructure expansion, growing surgical volumes, and comprehensive healthcare quality improvement initiatives driving the adoption of advanced antimicrobial therapeutics.
Growth is concentrated in major metropolitan healthcare centers and tertiary hospitals, including facilities in Mumbai, Delhi, Bangalore, and Chennai, where infectious disease specialists and hospital administrators are implementing modern HAI management protocols for enhanced patient safety and clinical outcomes.
Distribution channels through pharmaceutical distributors and hospital pharmacy networks expand therapeutic access across surgical departments and critical care facilities. The country's Ministry of Health and Family Welfare provides policy support for infection prevention initiatives, including comprehensive antimicrobial stewardship guidelines.
Key market factors:
- Hospital capacity expansion concentrated in urban centers and specialty facilities with comprehensive infection control programs
- Government support through national health missions and hospital quality improvement incentives
- Comprehensive pharmaceutical supply ecosystem, including established distributors with proven therapeutic delivery capabilities
- Clinical integration featuring advanced infection surveillance systems, antimicrobial stewardship platforms, and treatment optimization technologies
Why is China Emerging as a High-Growth Market for Hospital Acquired Infections Therapeutics?
In major healthcare centers including Beijing, Shanghai, Guangzhou, and Shenzhen, the adoption of comprehensive HAI therapeutic protocols is accelerating across tertiary hospitals and specialty medical facilities, driven by government healthcare quality initiatives and infection prevention mandates. The market demonstrates strong growth momentum with a CAGR of 8.0% through 2035, linked to comprehensive hospital system expansion and increasing focus on patient safety and infection reduction strategies.
Chinese healthcare providers are implementing advanced antimicrobial therapeutics and infection surveillance platforms to enhance clinical outcomes while meeting growing demand in expanding surgical and critical care sectors. The country's Healthy China 2030 initiative creates ongoing demand for HAI therapeutics, while increasing emphasis on antimicrobial resistance management drives adoption of novel therapeutic formulations.
Key development areas:
- Tertiary hospitals and specialty medical centers leading HAI therapeutic adoption with comprehensive infection management programs
- Pharmaceutical distribution channels providing integrated therapeutic solutions with high clinical compliance rates
- Strategic partnerships between international pharmaceutical manufacturers and Chinese healthcare enterprises expanding market reach
- Integration of digital health platforms and comprehensive antimicrobial stewardship systems
What is the Outlook for the Hospital Acquired Infections Therapeutics Market in the USA?
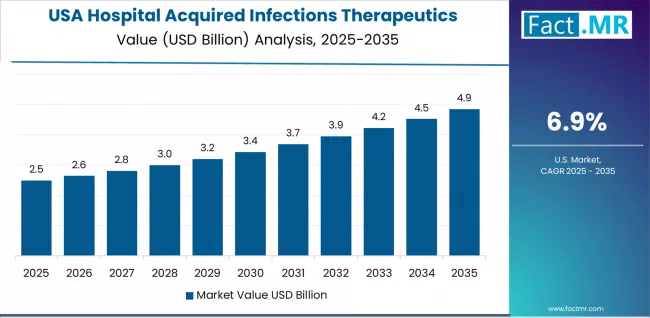
The USA hospital acquired infections therapeutics market’s expansion is driven by diverse hospital demand, including academic medical centers in major cities and comprehensive infection management protocols across multiple healthcare facility types. The country demonstrates strong growth potential with a CAGR of 6.9% through 2035, supported by federal HAI reduction initiatives and Centers for Medicare & Medicaid Services quality reporting requirements.
American healthcare providers face implementation challenges related to antimicrobial resistance patterns and formulary management complexities, requiring evidence-based therapeutic selection approaches and support from infectious disease specialists. However, growing infection prevention emphasis and regulatory compliance requirements create compelling clinical cases for HAI therapeutic adoption, particularly in surgical and intensive care applications where effective infection management has a direct impact on patient outcomes and hospital reimbursement rates.
Market characteristics:
- Academic medical centers and tertiary hospitals showing robust therapeutic utilization with substantial annual investment in infection prevention
- Regional adoption trends focused on antimicrobial stewardship programs in major metropolitan healthcare systems
- Future projections indicate the need for advanced diagnostic integration infrastructure and infectious disease specialist training programs
- Growing emphasis on patient safety metrics and infection rate reduction competitiveness in hospital quality rankings
Will the Drive to Achieve Clinical Excellence Enhance Prospects for Hospital Acquired Infections Therapeutics in Germany?
The market in Germany leads in evidence-based HAI management based on integration with national infection surveillance networks and rigorous antimicrobial stewardship protocols for enhanced therapeutic optimization. The country shows strong potential with a CAGR of 6.5% through 2035, driven by the modernization of hospital infection control infrastructure and the expansion of specialized antimicrobial interventions in major healthcare regions, including North Rhine-Westphalia, Bavaria, Baden-Württemberg, and Lower Saxony.
Hospitals and other healthcare settings in Germany are adopting guideline-based HAI therapeutics for infection reduction and regulatory compliance enhancement, particularly in regions with advanced healthcare quality systems and surgical applications demanding comprehensive antimicrobial protocols. Pharmaceutical distribution channels through established hospital pharmacy networks and specialty distributors expand coverage across university hospitals and healthcare facilities.
Leading market segments:
- University hospitals and academic medical centers implementing comprehensive infection management and antimicrobial stewardship programs
- Pharmaceutical partnerships with hospital pharmacy departments, achieving high guideline adherence rates
- Strategic collaborations between pharmaceutical manufacturers and hospital networks expanding therapeutic access
- Focus on combination therapy approaches and specialized infection management requirements
How to Emphasis on NHS Quality Standards Determine the Scope for Hospital Acquired Infections Therapeutics in the UK?
In the UK, especially in cities such as London, Manchester, and Birmingham, NHS hospital trusts are implementing comprehensive HAI therapeutic protocols to reduce infection rates and improve patient safety metrics, with documented evidence showing substantial improvement in clinical outcomes through targeted antimicrobial interventions. The market shows strong growth potential with a CAGR of 6.7% through 2035, linked to the ongoing NHS infection reduction initiatives, Quality Improvement programs, and emerging antimicrobial resistance surveillance in major healthcare regions.
Healthcare providers in the UK are adopting evidence-based HAI therapeutics and digital monitoring platforms to enhance treatment efficacy while maintaining standards demanded by NHS Quality Standards and Care Quality Commission requirements. The country's established healthcare infrastructure creates ongoing demand for therapeutic optimization and antimicrobial stewardship solutions that integrate with existing hospital pharmacy systems.
Market development factors:
- NHS hospital trusts and foundation trusts leading HAI therapeutic implementation across UK
- NICE guidelines and infection prevention programs providing clinical framework support for antimicrobial stewardship
- Strategic partnerships between British hospitals and international pharmaceutical providers expanding therapeutic capabilities
- Emphasis on infection surveillance integration and patient safety outcomes across hospital applications
How will Quality-Focused Implementation of Healthcare Standards Influence the Hospital Acquired Infections Therapeutics Landscape in Japan?
Japan's hospital acquired infections therapeutics market demonstrates sophisticated clinical protocols focused on infection prevention excellence and antimicrobial optimization, with documented integration of advanced surveillance systems achieving substantial improvement in infection control across hospital and specialty medical facilities.
The country maintains steady growth momentum with a CAGR of 7.2% through 2035, driven by healthcare facilities' emphasis on patient safety excellence and continuous quality improvement methodologies that align with Japanese medical standards applied to infection management.
Major healthcare regions, including Kanto, Kansai, Chubu, and Kyushu, showcase advanced implementation of antimicrobial therapeutics where HAI management integrates seamlessly with existing hospital protocols and comprehensive patient safety programs.
Key market characteristics:
- University hospitals and advanced treatment facilities driving sophisticated HAI therapeutic requirements with emphasis on clinical outcomes
- Quality partnerships enabling high protocol adherence with comprehensive infection surveillance programs
- Technology collaboration between Japanese hospitals and international pharmaceutical providers expanding therapeutic capabilities
- Emphasis on antimicrobial resistance monitoring requirements and continuous treatment optimization methodologies
How will the Hospital Acquired Infections Therapeutics Market Perform in Brazil?
In major metropolitan healthcare centers including São Paulo, Rio de Janeiro, Brasília, and Belo Horizonte, the adoption of modern HAI therapeutic protocols is expanding across public and private hospitals, driven by healthcare system modernization and growing infection prevention awareness. The market demonstrates growth potential with a CAGR of 7.1% through 2035, linked to comprehensive hospital capacity expansion and increasing focus on patient safety and infection control solutions.
Brazilian healthcare providers are implementing antimicrobial therapeutics and infection management platforms to enhance clinical performance while meeting growing demand in expanding surgical and critical care sectors. The country's national health system initiatives create ongoing demand for HAI therapeutics, while increasing emphasis on hospital quality standards drives adoption of evidence-based infection management approaches.
Key development areas:
- Public and private hospitals leading HAI therapeutic adoption with expanding infection prevention programs
- Pharmaceutical distribution channels providing therapeutic solutions with growing clinical implementation capabilities
- Strategic partnerships between international pharmaceutical companies and Brazilian healthcare systems expanding market reach
- Integration of infection surveillance platforms and antimicrobial stewardship frameworks
Europe Market Split by Country
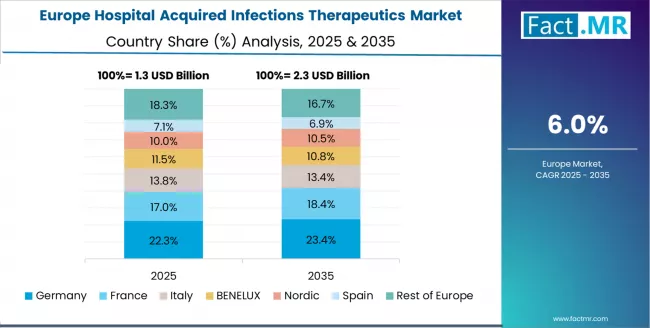
The hospital acquired infections therapeutics market in Europe is projected to grow from USD 1.7 billion in 2025 to USD 3.1 billion by 2035, registering a CAGR of 6.5% over the forecast period. Germany is expected to maintain its leadership position with a 28.5% market share in 2025, declining slightly to 27.8% by 2035, supported by its advanced healthcare infrastructure, comprehensive infection surveillance networks, and established antimicrobial stewardship programs serving major European hospital systems.
UK follows with a 22.3% share in 2025, projected to reach 22.7% by 2035, driven by comprehensive NHS infection reduction initiatives and Quality Improvement programs across hospital trusts. France holds a 19.8% share in 2025, expected to maintain 19.5% by 2035 through the ongoing development of hospital infection control protocols and healthcare quality networks.
Italy commands a 13.2% share, while Spain accounts for 10.4% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 5.8% to 6.3% by 2035, attributed to increasing HAI therapeutic adoption in Nordic healthcare systems and emerging Eastern European hospitals implementing infection prevention programs.
How will Stringent Hospital Protocols Implementation Influence Scope in Japan?
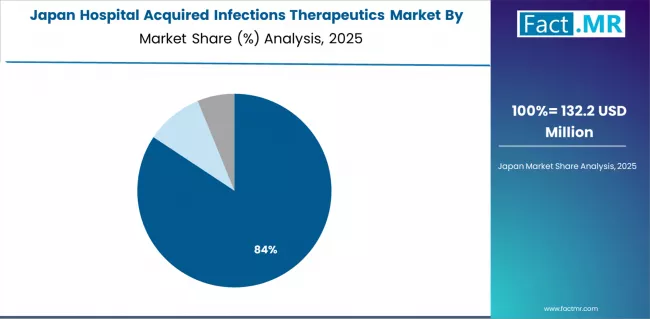
The Japanese hospital acquired infections therapeutics market demonstrates a mature and quality-focused landscape, characterized by sophisticated integration of evidence-based antimicrobial protocols with existing infection surveillance infrastructure across university hospitals, advanced treatment centers, and specialty medical facilities. Japan's emphasis on patient safety excellence and clinical quality drives demand for high-efficacy HAI therapeutics that support comprehensive infection prevention initiatives and regulatory requirements in hospital operations.
The market benefits from strong partnerships between international pharmaceutical providers like Pfizer Inc., Merck & Co., Inc., and domestic healthcare institutions, including established hospital networks and academic medical centers, creating comprehensive therapeutic ecosystems that prioritize clinical outcomes and antimicrobial stewardship programs. Healthcare centers in major urban regions showcase advanced HAI management implementations where therapeutic protocols achieve infection rate reductions through integrated monitoring systems.
How will Pharmaceutical Manufacturers Lead Hospital Acquired Infections Therapeutics in South Korea?
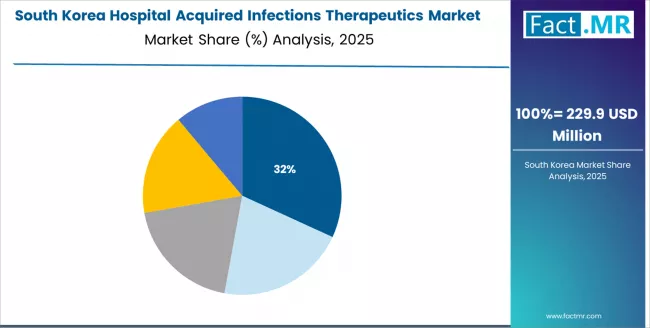
The South Korean hospital acquired infections therapeutics market is characterized by strong international pharmaceutical manufacturer presence, with companies like Pfizer Inc., GlaxoSmithKline Plc, and Bayer AG maintaining dominant positions through comprehensive product portfolios and clinical support capabilities for tertiary hospitals and specialty medical applications.
The market is demonstrating a growing emphasis on localized clinical evidence generation and rapid regulatory approval pathways, as South Korean healthcare providers increasingly demand therapeutic solutions that integrate with domestic hospital formularies and infection surveillance systems deployed across major medical centers and university hospitals.
Local pharmaceutical distributors and regional specialty pharmacy networks are gaining market share through strategic partnerships with global manufacturers, offering specialized services including clinical education programs and antimicrobial stewardship consultation services for infectious disease specialists.
The competitive landscape shows increasing collaboration between multinational pharmaceutical companies and Korean healthcare institutions, creating hybrid distribution models that combine international therapeutic expertise with local clinical knowledge and hospital relationship capabilities.
Competitive Landscape of the Hospital Acquired Infections Therapeutics Market
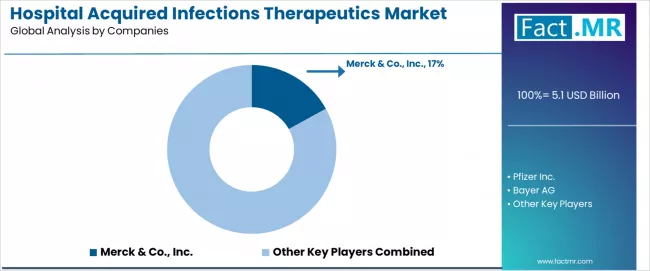
The hospital acquired infections therapeutics market features approximately 20-25 meaningful pharmaceutical players with moderate concentration, where the top three companies control roughly 40-45% of global market share through established drug portfolios and extensive hospital distribution networks. Competition centers on therapeutic efficacy, resistance profile management, and clinical evidence generation rather than price competition alone.
Market leaders include Merck & Co., Inc., Pfizer Inc., and Bayer AG, which maintain competitive advantages through comprehensive antimicrobial drug portfolios, advanced pharmaceutical manufacturing capabilities, and deep clinical expertise in hospital-acquired infection management, creating high prescriber loyalty among infectious disease specialists. These companies leverage established hospital pharmacy relationships and ongoing clinical research partnerships to defend market positions while expanding into adjacent antimicrobial categories and combination therapy applications.
Challengers encompass GlaxoSmithKline Plc and Daiichi Sankyo Company, Limited, which compete through specialized antimicrobial technologies and strong therapeutic presence in key hospital infection categories. Pharmaceutical specialists, including AbbVie Inc., F. Hoffmann-La Roche Ltd, and Allergan Plc, focus on specific infection types or therapeutic mechanisms, offering differentiated capabilities in resistant pathogen management, novel antibiotic development, and application-specific formulations.
Regional pharmaceutical manufacturers and emerging biotech companies create competitive pressure through innovative drug development approaches and rapid clinical trial capabilities, particularly in high-growth markets including India and China, where local regulatory expertise provides advantages in approval efficiency and hospital formulary access.
Market dynamics favor companies that combine advanced antimicrobial technologies with comprehensive clinical support services that address the complete infection management lifecycle from diagnosis support through treatment monitoring and antimicrobial stewardship consultation, while maintaining robust pipelines of next-generation therapeutics addressing evolving resistance patterns and unmet clinical needs in hospital-acquired infection management.
Key Players in the Hospital Acquired Infections Therapeutics Market
- Merck & Co., Inc.
- Pfizer Inc.
- Bayer AG
- GlaxoSmithKline Plc
- Daiichi Sankyo Company, Limited
- AbbVie Inc.
- F. Hoffmann-La Roche Ltd
- Allergan Plc
- Abbott Laboratories
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 5.1 Billion |
| Drug Class | Antibacterial Drugs, Antiviral Drugs, Antifungal Drugs |
| Infection Type | Surgical Site Infections, Urinary Tract Infections, Ventilator-associated Pneumonia, Bloodstream Infections, Other HAIs |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Country Covered | USA, Germany, UK, Japan, China, India, Brazil, and 40+ countries |
| Key Companies Profiled | Merck & Co., Inc., Pfizer Inc., Bayer AG, GlaxoSmithKline Plc, Daiichi Sankyo Company, Limited, AbbVie Inc., F. Hoffmann-La Roche Ltd, Allergan Plc, Abbott Laboratories |
| Additional Attributes | Revenue analysis by drug class and infection type categories, regional therapeutic adoption trends across North America, Europe, and Asia Pacific, competitive landscape with pharmaceutical manufacturers and distribution networks, drug efficacy specifications and resistance profile assessments, integration with hospital antimicrobial stewardship initiatives and infection surveillance platforms, innovations in antimicrobial development and combination therapy approaches, and development of specialized therapeutic applications with resistance mitigation and clinical outcome optimization capabilities. |
Hospital Acquired Infections Therapeutics Market by Segments
-
Drug Class :
- Antibacterial Drugs
- Antiviral Drugs
- Antifungal Drugs
-
Infection Type :
- Surgical Site Infections (SSIs)
- Urinary Tract Infections (UTIs)
- Ventilator-associated Pneumonia
- Bloodstream Infections
- Other HAIs
-
Region :
- North America
- USA
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Nordic
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Drug Class
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Drug Class, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Drug Class, 2025 to 2035
- Antibacterial Drugs
- Antiviral Drugs
- Antifungal Drugs
- Y to o to Y Growth Trend Analysis By Drug Class, 2020 to 2024
- Absolute $ Opportunity Analysis By Drug Class, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Infection Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Infection Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Infection Type, 2025 to 2035
- Surgical Site Infections (SSIs)
- Urinary Tract Infections (UTIs)
- Ventilator-associated Pneumonia
- Bloodstream Infections
- Other HAIs
- Y to o to Y Growth Trend Analysis By Infection Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Infection Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Drug Class
- By Infection Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Infection Type
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Drug Class
- By Infection Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Infection Type
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Drug Class
- By Infection Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Infection Type
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Drug Class
- By Infection Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Infection Type
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Drug Class
- By Infection Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Infection Type
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Drug Class
- By Infection Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Infection Type
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Drug Class
- By Infection Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Drug Class
- By Infection Type
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Drug Class
- By Infection Type
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Drug Class
- By Infection Type
- Competition Analysis
- Competition Deep Dive
- Merck & Co., Inc.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Pfizer Inc.
- Bayer AG
- GlaxoSmithKline Plc
- Daiichi Sankyo Company, Limited
- AbbVie Inc.
- F. Hoffmann-La Roche Ltd
- Allergan Plc
- Abbott Laboratories
- Merck & Co., Inc.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Infection Type, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Infection Type, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Infection Type, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by Infection Type, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by Infection Type, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by Infection Type, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by Infection Type, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Drug Class, 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by Infection Type, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Drug Class, 2025-2035
- Figure 5: Global Market Attractiveness Analysis by Drug Class
- Figure 6: Global Market Value Share and BPS Analysis by Infection Type, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Infection Type, 2025-2035
- Figure 8: Global Market Attractiveness Analysis by Infection Type
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025-2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025-2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025-2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025-2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025-2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Drug Class, 2025-2035
- Figure 22: North America Market Attractiveness Analysis by Drug Class
- Figure 23: North America Market Value Share and BPS Analysis by Infection Type, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Infection Type, 2025-2035
- Figure 25: North America Market Attractiveness Analysis by Infection Type
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Drug Class, 2025-2035
- Figure 29: Latin America Market Attractiveness Analysis by Drug Class
- Figure 30: Latin America Market Value Share and BPS Analysis by Infection Type, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by Infection Type, 2025-2035
- Figure 32: Latin America Market Attractiveness Analysis by Infection Type
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Drug Class, 2025-2035
- Figure 36: Western Europe Market Attractiveness Analysis by Drug Class
- Figure 37: Western Europe Market Value Share and BPS Analysis by Infection Type, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by Infection Type, 2025-2035
- Figure 39: Western Europe Market Attractiveness Analysis by Infection Type
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Drug Class, 2025-2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Drug Class
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by Infection Type, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by Infection Type, 2025-2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by Infection Type
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Drug Class, 2025-2035
- Figure 50: East Asia Market Attractiveness Analysis by Drug Class
- Figure 51: East Asia Market Value Share and BPS Analysis by Infection Type, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by Infection Type, 2025-2035
- Figure 53: East Asia Market Attractiveness Analysis by Infection Type
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Drug Class, 2025-2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Drug Class
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by Infection Type, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by Infection Type, 2025-2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by Infection Type
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Drug Class, 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Drug Class, 2025-2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Drug Class
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by Infection Type, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by Infection Type, 2025-2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by Infection Type
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the hospital acquired infections therapeutics market in 2025?
The global hospital acquired infections therapeutics market is estimated to be valued at USD 5.1 billion in 2025.
What will be the size of hospital acquired infections therapeutics market in 2035?
The market size for the hospital acquired infections therapeutics market is projected to reach USD 10.2 billion by 2035.
How much will be the hospital acquired infections therapeutics market growth between 2025 and 2035?
The hospital acquired infections therapeutics market is expected to grow at a 7.2% CAGR between 2025 and 2035.
What are the key product types in the hospital acquired infections therapeutics market?
The key product types in hospital acquired infections therapeutics market are antibacterial drugs , antiviral drugs and antifungal drugs.
Which infection type segment to contribute significant share in the hospital acquired infections therapeutics market in 2025?
In terms of infection type, surgical site infections (ssis) segment to command 31.8% share in the hospital acquired infections therapeutics market in 2025.