Peptide Therapeutics CDMO Market
Peptide Therapeutics CDMO Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
The Global Peptide Therapeutics CDMO Market Is Projected To Grow From USD 2.9 Billion In 2025 To USD 8.1 Billion By 2035, Advancing At A CAGR Of 10.8%. The API Manufacturing Type Is Expected To Lead The Market With A Significant Share Of 42.8% In 2025, While Large Pharmaceutical Companies Are Anticipated To Account For The Largest Portion Of 52.3% Of The End User Segment.
Peptide Therapeutics CDMO Market Forecast and Outlook 2025 to 2035
The peptide therapeutics CDMO industry stands at the threshold of a decade-long expansion trajectory that promises to reshape pharmaceutical contract manufacturing infrastructure and advanced peptide synthesis systems. The market's journey from USD 2.9 billion in 2025 to USD 8.1 billion by 2035 represents substantial growth, the market will rise at a CAGR of 10.8% which demonstrating the accelerating adoption of advanced API manufacturing systems and peptide development solutions across large pharmaceutical companies, biotechnology companies, and academic & research institutions sectors.
The first half of the decade (2025-2030) will witness the market climbing from USD 2.9 billion to approximately USD 4.8 billion, adding USD 1.9 billion in value, which constitutes 37% of the total forecast growth period. This phase will be characterised by the rapid adoption of API manufacturing systems, driven by increasing peptide drug development and therapeutic manufacturing requirements worldwide. Advanced solid phase peptide synthesis capabilities and sterile manufacturing features will become standard expectations rather than premium options.
The latter half (2030-2035) will witness sustained growth from USD 4.8 billion to USD 8.1 billion, representing an addition of USD 3.3 billion or 63% of the decade's expansion. This period will be defined by mass market penetration of commercial-scale manufacturing systems, integration with comprehensive pharmaceutical development platforms, and seamless compatibility with existing CDMO infrastructure. The market trajectory signals fundamental shifts in how pharmaceutical companies and biotech facilities approach peptide therapeutics manufacturing solutions, with participants positioned to benefit from sustained demand across multiple therapeutic application segments.
Quick Stats for Peptide Therapeutics CDMO Market
- Peptide Therapeutics CDMO Market Value (2025): USD 2.9 billion
- Peptide Therapeutics CDMO Market Forecast Value (2035): USD 8.1 billion
- Peptide Therapeutics CDMO Market Forecast CAGR: 10.8%
- Leading Product Type in Peptide Therapeutics CDMO Market: API Manufacturing
- Key Growth Regions in Peptide Therapeutics CDMO Market: Asia Pacific, North America, and Europe
- Top Key Players in Peptide Therapeutics CDMO Market: Bachem AG, PolyPeptide Group, CPC Scientific Inc., Lonza Group Ltd., CordenPharma International, GenScript Biotech Corporation
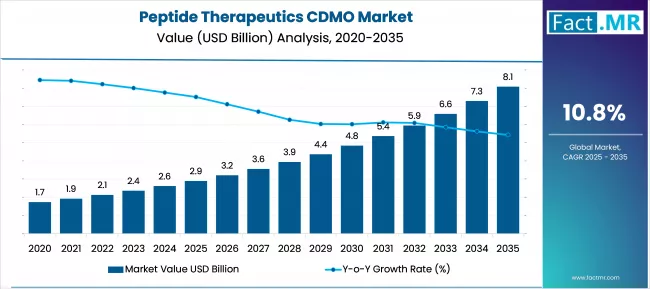
Peptide Therapeutics CDMO Market Year-over-Year Forecast 2025 to 2035
The Peptide Therapeutics CDMO market demonstrates distinct growth phases with varying market characteristics and competitive dynamics. Between 2025 and 2030, the market progresses through its technology adoption phase, expanding from USD 2.9 billion to USD 4.8 billion with steady annual increments averaging 11.5% growth. This period showcases the transition from basic peptide manufacturing units to advanced systems with enhanced synthesis capabilities and integrated pharmaceutical monitoring systems becoming mainstream features.
The 2025-2030 phase adds USD 1.9 billion to market value, representing 37% of total decade expansion. Market maturation factors include standardisation of peptide synthesis protocols, declining component costs for manufacturing arrays, and increasing pharmaceutical facility awareness of peptide therapeutics CDMO benefits reaching 85-90% effectiveness in drug development applications. Competitive landscape evolution during this period features established companies like Bachem AG and PolyPeptide Group expanding their peptide therapeutics CDMO portfolios while new entrants focus on compact designs and enhanced synthesis capabilities.
From 2030 to 2035, market dynamics shift toward advanced integration and multi-facility deployment, with growth accelerating from USD 4.8 billion to USD 8.1 billion, adding USD 3.3 billion or 63% of total expansion. This phase transition logic centres on commercial-scale manufacturing systems, integration with pharmaceutical automation networks, and deployment across diverse therapeutic scenarios, becoming standard rather than specialised applications. The competitive environment matures with focus shifting from basic manufacturing capability to comprehensive pharmaceutical development systems and integration with CDMO monitoring platforms.
Peptide Therapeutics CDMO Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 2.9 billion |
| Market Forecast (2035) ↑ | USD 8.1 billion |
| Growth Rate ★ | 10.8% CAGR |
| Leading Technology → | API Manufacturing |
| Primary Application → | Large Pharmaceutical Companies Segment |
The market demonstrates strong fundamentals with API manufacturing systems capturing a dominant share of 42.8% through advanced peptide synthesis capabilities and reliable pharmaceutical deployment capabilities. Large pharmaceutical companies drive primary demand with 52.3% share, supported by increasing facility spending on peptide therapeutics CDMO systems and drug development management solutions. Geographic expansion remains concentrated in developed markets with established pharmaceutical infrastructure, while emerging economies show accelerating adoption rates driven by facility modernisation and rising peptide therapeutics awareness.
Opportunity Pathways - Peptide Therapeutics CDMO Market
The peptide therapeutics CDMO market represents a compelling intersection of pharmaceutical technology, manufacturing modernization, and peptide synthesis management. With robust growth projected from USD 2.9 billion in 2025 to USD 8.1 billion by 2035 at an 10.8% CAGR, this market is driven by increasing peptide drug development, pharmaceutical infrastructure upgrades, and regulatory pressures for specialised manufacturing practices.
The market's expansion reflects a fundamental shift in how pharmaceutical companies and biotech facilities approach peptide manufacturing infrastructure. Strong growth opportunities exist across diverse applications, from API manufacturing operations requiring reliable synthesis quality to oncology facilities demanding advanced peptide standards. Geographic expansion is particularly pronounced in Asia-Pacific markets, led by India (15.8% CAGR) and China (14.2% CAGR), while established markets in North America and Europe drive premium positioning and technology innovation.
The dominance of API manufacturing systems and large pharmaceutical companies underscores the importance of proven synthesis technology and operational reliability in driving adoption. Implementation complexity and infrastructure compatibility remain key challenges, creating opportunities for companies that can simplify deployment while maintaining performance standards.
- Pathway A - Advanced Synthesis Technology Integration. Development of commercial-scale manufacturing systems with enhanced peptide synthesis capabilities, smart monitoring features, and extended system life can command premium pricing while meeting stringent pharmaceutical quality requirements. Integration with automated synthesis and process optimization capabilities creates competitive differentiation. Expected revenue pool: USD 1.6-2.4 billion.
- Pathway B - Geographic Expansion & Market Localization. Strong growth opportunities in India (15.8% CAGR) and China (14.2% CAGR) through local pharmaceutical partnerships, region-specific product variants, and compliance with local pharmaceutical codes. Localization reduces costs and addresses supply chain vulnerabilities while enabling market penetration. Revenue opportunity: USD 1.2-1.8 billion.
- Pathway C - Manufacturing Integration & Smart Pharmaceutical Compatibility. Developing systems that seamlessly integrate with pharmaceutical development platforms, offering comprehensive peptide manufacturing management, performance analytics, and maintenance optimization. Focus on compatibility with existing pharmaceutical infrastructure and simplified deployment processes. Pool: USD 1.0-1.5 billion.
- Pathway D - Biotechnology Applications Expansion. Specialized systems meeting biotech-grade synthesis standards for biotechnology companies, research institutions, and pharmaceutical operations where advanced peptide manufacturing and regulatory compliance justify premium pricing. Focus on enhanced flexibility features and biotech compliance capabilities. Revenue uplift: USD 0.8-1.2 billion.
- Pathway E - Sustainable Design & Pharmaceutical Certification. Systems designed for pharmaceutical compliance, energy efficiency, and waste reduction that appeal to environmentally conscious pharmaceutical facilities. Integration of sustainable components and reduced environmental footprint throughout system lifecycle. Expected upside: USD 0.6-0.9 billion.
- Pathway F - Modular & Retrofit Solutions. Simplified deployment systems designed for existing pharmaceutical facility retrofits, reducing complexity and installation costs while maintaining performance. Focus on minimal infrastructure modification requirements and plug-and-play compatibility. Innovation pool: USD 0.4-0.7 billion.
- Pathway G - Academic & Research Institution Specialization. Tailored solutions for research institutions, universities, and academic facilities with high-precision capacity, rapid synthesis processing, and integration with research requirements. Enhanced durability and easy maintenance for research environments. Strategic value: USD 0.3-0.5 billion.
Why is the Peptide Therapeutics CDMO Market Growing?
Peptide drug development demand creates compelling operational advantages through peptide therapeutics CDMO that provides immediate synthesis access without traditional manufacturing limitations, enabling facilities to promote therapeutic excellence while maintaining pharmaceutical convenience and reducing operational costs.
Pharmaceutical modernisation programs accelerate as pharmaceutical operators worldwide seek advanced synthesis management systems that complement traditional manufacturing infrastructure, enabling precise peptide solutions that align with development goals and quality requirements.
Pharmaceutical infrastructure enhancement drives adoption from pharmaceutical companies and biotech facilities requiring effective synthesis solutions that minimise maintenance requirements while maintaining manufacturing quality during high-usage operations.
However, growth faces headwinds from implementation complexity challenges that vary across pharmaceutical facility types regarding synthesis integration and regulatory requirements, potentially limiting deployment flexibility in certain pharmaceutical environments. Technical limitations also persist regarding synthesis capacity and maintenance intervals that may increase operational costs in high-volume applications with demanding pharmaceutical quality standards.
Segmental Analysis
The market segments by service type into API Manufacturing, Drug Product Manufacturing, and Development Services categories, representing the evolution from basic synthesis systems to advanced peptide manufacturing solutions for comprehensive pharmaceutical development.
End user segmentation divides the market into Large Pharmaceutical Companies, Biotechnology Companies, and Academic & Research Institutions sectors, reflecting distinct requirements for synthesis standards, pharmaceutical integration, and regulatory compliance.
Geographic distribution covers North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by pharmaceutical modernisation programs.
The segmentation structure reveals technology progression from API manufacturing systems toward integrated commercial-scale platforms with enhanced synthesis capabilities and monitoring features, while application diversity spans from pharmaceutical company operations to biotech facilities requiring precise peptide manufacturing solutions.
By Service Type, the API Manufacturing Segment Accounts for Dominant Market Share of 42.8%
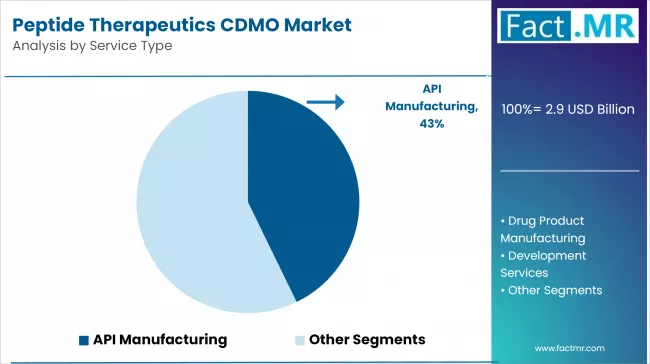
API Manufacturing systems command the leading position with 42.8% share in the Peptide Therapeutics CDMO market through advanced synthesis features, including comprehensive peptide manufacturing capabilities, extended system life, and cost-effective maintenance that enable pharmaceutical facilities to deploy reliable peptide therapeutics CDMO solutions across diverse pharmaceutical environments.
The segment benefits from pharmaceutical facility preference for proven synthesis technology that provides immediate drug development quality improvement without requiring complex infrastructure modifications. Cost-effective design features enable deployment in pharmaceutical environments, manufacturing facilities, and biotech applications where reliability and maintenance efficiency represent critical operational requirements.
API manufacturing systems differentiate through rapid deployment capability, proven synthesis performance, and integration with standard pharmaceutical systems that enhance facility automation while maintaining cost-effective operational profiles suitable for diverse pharmaceutical applications.
Key market characteristics:
- Advanced synthesis systems with multi-stage peptide manufacturing processing and automatic quality replacement indicators
- Extended system life enabling 6-12 months of continuous operation with minimal maintenance requirements
- Deployment accessories, including synthesis modules, integration connections, and maintenance kits for pharmaceutical operations
API Manufacturing Sub-segments:
- Large Scale Manufacturing: 24.7% market share - Leading sub-segment within API manufacturing
- Small Scale/Clinical Manufacturing: 18.1% market share
By End User, the Large Pharmaceutical Companies Segment Accounts for the Largest Market Share of 52.3%
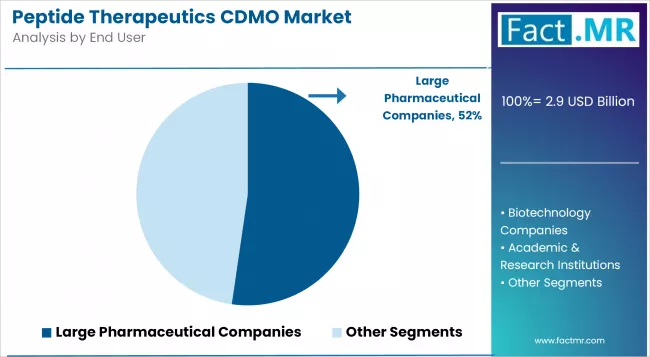
Large pharmaceutical companies dominate the Peptide Therapeutics CDMO market with 52.3% share due to widespread adoption of peptide therapeutics CDMO systems and increasing focus on pharmaceutical synthesis management, operational compliance, and drug development applications that minimise development timelines while maintaining operational efficiency.
Large pharmaceutical companies customers prioritise system reliability, synthesis consistency, and integration with existing pharmaceutical infrastructure that enables coordinated deployment across multiple development locations. The segment benefits from substantial pharmaceutical budgets and modernisation programs that emphasise operational efficiency for large pharmaceutical companies and pharmaceutical operations.
Pharmaceutical modernisation programs incorporate peptide therapeutics CDMO as standard equipment for pharmaceutical automation and operational compliance applications. At the same time, large pharmaceutical companies’ industry initiatives are increasing demand for efficient equipment that comply with pharmaceutical standards and minimise operational waste.
Large Pharmaceutical Companies Sub-segments:
- Large pharmaceutical companies encompass both multinational and regional pharmaceutical corporations requiring comprehensive peptide manufacturing solutions across their drug development pipelines.
Application dynamics include:
- Strong growth in large pharmaceutical companies and pharmaceutical facilities requiring peptide therapeutics CDMO solutions
- Increasing adoption in commercial pharmaceutical and development applications for synthesis efficiency
- Rising integration with pharmaceutical management systems for maintenance monitoring and performance tracking
What are the Drivers, Restraints, and Key Trends of the Peptide Therapeutics CDMO Market?
Pharmaceutical modernisation drives primary adoption as peptide therapeutics CDMO provides pharmaceutical synthesis capabilities that enable drug development access without traditional manufacturing limitations, supporting operational compliance and efficiency that require precise resource management.
Pharmaceutical infrastructure demand accelerates market expansion as pharmaceutical operators seek effective synthesis solutions that minimise maintenance costs while maintaining development quality during high-usage scenarios and pharmaceutical management operations. Peptide therapeutics spending increases worldwide, creating sustained demand for efficient systems that complement traditional pharmaceutical equipment and provide operational flexibility in complex pharmaceutical environments.
Implementation complexity challenges vary across pharmaceutical facility types regarding synthesis integration and regulatory requirements, which may limit deployment flexibility and market penetration in facilities with restrictive infrastructure modifications.
Technical performance limitations persist regarding synthesis capacity and maintenance intervals that may increase operational costs in high-volume applications with demanding pharmaceutical quality standards and frequent usage patterns. Market fragmentation across multiple pharmaceutical codes and deployment standards creates compatibility concerns between different system manufacturers and existing pharmaceutical infrastructure.
Adoption accelerates in pharmaceutical companies and biotech sectors where peptide therapeutics CDMO justifies system costs, with geographic concentration in developed markets transitioning toward mainstream adoption in emerging economies driven by pharmaceutical modernisation and peptide infrastructure development.
Technology development focuses on enhanced synthesis capabilities, improved peptide technology, and integration with pharmaceutical management systems that optimise maintenance scheduling and development quality monitoring. The market could face disruption if alternative synthesis technologies or regulatory restrictions significantly limit peptide therapeutics CDMO deployment in pharmaceutical or biotech applications.
Analysis of the Peptide Therapeutics CDMO Market by Key Country
The peptide therapeutics CDMO market demonstrates varied regional dynamics with Growth Leaders including India (15.8% CAGR) and China (14.2% CAGR) driving expansion through pharmaceutical modernisation and peptide therapeutics CDMO infrastructure development.
Steady performers encompass South Korea (12.9% CAGR), Japan (11.7% CAGR), and Germany (10.8% CAGR), benefiting from established pharmaceutical sectors and advanced pharmaceutical management adoption. Emerging Markets feature Switzerland (10.1% CAGR) and USA (9.6% CAGR), where specialised pharmaceutical applications and synthesis technology integration support consistent growth patterns.
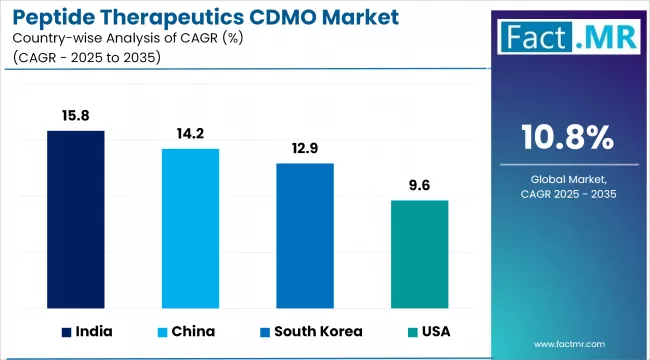
| Country | CAGR (2025-2035) |
|---|---|
| India | 15.8% |
| China | 14.2% |
| South Korea | 12.9% |
| Japan | 11.7% |
| Germany | 10.8% |
| Switzerland | 10.1% |
| USA | 9.6% |
Regional synthesis reveals Asia-Pacific markets leading growth through pharmaceutical modernisation and peptide therapeutics CDMO infrastructure development, while European countries maintain steady expansion supported by synthesis technology advancement and pharmaceutical modernisation requirements. North American markets show moderate growth driven by peptide therapeutics CDMO applications and pharmaceutical facility integration trends.
India Drives Global Market Leadership
India establishes market leadership through aggressive pharmaceutical modernisation programs and comprehensive peptide therapeutics CDMO infrastructure development, integrating advanced peptide therapeutics CDMO as standard components in API manufacturing and pharmaceutical applications.
The country's 15.8% CAGR through 2035 reflects government initiatives promoting pharmaceutical technology and domestic pharmaceutical management capabilities that mandate the use of peptide therapeutics CDMO systems in pharmaceutical companies and biotech installations. Growth concentrates in major pharmaceutical centres, including Hyderabad, Mumbai, and Ahmedabad, where pharmaceutical modernisation showcases integrated peptide CDMO systems that appeal to domestic operators seeking advanced synthesis solutions and operational efficiency applications.
Indian pharmaceutical providers are developing cost-effective peptide therapeutics CDMO solutions that combine domestic production advantages with advanced features, including multi-stage synthesis systems and extended operational capacity.
Strategic Market Indicators:
- Large pharmaceutical companies facilities leading adoption with 60% deployment rate in pharmaceutical manufacturing and biotech buildings
- Government pharmaceutical programs providing substantial funding for domestic pharmaceutical technology development
- Local providers capturing 45% market share through competitive pricing and localised support
- Biotech segment growth driven by pharmaceutical companies and biotech facility requirements for peptide therapeutics CDMO systems
- Export market development for cost-effective peptide therapeutics CDMO solutions targeting emerging pharmaceutical markets
China Emerges as High-Growth Market
In Beijing, Shanghai, and Shenzhen, pharmaceutical companies and biotech facilities are implementing advanced peptide therapeutics CDMO as standard equipment for pharmaceutical synthesis operations and operational compliance applications, driven by increasing pharmaceutical facility spending and modernisation programs that emphasise efficient infrastructure capabilities. The market is projected to demonstrate a 14.2% CAGR through 2035, supported by government pharmaceutical initiatives and pharmaceutical infrastructure development programs that promote advanced peptide CDMO systems for pharmaceutical companies and biotech facilities. Chinese pharmaceutical facility operators are adopting peptide therapeutics CDMO systems that provide reliable synthesis management and pharmaceutical automation features, particularly appealing in pharmaceutical developments where operational compliance represents critical requirements.
Market expansion benefits from growing pharmaceutical construction capabilities and international technology transfer agreements that enable domestic production of advanced peptide CDMO systems for pharmaceutical companies and biotech applications.
Market Intelligence Brief:
- Large pharmaceutical companies and biotech segments are driving initial adoption with 40% annual growth in installations
- Pharmaceutical modernisation programs emphasising peptide therapeutics CDMO systems for operational compliance
- Local manufacturers partnering with international technology providers for system development
- Pharmaceutical and biotech facilities implementing peptide therapeutics CDMO for pharmaceutical automation and quality management
South Korea Shows Strong Technology Innovation
South Korea's advanced pharmaceutical technology market demonstrates sophisticated peptide therapeutics CDMO deployment with documented operational effectiveness in pharmaceutical manufacturing facilities and biotech applications through integration with existing pharmaceutical management systems and synthesis infrastructure. The country leverages engineering expertise in synthesis technology and pharmaceutical systems integration to maintain a 12.9% CAGR through 2035. Pharmaceutical centres, including Seoul, Incheon, and Busan, showcase premium installations where peptide therapeutics CDMO integrates with comprehensive pharmaceutical platforms and facility management systems to optimise development quality and synthesis effectiveness.
South Korean pharmaceutical contractors prioritise system reliability and operational compliance in peptide therapeutics CDMO development, creating demand for premium systems with advanced features, including enhanced synthesis durability and integration with pharmaceutical automation systems. The market benefits from established pharmaceutical infrastructure and a willingness to invest in advanced synthesis technologies that provide long-term operational benefits and compliance with pharmaceutical regulations.
Market Intelligence Brief:
- Engineering focuses on pharmaceutical standards and system integration, driving premium segment growth
- Pharmaceutical contractor partnerships providing 40% faster technology deployment cycles
- Technology collaboration between Korean synthesis manufacturers and international pharmaceutical companies
- Pharmaceutical management programs expanding peptide therapeutics CDMO integration in pharmaceutical and biotech scenarios
Japan Demonstrates Technology Excellence
Japan's market expansion benefits from diverse pharmaceutical demand, including pharmaceutical modernisation in Tokyo and Osaka, pharmaceutical manufacturing equipment upgrades, and government pharmaceutical programs that increasingly incorporate efficient synthesis solutions for pharmaceutical applications. The country maintains an 11.7% CAGR through 2035, driven by rising pharmaceutical technology awareness and increasing recognition of peptide therapeutics CDMO technology benefits, including reliable development quality and reduced operational waste capabilities.
Market dynamics focus on cost-effective peptide therapeutics CDMO solutions that balance advanced synthesis features with performance considerations important to Japanese pharmaceutical facility operators. Growing pharmaceutical development creates sustained demand for modern synthesis systems in new pharmaceutical facility infrastructure and pharmaceutical manufacturing equipment modernisation projects.
Strategic Market Considerations:
- Large pharmaceutical companies and biotech segments leading growth with focus on peptide therapeutics CDMO and quality applications
- Regional pharmaceutical facility requirements driving a diverse product portfolio from basic synthesis to advanced multi-stage systems
- Import dependency challenges offset by potential local assembly partnerships with international manufacturers
- Government pharmaceutical initiatives beginning to influence procurement standards and operational requirements
Germany Maintains Technology Leadership
Germany's advanced pharmaceutical technology market demonstrates sophisticated peptide therapeutics CDMO deployment with documented operational effectiveness in pharmaceutical manufacturing facilities and biotech applications through integration with existing pharmaceutical management systems and synthesis infrastructure. The country leverages engineering expertise in synthesis technology and pharmaceutical systems integration to maintain a 10.8% CAGR through 2035. Pharmaceutical centres, including Munich, Frankfurt, and Berlin, showcase premium installations where peptide therapeutics CDMO integrates with comprehensive pharmaceutical platforms and facility management systems to optimise development quality and synthesis effectiveness.
German pharmaceutical contractors prioritise system reliability and operational compliance in peptide therapeutics CDMO development, creating demand for premium systems with advanced features, including enhanced synthesis durability and integration with pharmaceutical automation systems. The market benefits from established pharmaceutical infrastructure and a willingness to invest in advanced synthesis technologies that provide long-term operational benefits and compliance with pharmaceutical regulations.
Market Intelligence Brief:
- Engineering focuses on pharmaceutical standards and system integration, driving premium segment growth
- Pharmaceutical contractor partnerships providing 35% faster technology deployment cycles
- Technology collaboration between German synthesis manufacturers and international pharmaceutical companies
- Pharmaceutical management programs expanding peptide therapeutics CDMO integration in pharmaceutical and biotech scenarios
Switzerland Shows Strong Regional Leadership
Switzerland's market expansion benefits from diverse pharmaceutical demand, including pharmaceutical modernisation in Basel and Zurich, pharmaceutical manufacturing equipment upgrades, and government pharmaceutical programs that increasingly incorporate efficient synthesis solutions for pharmaceutical applications. The country maintains a 10.1% CAGR through 2035, driven by rising pharmaceutical technology awareness and increasing recognition of peptide therapeutics CDMO technology benefits, including reliable development quality and reduced operational waste capabilities.
Market dynamics focus on cost-effective peptide therapeutics CDMO solutions that balance advanced synthesis features with performance considerations important to Swiss pharmaceutical facility operators. Growing pharmaceutical development creates sustained demand for modern synthesis systems in new pharmaceutical facility infrastructure and pharmaceutical manufacturing equipment modernisation projects.
Strategic Market Considerations:
- Large pharmaceutical companies and biotech segments leading growth with focus on peptide therapeutics CDMO and quality applications
- Regional pharmaceutical facility requirements driving a diverse product portfolio from basic synthesis to advanced multi-stage systems
- Import dependency challenges offset by potential local assembly partnerships with international manufacturers
- Government pharmaceutical initiatives beginning to influence procurement standards and operational requirements
United States Demonstrates Technology Innovation
The U.S. market emphasises advanced peptide therapeutics CDMO features, including precision synthesis systems and integration with comprehensive pharmaceutical management platforms that manage development quality, maintenance scheduling, and synthesis tracking applications through unified pharmaceutical systems.
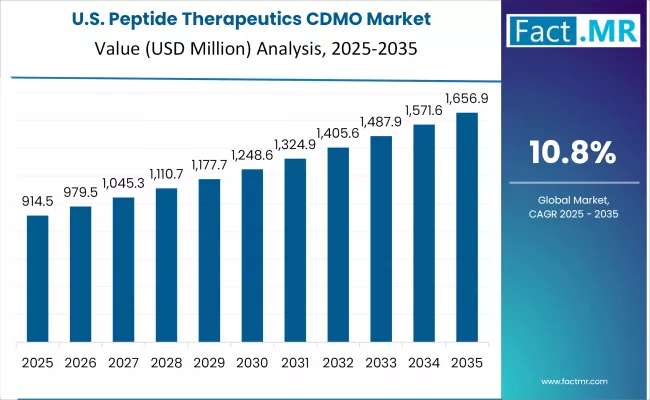
The country is projected to show a 9.6% CAGR through 2035, driven by pharmaceutical modernisation under synthesis equipment upgrades and biotech demand for reliable, peptide therapeutics CDMO systems. American pharmaceutical facility operators prioritise operational effectiveness with peptide therapeutics CDMO delivering consistent development quality through advanced synthesis algorithms and pharmaceutical monitoring capabilities.
Technology deployment channels include major pharmaceutical synthesis equipment suppliers, specialised pharmaceutical management companies, and biotech procurement programs that support professional installation for complex pharmaceutical applications.
Performance Metrics:
- Large pharmaceutical companies facilities in California, Massachusetts, and New Jersey leading peptide therapeutics CDMO adoption for pharmaceutical operations
- Equipment supplier channel maintaining 70% market share for complex pharmaceutical integration applications
- Biotech procurement programs supporting 30% of peptide therapeutics CDMO acquisitions across pharmaceutical companies and biotech facilities
- Pharmaceutical platform compatibility with major pharmaceutical management systems, driving procurement selection criteria
Europe Market Split by Country
The peptide therapeutics CDMO market in Europe is projected to grow from USD 0.8 billion in 2025 to USD 2.1 billion by 2035, registering a CAGR of 10.7% over the forecast period. Switzerland is expected to maintain its leadership position with a 24.8% market share in 2025, declining slightly to 23.9% by 2035, supported by its advanced pharmaceutical infrastructure and major synthesis management centres, including Basel and Zurich.
Germany follows with a 21.7% share in 2025, projected to reach 22.4% by 2035, driven by comprehensive pharmaceutical modernisation programs and synthesis technology development initiatives. The United Kingdom holds a 16.4% share in 2025, expected to maintain 15.8% by 2035 through specialised pharmaceutical applications and operational compliance requirements.
France commands a 13.9% share, while Italy accounts for 11.2% in 2025. Netherlands maintains 8.3% market share, while BENELUX (excluding Netherlands) holds 3.4% in 2025. The Rest of Western Europe region is anticipated to maintain its share at 0.3% by 2035, attributed to increasing peptide therapeutics CDMO adoption in Nordic countries and emerging Eastern European pharmaceutical facilities implementing synthesis programs.
API Manufacturing Dominates Peptide Therapeutics CDMO System Demand in Japan
In Japan, the peptide therapeutics CDMO market prioritises API manufacturing applications, which capture 42.8% share of pharmaceutical and synthesis installations through advanced features, including precision synthesis optimisation and seamless integration with existing pharmaceutical management infrastructure.
Japanese pharmaceutical operators emphasise reliability, development quality, and long-term operational excellence, creating demand for API manufacturing systems that provide peptide therapeutics CDMO capabilities and adaptive synthesis control based on operational requirements and quality scenarios.
Drug product manufacturing maintains a secondary market position with 31.7% share primarily in specialised applications and pharmaceutical installations where advanced synthesis functionality meets operational requirements without complex maintenance features.
Market Characteristics:
- Premium focus on API manufacturing systems with advanced synthesis algorithms and precision development quality capabilities
- Integration requirements with existing pharmaceutical platforms and facility management systems
- Emphasis on operational reliability and long-term durability in pharmaceutical applications
API Manufacturing Equipment Suppliers Lead Peptide Therapeutics CDMO System Services in South Korea
In South Korea, the market structure favours API Manufacturing applications, which maintain 38.4% share through comprehensive synthesis requirements and established pharmaceutical procurement networks supporting both pharmaceutical companies and biotech installations.
These applications offer integrated solutions combining advanced peptide therapeutics CDMO systems with professional deployment services and ongoing technical support that appeal to Korean pharmaceutical facility operators seeking reliable, pharmaceutical automation systems.
Drug product manufacturing applications capture 34.7% market share by providing localised service capabilities and competitive solutions for standard pharmaceutical installations, while Development Services focus on specialised applications with 26.9% share and cost-effective solutions tailored to Korean pharmaceutical characteristics.
Application Insights:
- API Manufacturing applications maintaining dominant market positioning through advanced technology offerings
- Local pharmaceutical service networks expanding to support growing demand for professional installation and maintenance
- System integration capabilities are becoming a key differentiator for pharmaceutical building and biotech applications
Competitive Landscape of the Peptide Therapeutics CDMO Market
The peptide therapeutics CDMO market operates with moderate concentration, featuring approximately 10-15 meaningful participants, where leading companies control roughly 35-40% of the global market share through established pharmaceutical facility relationships and comprehensive technology portfolios. Competition emphasises advanced synthesis capabilities, system reliability, and pharmaceutical integration rather than price-based rivalry.
Market leaders encompass Bachem AG with 16.8% market share, PolyPeptide Group, and CPC Scientific Inc., which maintain competitive advantages through extensive pharmaceutical management expertise, global pharmaceutical synthesis equipment networks, and comprehensive system integration capabilities that create customer switching costs and support premium pricing. These companies leverage decades of pharmaceutical equipment experience and ongoing research investments to develop advanced peptide therapeutics CDMO systems with enhanced synthesis and pharmaceutical management features.
Technology challengers include Lonza Group Ltd., CordenPharma International, and GenScript Biotech Corporation, which compete through specialised synthesis technology focus and innovative deployment interfaces that appeal to pharmaceutical customers seeking advanced development quality capabilities and operational flexibility. These companies differentiate through rapid technology development cycles and specialised pharmaceutical application focus.
Market dynamics favour participants that combine reliable synthesis hardware with advanced monitoring software, including development quality tracking and automatic maintenance alert capabilities. Competitive pressure intensifies as traditional pharmaceutical equipment manufacturers expand into peptide therapeutics CDMO systems. At the same time, specialised synthesis companies challenge established players through innovative pharmaceutical solutions and cost-effective platforms targeting specialised pharmaceutical segments.
Key Players in the Peptide Therapeutics CDMO Market
- Bachem AG
- PolyPeptide Group
- CPC Scientific Inc.
- Lonza Group Ltd.
- CordenPharma International
- GenScript Biotech Corporation
- Almac Group
- Thermo Fisher Scientific Inc.
- AmbioPharm Inc.
- Peptides International Inc.
Scope of the Report
| Item | Value |
|---|---|
| Quantitative Units | USD 2.9 Billion |
| Service Type | API Manufacturing, Drug Product Manufacturing, Development Services |
| End User | Large Pharmaceutical Companies, Biotechnology Companies, Academic & Research Institutions |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | India, China, South Korea, Japan, Germany, Switzerland, USA, and 25+ additional countries |
| Key Companies Profiled | Bachem AG, PolyPeptide Group, CPC Scientific Inc., Lonza Group Ltd., CordenPharma International, GenScript Biotech Corporation |
| Additional Attributes | Dollar sales by service type and end user categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with pharmaceutical equipment manufacturers and technology providers, facility preferences for development quality and system reliability, integration with pharmaceutical management platforms and maintenance systems, innovations in synthesis technology and deployment efficiency, and development of API manufacturing solutions with enhanced peptide synthesis and monitoring capabilities. |
Peptide Therapeutics CDMO Market by Segments
-
Service Type :
- API Manufacturing
- Drug Product Manufacturing
- Development Services
-
Scale of Operation :
- Preclinical/Clinical
- Commercial
-
Synthesis Method :
- Solid Phase Peptide Synthesis (SPPS)
- Liquid Phase Peptide Synthesis (LPPS)
- Hybrid Methods
-
Peptide Length :
- Short Peptides (2-10 amino acids)
- Medium Peptides (11-40 amino acids)
- Long Peptides (40+ amino acids)
-
Therapeutic Application :
- Oncology
- Metabolic Disorders
- Cardiovascular Diseases
- Infectious Diseases
- Others
-
End User :
- Large Pharmaceutical Companies
- Biotechnology Companies
- Academic & Research Institutions
-
Region :
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Union
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand-side Trends
- Supply-side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020-2024 and Forecast, 2025-2035
- Historical Market Size Value (USD Billion) & Units Analysis, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Projections, 2025-2035
- Y-o-Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020-2024 and Forecast 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Service Type
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By Service Type, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By Service Type, 2025-2035
- API Manufacturing
- Drug Product Manufacturing
- Development Services
- Y-o-Y Growth Trend Analysis By Service Type, 2020-2024
- Absolute $ Opportunity Analysis By Service Type, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Scale of Operation
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By Scale of Operation, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By Scale of Operation, 2025-2035
- Preclinical/Clinical
- Commercial
- Y-o-Y Growth Trend Analysis By Scale of Operation, 2020-2024
- Absolute $ Opportunity Analysis By Scale of Operation, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Synthesis Method
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By Synthesis Method, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By Synthesis Method, 2025-2035
- Solid Phase Peptide Synthesis (SPPS)
- Liquid Phase Peptide Synthesis (LPPS)
- Hybrid Methods
- Y-o-Y Growth Trend Analysis By Synthesis Method, 2020-2024
- Absolute $ Opportunity Analysis By Synthesis Method, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Peptide Length
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By Peptide Length, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By Peptide Length, 2025-2035
- Short Peptides (2-10 amino acids)
- Medium Peptides (11-40 amino acids)
- Long Peptides (40+ amino acids)
- Y-o-Y Growth Trend Analysis By Peptide Length, 2020-2024
- Absolute $ Opportunity Analysis By Peptide Length, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Therapeutic Application
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By Therapeutic Application, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By Therapeutic Application, 2025-2035
- Oncology
- Metabolic Disorders
- Cardiovascular Diseases
- Infectious Diseases
- Others
- Y-o-Y Growth Trend Analysis By Therapeutic Application, 2020-2024
- Absolute $ Opportunity Analysis By Therapeutic Application, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By End User
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By End User, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By End User, 2025-2035
- Large Pharmaceutical Companies
- Biotechnology Companies
- Academic & Research Institutions
- Y-o-Y Growth Trend Analysis By End User, 2020-2024
- Absolute $ Opportunity Analysis By End User, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Region
- Introduction
- Historical Market Size Value (USD Billion) & Units Analysis By Region, 2020-2024
- Current Market Size Value (USD Billion) & Units Analysis and Forecast By Region, 2025-2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia & Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- U.S.
- Canada
- Mexico
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Key Takeaways
- Latin America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Key Takeaways
- Western Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Germany
- U.K.
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Europe
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Key Takeaways
- Eastern Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltics
- Rest of Eastern Europe
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Key Takeaways
- East Asia Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- China
- Japan
- South Korea
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Key Takeaways
- South Asia & Pacific Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia & Pacific
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Key Takeaways
- Middle East & Africa Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Key Takeaways
- Key Countries Market Analysis
- U.S.
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- U.K.
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Nordic
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- BENELUX
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Balkan & Baltics
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- U.S.
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Service Type
- By Scale of Operation
- By Synthesis Method
- By Peptide Length
- By Therapeutic Application
- By End User
- Competition Analysis
- Competition Deep Dive
- Bachem AG
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- PolyPeptide Group
- CPC Scientific Inc.
- Lonza Group Ltd.
- CordenPharma International
- GenScript Biotech Corporation
- Almac Group
- Thermo Fisher Scientific Inc.
- AmbioPharm Inc.
- Peptides International Inc.
- Bachem AG
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Billion) Forecast by Region, 2020 to 2035
- Table 2: Global Market Units Forecast by Region, 2020 to 2035
- Table 3: Global Market Value (USD Billion) Forecast by Service Type, 2020 to 2035
- Table 4: Global Market Units Forecast by Service Type, 2020 to 2035
- Table 5: Global Market Value (USD Billion) Forecast by Scale of Operation, 2020 to 2035
- Table 6: Global Market Units Forecast by Scale of Operation, 2020 to 2035
- Table 7: Global Market Value (USD Billion) Forecast by Synthesis Method, 2020 to 2035
- Table 8: Global Market Units Forecast by Synthesis Method, 2020 to 2035
- Table 9: Global Market Value (USD Billion) Forecast by Peptide Length, 2020 to 2035
- Table 10: Global Market Units Forecast by Peptide Length, 2020 to 2035
- Table 11: Global Market Value (USD Billion) Forecast by Therapeutic Application, 2020 to 2035
- Table 12: Global Market Units Forecast by Therapeutic Application, 2020 to 2035
- Table 13: Global Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 14: Global Market Units Forecast by End User, 2020 to 2035
- Table 15: North America Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 16: North America Market Units Forecast by Country, 2020 to 2035
- Table 17: North America Market Value (USD Billion) Forecast by Service Type, 2020 to 2035
- Table 18: North America Market Units Forecast by Service Type, 2020 to 2035
- Table 19: North America Market Value (USD Billion) Forecast by Scale of Operation, 2020 to 2035
- Table 20: North America Market Units Forecast by Scale of Operation, 2020 to 2035
- Table 21: North America Market Value (USD Billion) Forecast by Synthesis Method, 2020 to 2035
- Table 22: North America Market Units Forecast by Synthesis Method, 2020 to 2035
- Table 23: North America Market Value (USD Billion) Forecast by Peptide Length, 2020 to 2035
- Table 24: North America Market Units Forecast by Peptide Length, 2020 to 2035
- Table 25: North America Market Value (USD Billion) Forecast by Therapeutic Application, 2020 to 2035
- Table 26: North America Market Units Forecast by Therapeutic Application, 2020 to 2035
- Table 27: North America Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 28: North America Market Units Forecast by End User, 2020 to 2035
- Table 29: Latin America Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 30: Latin America Market Units Forecast by Country, 2020 to 2035
- Table 31: Latin America Market Value (USD Billion) Forecast by Service Type, 2020 to 2035
- Table 32: Latin America Market Units Forecast by Service Type, 2020 to 2035
- Table 33: Latin America Market Value (USD Billion) Forecast by Scale of Operation, 2020 to 2035
- Table 34: Latin America Market Units Forecast by Scale of Operation, 2020 to 2035
- Table 35: Latin America Market Value (USD Billion) Forecast by Synthesis Method, 2020 to 2035
- Table 36: Latin America Market Units Forecast by Synthesis Method, 2020 to 2035
- Table 37: Latin America Market Value (USD Billion) Forecast by Peptide Length, 2020 to 2035
- Table 38: Latin America Market Units Forecast by Peptide Length, 2020 to 2035
- Table 39: Latin America Market Value (USD Billion) Forecast by Therapeutic Application, 2020 to 2035
- Table 40: Latin America Market Units Forecast by Therapeutic Application, 2020 to 2035
- Table 41: Latin America Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 42: Latin America Market Units Forecast by End User, 2020 to 2035
- Table 43: Western Europe Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 44: Western Europe Market Units Forecast by Country, 2020 to 2035
- Table 45: Western Europe Market Value (USD Billion) Forecast by Service Type, 2020 to 2035
- Table 46: Western Europe Market Units Forecast by Service Type, 2020 to 2035
- Table 47: Western Europe Market Value (USD Billion) Forecast by Scale of Operation, 2020 to 2035
- Table 48: Western Europe Market Units Forecast by Scale of Operation, 2020 to 2035
- Table 49: Western Europe Market Value (USD Billion) Forecast by Synthesis Method, 2020 to 2035
- Table 50: Western Europe Market Units Forecast by Synthesis Method, 2020 to 2035
- Table 51: Western Europe Market Value (USD Billion) Forecast by Peptide Length, 2020 to 2035
- Table 52: Western Europe Market Units Forecast by Peptide Length, 2020 to 2035
- Table 53: Western Europe Market Value (USD Billion) Forecast by Therapeutic Application, 2020 to 2035
- Table 54: Western Europe Market Units Forecast by Therapeutic Application, 2020 to 2035
- Table 55: Western Europe Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 56: Western Europe Market Units Forecast by End User, 2020 to 2035
- Table 57: Eastern Europe Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 58: Eastern Europe Market Units Forecast by Country, 2020 to 2035
- Table 59: Eastern Europe Market Value (USD Billion) Forecast by Service Type, 2020 to 2035
- Table 60: Eastern Europe Market Units Forecast by Service Type, 2020 to 2035
- Table 61: Eastern Europe Market Value (USD Billion) Forecast by Scale of Operation, 2020 to 2035
- Table 62: Eastern Europe Market Units Forecast by Scale of Operation, 2020 to 2035
- Table 63: Eastern Europe Market Value (USD Billion) Forecast by Synthesis Method, 2020 to 2035
- Table 64: Eastern Europe Market Units Forecast by Synthesis Method, 2020 to 2035
- Table 65: Eastern Europe Market Value (USD Billion) Forecast by Peptide Length, 2020 to 2035
- Table 66: Eastern Europe Market Units Forecast by Peptide Length, 2020 to 2035
- Table 67: Eastern Europe Market Value (USD Billion) Forecast by Therapeutic Application, 2020 to 2035
- Table 68: Eastern Europe Market Units Forecast by Therapeutic Application, 2020 to 2035
- Table 69: Eastern Europe Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 70: Eastern Europe Market Units Forecast by End User, 2020 to 2035
- Table 71: East Asia Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 72: East Asia Market Units Forecast by Country, 2020 to 2035
- Table 73: East Asia Market Value (USD Billion) Forecast by Service Type, 2020 to 2035
- Table 74: East Asia Market Units Forecast by Service Type, 2020 to 2035
- Table 75: East Asia Market Value (USD Billion) Forecast by Scale of Operation, 2020 to 2035
- Table 76: East Asia Market Units Forecast by Scale of Operation, 2020 to 2035
- Table 77: East Asia Market Value (USD Billion) Forecast by Synthesis Method, 2020 to 2035
- Table 78: East Asia Market Units Forecast by Synthesis Method, 2020 to 2035
- Table 79: East Asia Market Value (USD Billion) Forecast by Peptide Length, 2020 to 2035
- Table 80: East Asia Market Units Forecast by Peptide Length, 2020 to 2035
- Table 81: East Asia Market Value (USD Billion) Forecast by Therapeutic Application, 2020 to 2035
- Table 82: East Asia Market Units Forecast by Therapeutic Application, 2020 to 2035
- Table 83: East Asia Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 84: East Asia Market Units Forecast by End User, 2020 to 2035
- Table 85: South Asia & Pacific Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 86: South Asia & Pacific Market Units Forecast by Country, 2020 to 2035
- Table 87: South Asia & Pacific Market Value (USD Billion) Forecast by Service Type, 2020 to 2035
- Table 88: South Asia & Pacific Market Units Forecast by Service Type, 2020 to 2035
- Table 89: South Asia & Pacific Market Value (USD Billion) Forecast by Scale of Operation, 2020 to 2035
- Table 90: South Asia & Pacific Market Units Forecast by Scale of Operation, 2020 to 2035
- Table 91: South Asia & Pacific Market Value (USD Billion) Forecast by Synthesis Method, 2020 to 2035
- Table 92: South Asia & Pacific Market Units Forecast by Synthesis Method, 2020 to 2035
- Table 93: South Asia & Pacific Market Value (USD Billion) Forecast by Peptide Length, 2020 to 2035
- Table 94: South Asia & Pacific Market Units Forecast by Peptide Length, 2020 to 2035
- Table 95: South Asia & Pacific Market Value (USD Billion) Forecast by Therapeutic Application, 2020 to 2035
- Table 96: South Asia & Pacific Market Units Forecast by Therapeutic Application, 2020 to 2035
- Table 97: South Asia & Pacific Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 98: South Asia & Pacific Market Units Forecast by End User, 2020 to 2035
- Table 99: Middle East & Africa Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 100: Middle East & Africa Market Units Forecast by Country, 2020 to 2035
- Table 101: Middle East & Africa Market Value (USD Billion) Forecast by Service Type, 2020 to 2035
- Table 102: Middle East & Africa Market Units Forecast by Service Type, 2020 to 2035
- Table 103: Middle East & Africa Market Value (USD Billion) Forecast by Scale of Operation, 2020 to 2035
- Table 104: Middle East & Africa Market Units Forecast by Scale of Operation, 2020 to 2035
- Table 105: Middle East & Africa Market Value (USD Billion) Forecast by Synthesis Method, 2020 to 2035
- Table 106: Middle East & Africa Market Units Forecast by Synthesis Method, 2020 to 2035
- Table 107: Middle East & Africa Market Value (USD Billion) Forecast by Peptide Length, 2020 to 2035
- Table 108: Middle East & Africa Market Units Forecast by Peptide Length, 2020 to 2035
- Table 109: Middle East & Africa Market Value (USD Billion) Forecast by Therapeutic Application, 2020 to 2035
- Table 110: Middle East & Africa Market Units Forecast by Therapeutic Application, 2020 to 2035
- Table 111: Middle East & Africa Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 112: Middle East & Africa Market Units Forecast by End User, 2020 to 2035
List Of Figures
- Figure 1: Global Market Units Forecast 2020 to 2035
- Figure 2: Global Market Pricing Analysis
- Figure 3: Global Market Value (USD Billion) Forecast 2020 to 2035
- Figure 4: Global Market Value Share and BPS Analysis by Service Type, 2025 and 2035
- Figure 5: Global Market Y-o-Y Growth Comparison by Service Type, 2025 to 2035
- Figure 6: Global Market Attractiveness Analysis by Service Type
- Figure 7: Global Market Value Share and BPS Analysis by Scale of Operation, 2025 and 2035
- Figure 8: Global Market Y-o-Y Growth Comparison by Scale of Operation, 2025 to 2035
- Figure 9: Global Market Attractiveness Analysis by Scale of Operation
- Figure 10: Global Market Value Share and BPS Analysis by Synthesis Method, 2025 and 2035
- Figure 11: Global Market Y-o-Y Growth Comparison by Synthesis Method, 2025 to 2035
- Figure 12: Global Market Attractiveness Analysis by Synthesis Method
- Figure 13: Global Market Value Share and BPS Analysis by Peptide Length, 2025 and 2035
- Figure 14: Global Market Y-o-Y Growth Comparison by Peptide Length, 2025 to 2035
- Figure 15: Global Market Attractiveness Analysis by Peptide Length
- Figure 16: Global Market Value Share and BPS Analysis by Therapeutic Application, 2025 and 2035
- Figure 17: Global Market Y-o-Y Growth Comparison by Therapeutic Application, 2025 to 2035
- Figure 18: Global Market Attractiveness Analysis by Therapeutic Application
- Figure 19: Global Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 20: Global Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 21: Global Market Attractiveness Analysis by End User
- Figure 22: Global Market Value (USD Billion) Share and BPS Analysis by Region, 2025 and 2035
- Figure 23: Global Market Y-o-Y Growth Comparison by Region, 2025 to 2035
- Figure 24: Global Market Attractiveness Analysis by Region
- Figure 25: North America Market Incremental $ Opportunity, 2025 to 2035
- Figure 26: Latin America Market Incremental $ Opportunity, 2025 to 2035
- Figure 27: Western Europe Market Incremental $ Opportunity, 2025 to 2035
- Figure 28: Eastern Europe Market Incremental $ Opportunity, 2025 to 2035
- Figure 29: East Asia Market Incremental $ Opportunity, 2025 to 2035
- Figure 30: South Asia & Pacific Market Incremental $ Opportunity, 2025 to 2035
- Figure 31: Middle East & Africa Market Incremental $ Opportunity, 2025 to 2035
- Figure 32: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: North America Market Value Share and BPS Analysis by Service Type, 2025 and 2035
- Figure 34: North America Market Y-o-Y Growth Comparison by Service Type, 2025 to 2035
- Figure 35: North America Market Attractiveness Analysis by Service Type
- Figure 36: North America Market Value Share and BPS Analysis by Scale of Operation, 2025 and 2035
- Figure 37: North America Market Y-o-Y Growth Comparison by Scale of Operation, 2025 to 2035
- Figure 38: North America Market Attractiveness Analysis by Scale of Operation
- Figure 39: North America Market Value Share and BPS Analysis by Synthesis Method, 2025 and 2035
- Figure 40: North America Market Y-o-Y Growth Comparison by Synthesis Method, 2025 to 2035
- Figure 41: North America Market Attractiveness Analysis by Synthesis Method
- Figure 42: North America Market Value Share and BPS Analysis by Peptide Length, 2025 and 2035
- Figure 43: North America Market Y-o-Y Growth Comparison by Peptide Length, 2025 to 2035
- Figure 44: North America Market Attractiveness Analysis by Peptide Length
- Figure 45: North America Market Value Share and BPS Analysis by Therapeutic Application, 2025 and 2035
- Figure 46: North America Market Y-o-Y Growth Comparison by Therapeutic Application, 2025 to 2035
- Figure 47: North America Market Attractiveness Analysis by Therapeutic Application
- Figure 48: North America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 49: North America Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 50: North America Market Attractiveness Analysis by End User
- Figure 51: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 52: Latin America Market Value Share and BPS Analysis by Service Type, 2025 and 2035
- Figure 53: Latin America Market Y-o-Y Growth Comparison by Service Type, 2025 to 2035
- Figure 54: Latin America Market Attractiveness Analysis by Service Type
- Figure 55: Latin America Market Value Share and BPS Analysis by Scale of Operation, 2025 and 2035
- Figure 56: Latin America Market Y-o-Y Growth Comparison by Scale of Operation, 2025 to 2035
- Figure 57: Latin America Market Attractiveness Analysis by Scale of Operation
- Figure 58: Latin America Market Value Share and BPS Analysis by Synthesis Method, 2025 and 2035
- Figure 59: Latin America Market Y-o-Y Growth Comparison by Synthesis Method, 2025 to 2035
- Figure 60: Latin America Market Attractiveness Analysis by Synthesis Method
- Figure 61: Latin America Market Value Share and BPS Analysis by Peptide Length, 2025 and 2035
- Figure 62: Latin America Market Y-o-Y Growth Comparison by Peptide Length, 2025 to 2035
- Figure 63: Latin America Market Attractiveness Analysis by Peptide Length
- Figure 64: Latin America Market Value Share and BPS Analysis by Therapeutic Application, 2025 and 2035
- Figure 65: Latin America Market Y-o-Y Growth Comparison by Therapeutic Application, 2025 to 2035
- Figure 66: Latin America Market Attractiveness Analysis by Therapeutic Application
- Figure 67: Latin America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 68: Latin America Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 69: Latin America Market Attractiveness Analysis by End User
- Figure 70: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 71: Western Europe Market Value Share and BPS Analysis by Service Type, 2025 and 2035
- Figure 72: Western Europe Market Y-o-Y Growth Comparison by Service Type, 2025 to 2035
- Figure 73: Western Europe Market Attractiveness Analysis by Service Type
- Figure 74: Western Europe Market Value Share and BPS Analysis by Scale of Operation, 2025 and 2035
- Figure 75: Western Europe Market Y-o-Y Growth Comparison by Scale of Operation, 2025 to 2035
- Figure 76: Western Europe Market Attractiveness Analysis by Scale of Operation
- Figure 77: Western Europe Market Value Share and BPS Analysis by Synthesis Method, 2025 and 2035
- Figure 78: Western Europe Market Y-o-Y Growth Comparison by Synthesis Method, 2025 to 2035
- Figure 79: Western Europe Market Attractiveness Analysis by Synthesis Method
- Figure 80: Western Europe Market Value Share and BPS Analysis by Peptide Length, 2025 and 2035
- Figure 81: Western Europe Market Y-o-Y Growth Comparison by Peptide Length, 2025 to 2035
- Figure 82: Western Europe Market Attractiveness Analysis by Peptide Length
- Figure 83: Western Europe Market Value Share and BPS Analysis by Therapeutic Application, 2025 and 2035
- Figure 84: Western Europe Market Y-o-Y Growth Comparison by Therapeutic Application, 2025 to 2035
- Figure 85: Western Europe Market Attractiveness Analysis by Therapeutic Application
- Figure 86: Western Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 87: Western Europe Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 88: Western Europe Market Attractiveness Analysis by End User
- Figure 89: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 90: Eastern Europe Market Value Share and BPS Analysis by Service Type, 2025 and 2035
- Figure 91: Eastern Europe Market Y-o-Y Growth Comparison by Service Type, 2025 to 2035
- Figure 92: Eastern Europe Market Attractiveness Analysis by Service Type
- Figure 93: Eastern Europe Market Value Share and BPS Analysis by Scale of Operation, 2025 and 2035
- Figure 94: Eastern Europe Market Y-o-Y Growth Comparison by Scale of Operation, 2025 to 2035
- Figure 95: Eastern Europe Market Attractiveness Analysis by Scale of Operation
- Figure 96: Eastern Europe Market Value Share and BPS Analysis by Synthesis Method, 2025 and 2035
- Figure 97: Eastern Europe Market Y-o-Y Growth Comparison by Synthesis Method, 2025 to 2035
- Figure 98: Eastern Europe Market Attractiveness Analysis by Synthesis Method
- Figure 99: Eastern Europe Market Value Share and BPS Analysis by Peptide Length, 2025 and 2035
- Figure 100: Eastern Europe Market Y-o-Y Growth Comparison by Peptide Length, 2025 to 2035
- Figure 101: Eastern Europe Market Attractiveness Analysis by Peptide Length
- Figure 102: Eastern Europe Market Value Share and BPS Analysis by Therapeutic Application, 2025 and 2035
- Figure 103: Eastern Europe Market Y-o-Y Growth Comparison by Therapeutic Application, 2025 to 2035
- Figure 104: Eastern Europe Market Attractiveness Analysis by Therapeutic Application
- Figure 105: Eastern Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 106: Eastern Europe Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 107: Eastern Europe Market Attractiveness Analysis by End User
- Figure 108: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 109: East Asia Market Value Share and BPS Analysis by Service Type, 2025 and 2035
- Figure 110: East Asia Market Y-o-Y Growth Comparison by Service Type, 2025 to 2035
- Figure 111: East Asia Market Attractiveness Analysis by Service Type
- Figure 112: East Asia Market Value Share and BPS Analysis by Scale of Operation, 2025 and 2035
- Figure 113: East Asia Market Y-o-Y Growth Comparison by Scale of Operation, 2025 to 2035
- Figure 114: East Asia Market Attractiveness Analysis by Scale of Operation
- Figure 115: East Asia Market Value Share and BPS Analysis by Synthesis Method, 2025 and 2035
- Figure 116: East Asia Market Y-o-Y Growth Comparison by Synthesis Method, 2025 to 2035
- Figure 117: East Asia Market Attractiveness Analysis by Synthesis Method
- Figure 118: East Asia Market Value Share and BPS Analysis by Peptide Length, 2025 and 2035
- Figure 119: East Asia Market Y-o-Y Growth Comparison by Peptide Length, 2025 to 2035
- Figure 120: East Asia Market Attractiveness Analysis by Peptide Length
- Figure 121: East Asia Market Value Share and BPS Analysis by Therapeutic Application, 2025 and 2035
- Figure 122: East Asia Market Y-o-Y Growth Comparison by Therapeutic Application, 2025 to 2035
- Figure 123: East Asia Market Attractiveness Analysis by Therapeutic Application
- Figure 124: East Asia Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 125: East Asia Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 126: East Asia Market Attractiveness Analysis by End User
- Figure 127: South Asia & Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 128: South Asia & Pacific Market Value Share and BPS Analysis by Service Type, 2025 and 2035
- Figure 129: South Asia & Pacific Market Y-o-Y Growth Comparison by Service Type, 2025 to 2035
- Figure 130: South Asia & Pacific Market Attractiveness Analysis by Service Type
- Figure 131: South Asia & Pacific Market Value Share and BPS Analysis by Scale of Operation, 2025 and 2035
- Figure 132: South Asia & Pacific Market Y-o-Y Growth Comparison by Scale of Operation, 2025 to 2035
- Figure 133: South Asia & Pacific Market Attractiveness Analysis by Scale of Operation
- Figure 134: South Asia & Pacific Market Value Share and BPS Analysis by Synthesis Method, 2025 and 2035
- Figure 135: South Asia & Pacific Market Y-o-Y Growth Comparison by Synthesis Method, 2025 to 2035
- Figure 136: South Asia & Pacific Market Attractiveness Analysis by Synthesis Method
- Figure 137: South Asia & Pacific Market Value Share and BPS Analysis by Peptide Length, 2025 and 2035
- Figure 138: South Asia & Pacific Market Y-o-Y Growth Comparison by Peptide Length, 2025 to 2035
- Figure 139: South Asia & Pacific Market Attractiveness Analysis by Peptide Length
- Figure 140: South Asia & Pacific Market Value Share and BPS Analysis by Therapeutic Application, 2025 and 2035
- Figure 141: South Asia & Pacific Market Y-o-Y Growth Comparison by Therapeutic Application, 2025 to 2035
- Figure 142: South Asia & Pacific Market Attractiveness Analysis by Therapeutic Application
- Figure 143: South Asia & Pacific Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 144: South Asia & Pacific Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 145: South Asia & Pacific Market Attractiveness Analysis by End User
- Figure 146: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 147: Middle East & Africa Market Value Share and BPS Analysis by Service Type, 2025 and 2035
- Figure 148: Middle East & Africa Market Y-o-Y Growth Comparison by Service Type, 2025 to 2035
- Figure 149: Middle East & Africa Market Attractiveness Analysis by Service Type
- Figure 150: Middle East & Africa Market Value Share and BPS Analysis by Scale of Operation, 2025 and 2035
- Figure 151: Middle East & Africa Market Y-o-Y Growth Comparison by Scale of Operation, 2025 to 2035
- Figure 152: Middle East & Africa Market Attractiveness Analysis by Scale of Operation
- Figure 153: Middle East & Africa Market Value Share and BPS Analysis by Synthesis Method, 2025 and 2035
- Figure 154: Middle East & Africa Market Y-o-Y Growth Comparison by Synthesis Method, 2025 to 2035
- Figure 155: Middle East & Africa Market Attractiveness Analysis by Synthesis Method
- Figure 156: Middle East & Africa Market Value Share and BPS Analysis by Peptide Length, 2025 and 2035
- Figure 157: Middle East & Africa Market Y-o-Y Growth Comparison by Peptide Length, 2025 to 2035
- Figure 158: Middle East & Africa Market Attractiveness Analysis by Peptide Length
- Figure 159: Middle East & Africa Market Value Share and BPS Analysis by Therapeutic Application, 2025 and 2035
- Figure 160: Middle East & Africa Market Y-o-Y Growth Comparison by Therapeutic Application, 2025 to 2035
- Figure 161: Middle East & Africa Market Attractiveness Analysis by Therapeutic Application
- Figure 162: Middle East & Africa Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 163: Middle East & Africa Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 164: Middle East & Africa Market Attractiveness Analysis by End User
- Figure 165: Global Market - Tier Structure Analysis
- Figure 166: Global Market - Company Share Analysis
- FAQs -
How big is the Peptide Therapeutics CDMO Market in 2025?
The global Peptide Therapeutics CDMO Market is valued at USD 2.9 billion in 2025.
What will be the size of the Peptide Therapeutics CDMO Market in 2035?
The size of the Peptide Therapeutics CDMO Market is projected to reach USD 8.1 billion by 2035.
How much will the Peptide Therapeutics CDMO Market grow between 2025 and 2035?
The Peptide Therapeutics CDMO Market is expected to grow at a 10.8?GR between 2025 and 2035.
Who are the prominent players in the Peptide Therapeutics CDMO Market?
Prominent players in the market include Bachem AG, PolyPeptide Group, CPC Scientific Inc., Lonza Group Ltd., CordenPharma International, and GenScript Biotech Corporation.