Liquid Embolic Agent Market
Liquid Embolic Agent Market Size and Share Forecast Outlook 2025 to 2035
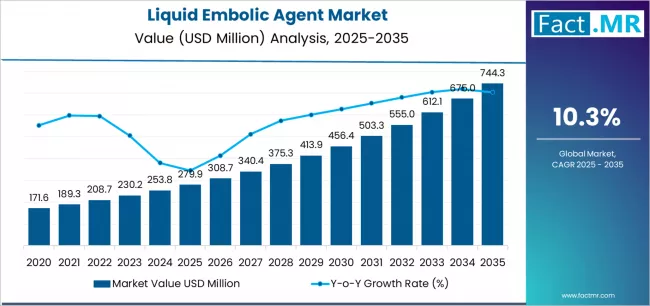
Liquid embolic agent market is projected to grow from USD 279.9 million in 2025 to USD 744.3 million by 2035, at a CAGR of 10.3%. EVOH will dominate with a 51.2% market share, while avm will lead the application segment with a 46.7% share.
Liquid Embolic Agent Market Forecast and Outlook 2025 to 2035
The global liquid embolic agent market is projected to reach USD 744.3 million by 2035, recording an absolute increase of USD 464.4 million over the forecast period. The market is valued at USD 279.9 million in 2025 and is set to rise at a CAGR of 10.3% during the assessment period.
The overall market size is expected to grow by approximately 2.7 times during the same period, supported by increasing prevalence of neurovascular disorders and expanding minimally invasive treatment adoption across interventional radiology and neurosurgery disciplines worldwide, driving demand for advanced embolization materials and increasing investments in next-generation polymer formulations with enhanced visibility and controlled delivery capabilities across arteriovenous malformation, tumor embolization, and hemorrhage management applications globally.
Quick Stats for Liquid Embolic Agent Market
- Liquid Embolic Agent Market Value (2025): USD 279.9 million
- Liquid Embolic Agent Market Forecast Value (2035): USD 744.3 million
- Liquid Embolic Agent Market Forecast CAGR: 10.3%
- Leading Product Type in Liquid Embolic Agent Market: EVOH (51.2%)
- Key Growth Regions in Liquid Embolic Agent Market: Asia Pacific, North America, and Europe
- Top Players in Liquid Embolic Agent Market: Medtronic, Johnson & Johnson (CERENOVUS), B. Braun SE, Terumo, Boston Scientific, Meril, Gem srl, Balt, Sirtex / BlackSwan, INVAMED
Interventional physicians face mounting pressure to achieve complete vessel occlusion while minimizing procedure complexity and reducing radiation exposure, with modern liquid embolic agents providing documented clinical benefits including precise flow control, permanent occlusion, and reduced procedural time compared to conventional embolic materials alone.
Rising incidence of brain arteriovenous malformations and expanding catheter-based intervention capabilities enabling complex neurovascular treatments create substantial opportunities for medical device manufacturers and healthcare providers. However, high product costs and limited reimbursement coverage may pose obstacles to widespread adoption and market penetration.
The EVOH product type segment dominates market activity, driven by superior biocompatibility and non-adhesive properties delivering predictable embolization across complex neurovascular anatomy worldwide. Physicians increasingly recognize the clinical advantages of EVOH-based liquid embolic agents, with established products providing controlled injection characteristics and excellent radiopacity at competitive procedural costs through proven delivery systems.
The AVM application segment demonstrates the strongest market presence, supported by high clinical need for definitive treatment and established protocols for liquid embolic agent utilization in brain arteriovenous malformation management. Hospitals emerge as the dominant end-use sector, reflecting concentrated expertise for complex neurovascular procedures and comprehensive interventional infrastructure supporting advanced embolization treatments. India represents the fastest-growing market, driven by increasing neurovascular disease burden and expanding interventional radiology capacity supporting improved treatment access.
Regional dynamics show Asia Pacific maintaining strong growth momentum, supported by large patient populations and healthcare infrastructure investments driving interventional treatment adoption across developing economies. China demonstrates robust expansion potential driven by regulatory approvals for domestic products and expanding stroke center network requiring advanced embolization solutions, while USA emphasizes established clinical adoption and comprehensive insurance coverage.
Germany leads European consumption through research-driven innovation and advanced neurointerventional expertise, followed by UK supported by strong National Health Service protocols and specialized treatment centers.
The competitive landscape features moderate concentration with Medtronic maintaining market leadership position at 16.8% market share, while established players including Johnson & Johnson (CERENOVUS), B. Braun SE, Terumo, and Boston Scientific compete through comprehensive product portfolios and clinical evidence generation across diverse neurovascular applications.
Liquid Embolic Agent Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the liquid embolic agent market is projected to expand from USD 279.9 million to USD 411.9 million, resulting in a value increase of USD 132.0 million, which represents 28.4% of the total forecast growth for the period. This phase of development will be shaped by rising prevalence of cerebral arteriovenous malformations requiring definitive treatment, product innovation in biocompatible polymer formulations with enhanced radiopacity and flow characteristics, as well as expanding training programs for interventional radiologists and neurosurgeons in liquid embolic agent techniques. Companies are establishing competitive positions through investment in clinical trial programs, advanced delivery system development, and strategic partnerships across teaching hospitals, specialized neurovascular centers, and interventional radiology departments.
From 2029 to 2035, the market is forecast to grow from USD 411.9 million to USD 744.3 million, adding another USD 332.4 million, which constitutes 71.6% of the overall expansion. This period is expected to be characterized by the expansion of specialized applications, including peripheral vascular embolization for trauma hemorrhage and hypervascular tumor treatments in oncology settings tailored for specific anatomical requirements, strategic collaborations between medical device companies and academic medical centers, and an enhanced focus on combination therapies and adjunctive treatment protocols. The growing emphasis on outpatient endovascular procedures and rising adoption of liquid embolic agents in ambulatory surgery centers will drive demand for user-friendly formulations with simplified preparation and enhanced safety profiles across diverse clinical environments.
Liquid Embolic Agent Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 279.9 million |
| Market Forecast Value (2035) | USD 744.3 million |
| Forecast CAGR (2025-2035) | 10.3% |
Why is the Liquid Embolic Agent Market Growing?
The liquid embolic agent market grows by enabling interventional physicians and neurosurgeons to achieve definitive vascular occlusion and treat complex neurovascular pathologies while minimizing procedural invasiveness and improving patient outcomes without extensive surgical exposure and traditional treatment complications.
Physicians face mounting pressure to manage intricate arteriovenous malformations and achieve complete nidus penetration while reducing procedure duration, with modern liquid embolic agents typically providing essential performance characteristics including controlled flow dynamics, permanent vessel occlusion, and minimal inflammatory response compared to conventional embolic materials including coils and particles alone, making liquid embolics essential for comprehensive neurovascular intervention.
The interventional medicine field's need for versatile embolization materials and predictable occlusion outcomes creates demand for specialized liquid embolic products that can navigate tortuous anatomy, penetrate complex vascular networks, and provide durable treatment results without compromising safety profiles or procedural efficiency.
Clinical evidence and treatment guideline recommendations drive adoption in neurovascular environments, interventional radiology suites, and hybrid operating rooms, where treatment efficacy has direct impact on patient morbidity and long-term outcomes. The increasing incidence of brain arteriovenous malformations and cerebral aneurysms, affecting patient populations across neurology and neurosurgery disciplines, creates expanding treatment volumes requiring advanced embolization technologies.
Rising awareness about minimally invasive treatment options and catheter-based interventions enables informed treatment selection and adoption of liquid embolic approaches over traditional surgical resection. However, technical expertise requirements and learning curve considerations may limit adoption among less experienced operators or facilities with limited interventional neurovascular volumes.
Segmental Analysis
The market is segmented by product type, application, end-use, and region. By product type, the market is divided into EVOH, cyanoacrylates, N-BCA, N-HCA, and others. Based on application, the market is categorized into AVM, hypervascular tumors, peripheral vasculature hemorrhage, and others. By end-use, the market includes hospitals, ASCs, specialty clinics, and others. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Product Type, Which Segment Accounts for the Dominant Market Share?
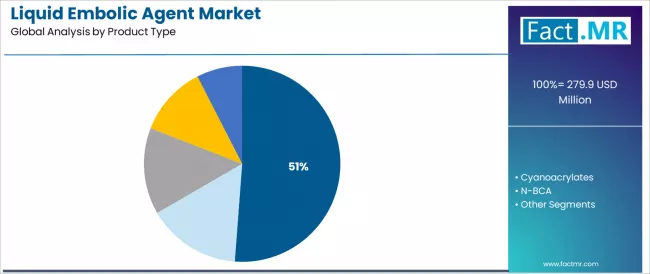
The EVOH product type segment represents the dominant force in the liquid embolic agent market, capturing 51.2% of the total market share in 2025. This established polymer category encompasses solutions featuring ethylene vinyl alcohol copolymer formulations with superior biocompatibility properties, including advanced tantalum powder integration and DMSO solvent systems that enable controlled precipitation and predictable occlusion across brain arteriovenous malformations and complex neurovascular lesions worldwide.
The EVOH segment's market leadership stems from its superior handling characteristics, with solutions capable of providing non-adhesive catheter delivery, cohesive mass formation, and permanent vessel occlusion while maintaining excellent radiopacity and controlled injection dynamics across diverse anatomical challenges.
The cyanoacrylates segment maintains a substantial market share at 34.6%, serving established clinical applications requiring rapid polymerization and immediate vessel occlusion across neurovascular and peripheral vascular embolization procedures. These solutions offer proven clinical efficacy for high-flow vascular malformations while providing sufficient performance justification through decades of clinical experience. The cyanoacrylates segment demonstrates steady presence, driven by cost considerations and physician familiarity with established products.
Within the product type category, N-BCA (N-butyl cyanoacrylate) demonstrates specialized adoption for specific neurovascular applications, while N-HCA (N-hexyl cyanoacrylate) serves applications requiring slower polymerization characteristics. Others category includes emerging polymer formulations and investigational agents.
Key clinical advantages driving the EVOH segment include:
- Non-adhesive properties with microcatheter compatibility enabling safe delivery and reduced risk of catheter entrapment during complex neurovascular procedures without premature polymerization concerns
- Controlled precipitation characteristics allowing precise flow modulation and predictable occlusion patterns through adjusted injection rates and technique modifications
- Superior biocompatibility providing minimal inflammatory response and excellent long-term safety profiles while maintaining permanent vessel occlusion without material degradation
- Enhanced radiopacity features ensuring optimal visualization under fluoroscopy enabling real-time monitoring and precise delivery control across intricate vascular anatomy
By Application, Which Segment Accounts for the Largest Market Share?
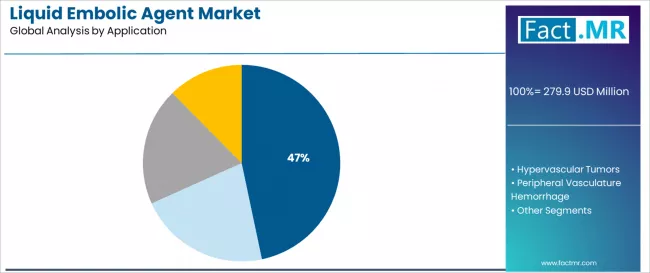
AVM dominates the liquid embolic agent application landscape with a 46.7% market share in 2025, reflecting the critical role of embolization in treating brain arteriovenous malformations and achieving nidus obliteration across neurovascular patient populations worldwide.
The AVM segment's market leadership is reinforced by established treatment protocols, comprehensive clinical evidence, and recognition as preferred therapy for specific AVM presentations requiring pre-surgical embolization or definitive endovascular treatment.
Within this segment, brain AVM represents the primary indication category, driven by high clinical need and well-established interventional approaches. This sub-segment benefits from specialized neurovascular expertise and concentrated treatment volumes at comprehensive stroke centers and academic medical institutions.
The hypervascular tumors segment represents an important application category at 29.8% market share with robust growth potential, demonstrating expansion through pre-surgical embolization for meningiomas, renal cell carcinomas, and other highly vascularized neoplasms. This segment benefits from oncology integration and multidisciplinary treatment planning requiring vascular intervention.
The peripheral vasculature hemorrhage segment maintains meaningful presence at 14.9% through trauma applications and emergency embolization for life-threatening bleeding, while others category addresses specialized indications including varicocele embolization and vascular malformation treatments.
Key market dynamics supporting application segment growth include:
- AVM application expansion driven by increasing diagnosis rates and comprehensive treatment guidelines, requiring specialized embolization materials and expert technique
- Hypervascular tumors category growth trends requiring pre-surgical devascularization and symptom management through selective embolization
- Integration of liquid embolic agents into multidisciplinary treatment protocols enabling comprehensive vascular intervention strategies
- Growing emphasis on trauma embolization and hemorrhage control driving emergency application adoption in acute care settings
By End-use, Which Segment Accounts for a Significant Market Share?
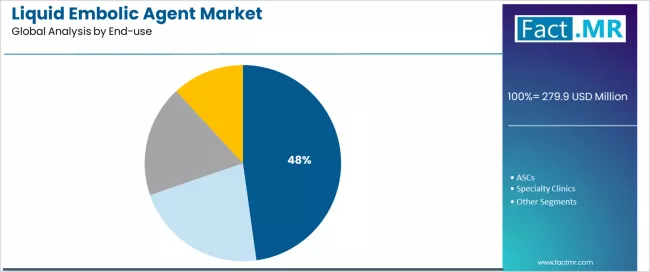
Hospitals represent the leading end-use segment in the liquid embolic agent market with a 47.8% market share in 2025, reflecting the concentrated expertise and comprehensive infrastructure required for complex neurovascular interventions and embolization procedures. The hospitals segment demonstrates consistent demand driven by the need for specialized interventional suites, advanced imaging capabilities, and multidisciplinary neurovascular teams supporting safe liquid embolic agent utilization.
The ASCs segment emerges as an important end-use category at 32.4% market share with substantial growth potential, driven by healthcare cost containment initiatives and expanding outpatient interventional capabilities for selected vascular procedures.
Ambulatory surgery centers pursuing peripheral vascular embolization and lower-complexity treatments require streamlined liquid embolic protocols and enhanced safety features supporting independent facility operations.
Within end-use applications, specialty clinics demonstrates focused adoption through dedicated interventional radiology practices and neurovascular treatment centers. Others category addresses research institutions, teaching hospitals, and hybrid care models supporting advanced interventional procedures.
Key end-use dynamics include:
- Hospitals requirements maintaining dominance through complex neurovascular cases with emphasis on comprehensive stroke center capabilities and 24/7 interventional availability
- ASCs applications showing growth through peripheral vascular procedures and selected neurovascular treatments supporting cost-effective outpatient models
- Specialty clinics emphasizing dedicated interventional expertise and focused procedural volumes addressing specific vascular pathologies
- Academic medical centers driving innovation and clinical research supporting evidence generation and technique refinement
What are the Drivers, Restraints, and Key Trends of the Liquid Embolic Agent Market?
The market is driven by three concrete demand factors tied to clinical treatment outcomes. First, increasing prevalence of neurovascular disorders including brain arteriovenous malformations and cerebral aneurysms creates expanding patient populations requiring definitive embolization treatments, with liquid embolic agents representing superior alternatives for complex anatomy and high-flow lesions in interventional settings, requiring widespread product availability. Second, growing adoption of minimally invasive neurovascular interventions drives systematic physician training and procedural volume growth, with liquid embolic agents demonstrating significant advantages in complete nidus penetration, reduced procedure time, and improved occlusion rates through advanced polymer formulations by 2030. Third, expanding interventional radiology and neurointerventional infrastructure in emerging markets enables increasing treatment access that improves patient outcomes while supporting healthcare system efficiency and cost-effectiveness objectives.
Market restraints include high product costs and procedural expenses that can challenge healthcare budgets and reimbursement adequacy, particularly in price-sensitive markets where alternative embolic materials provide functional alternatives and cost justification proves difficult for routine applications. Technical complexity and operator learning curves pose another significant obstacle, as liquid embolic agent utilization requires specialized training in microcatheter techniques and flow dynamics understanding, potentially affecting adoption rates among general interventionalists with limited neurovascular experience. Limited long-term outcome data for certain applications and anatomical locations create additional barriers for expanding clinical indications, demanding comprehensive registry studies and comparative effectiveness research supporting evidence-based utilization.
Key trends indicate accelerated next-generation product development in developed markets, particularly North America and Europe, where manufacturers demonstrate focus on ready-to-use formulations, enhanced radiopacity systems, and improved delivery devices offering reduced preparation time and enhanced procedural efficiency. Combination therapy trends toward liquid embolic agents with adjunctive coil embolization or balloon assistance enable complex treatment strategies addressing challenging vascular anatomy and achieving superior occlusion rates. However, the market thesis could face disruption if significant advances in alternative embolization technologies or major shifts in neurovascular treatment paradigms reduce reliance on traditional liquid embolic approaches.
Analysis of the Liquid Embolic Agent Market by Key Countries
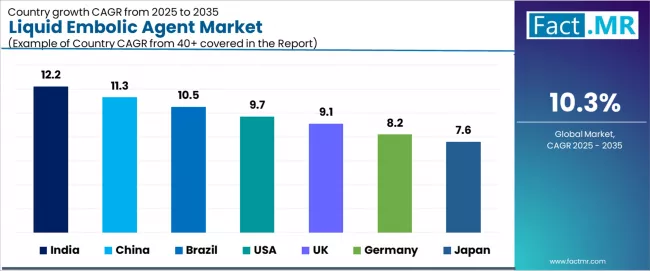
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 12.2% |
| China | 11.3% |
| Brazil | 10.5% |
| USA | 9.7% |
| UK | 9.1% |
| Germany | 8.2% |
| Japan | 7.6% |
The global liquid embolic agent market is expanding rapidly, with India leading at a 12.2% CAGR through 2035, driven by fastest adoption in emerging markets supporting expanding interventional radiology infrastructure and neurovascular treatment capabilities. China follows at 11.3%, supported by regulatory speed and local manufacturing enabling domestic product availability and cost-competitive treatment access. Brazil records 10.5%, reflecting fastest LATAM growth with expanding stroke center network and interventional expertise development.
USA advances at 9.7%, leveraging strong clinical adoption and comprehensive training programs supporting widespread physician expertise. UK posts 9.1%, focusing on strong neurointerventional demand and National Health Service treatment protocols, while Germany grows steadily at 8.2%, emphasizing research-driven but slower adoption patterns. Japan demonstrates 7.6% growth, anchored by aging population but slower diffusion of advanced embolization techniques.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the liquid embolic agent market with a CAGR of 12.2% through 2035. The country's leadership position stems from rapidly expanding interventional radiology capacity, increasing neurovascular disease awareness, and comprehensive stroke center development programs driving advanced embolization treatment adoption across metropolitan regions and tertiary care institutions.
Growth is concentrated in major medical hubs and teaching hospitals, including Delhi, Mumbai, Bangalore, and Chennai, where interventional physicians are increasingly utilizing liquid embolic agents for brain arteriovenous malformation treatment beyond traditional surgical approaches.
Clinical channels through comprehensive stroke centers, specialized neurovascular units, and interventional radiology departments expand liquid embolic agent utilization across complex vascular pathologies. The country's growing medical tourism sector and increasing insurance coverage provide strong momentum for advanced interventional treatments, including comprehensive adoption across corporate hospitals and specialty neuroscience centers seeking international quality standards.
Key market factors:
- Interventional expertise concentrated in metropolitan teaching hospitals with expanding neurovascular intervention capabilities
- Healthcare infrastructure investment through both public sector hospitals and private specialty institutions
- Comprehensive stroke management programs, including mechanical thrombectomy capabilities with proven interventional radiology expertise
- Domestic medical device manufacturing initiatives featuring partnerships with international companies offering cost-effective products
Why is China Emerging as a High-Growth Market?
In major metropolitan centers including Beijing, Shanghai, Guangzhou, and Chengdu, the utilization of liquid embolic agents is accelerating across comprehensive stroke centers, driven by regulatory approvals for domestic products and government initiatives promoting advanced neurovascular treatment capabilities. The market demonstrates strong growth momentum with a CAGR of 11.3% through 2035, linked to comprehensive healthcare system modernization and increasing emphasis on interventional treatment adoption in major teaching hospitals.
Chinese interventional physicians are implementing liquid embolic techniques integrated with multidisciplinary neurovascular teams to achieve definitive treatment outcomes while meeting growing patient expectations in stroke care quality. The country's expanding stroke center network and specialized neurointerventional training programs create ongoing demand for advanced embolization materials, while increasing emphasis on domestic product development drives competitive pricing and market accessibility.
Key development areas:
- Teaching hospitals and comprehensive stroke centers leading liquid embolic agent adoption with emphasis on complex neurovascular cases
- Regulatory environment enabling faster domestic product approvals and clinical trial facilitation
- Training program expansion through national societies and international collaborations building interventional expertise
- Growing preference for domestic manufacturers addressing cost requirements alongside imported premium products offering established clinical evidence
What drives the USA Market’s Clinical Leadership?
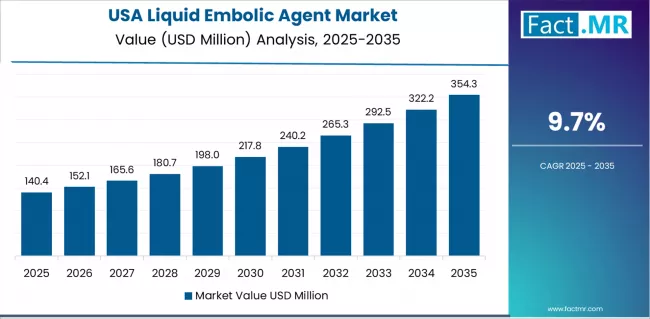
USA’s market expansion is driven by established clinical adoption patterns, including comprehensive physician training programs and extensive insurance coverage supporting liquid embolic agent utilization across diverse neurovascular applications. The country demonstrates steady growth potential with a CAGR of 9.7% through 2035, supported by robust clinical evidence generation and continuous product innovation from established medical device manufacturers.
American physicians face evolving treatment landscapes requiring updated techniques and emerging indications, necessitating ongoing education and protocol development. However, established comprehensive stroke center networks and fellowship-trained neurointerventional specialists create stable baseline demand for liquid embolic agents, particularly among facilities pursuing advanced certification where sophisticated embolization capabilities drive quality metrics and patient referral patterns.
Market characteristics:
- Academic medical centers and comprehensive stroke centers showing robust utilization with substantial procedural volumes
- Regional practice patterns varying between high-volume urban centers and expanding community hospital capabilities
- Future projections indicate continued application expansion with emphasis on peripheral vascular and oncologic indications
- Growing emphasis on standardized protocols and quality registries supporting outcome tracking and technique refinement
How does Germany Demonstrate Research Excellence Leadership?
The market in Germany leads in clinical research and evidence generation based on comprehensive academic programs and collaborative research networks advancing liquid embolic agent applications and technique optimization. The country shows strong potential with a CAGR of 8.2% through 2035, driven by university hospital leadership and advanced neurointerventional expertise in major medical centers across Bavaria, Baden-Württemberg, and North Rhine-Westphalia.
German physicians are adopting liquid embolic agents through rigorous clinical protocols and systematic outcome assessment for evidence-based practice, particularly in complex arteriovenous malformation cases and research-driven applications demanding comprehensive documentation. Clinical channels through university hospitals, specialized neurovascular centers, and research collaboratives expand utilization across carefully selected patient populations.
Leading market segments:
- University hospitals in major academic centers implementing comprehensive liquid embolic programs with research integration
- Clinical research networks achieving systematic outcome tracking and comparative effectiveness studies
- Strategic collaborations between medical device companies and academic institutions advancing product development and technique innovation
- Focus on long-term outcome studies and registry participation addressing evidence gaps and clinical guideline development
What Positions UK for National Health Service Integration?
In major neuroscience centers including London, Manchester, Birmingham, and Edinburgh, interventional physicians are implementing liquid embolic agents through comprehensive stroke networks and National Health Service treatment pathways, with documented adoption showing systematic utilization for appropriate arteriovenous malformation presentations and established clinical protocols. The market shows solid growth potential with a CAGR of 9.1% through 2035, linked to strong neurointerventional demand, specialized training programs, and comprehensive stroke care integration in regional neurovascular centers.
Physicians are adopting liquid embolic techniques with systematic case selection to optimize treatment outcomes while maintaining standards demanded by National Health Service clinical governance and cost-effectiveness requirements. The country's established neurovascular expertise and regional hub-and-spoke models create ongoing opportunities for liquid embolic utilization that support evidence-based practice and equitable treatment access.
Market development factors:
- Regional neuroscience centers leading liquid embolic adoption across UK through specialized neurointerventional services
- National Health Service procurement frameworks providing systematic product evaluation and cost-effectiveness assessment
- Strategic clinical networks enabling knowledge sharing and technique dissemination across regional centers
- Emphasis on outcome measurement and quality improvement supporting continuous practice refinement and guideline adherence
How does Japan Show Demographic-Driven Growth?
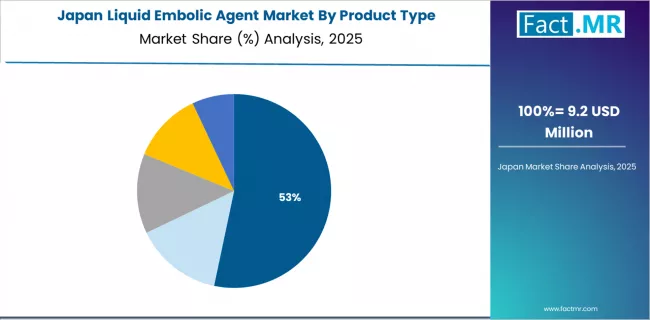
Japan's liquid embolic agent market demonstrates steady expansion focused on aging population needs and established interventional radiology infrastructure, with documented integration of embolization techniques achieving reliable treatment outcomes in neurovascular and peripheral vascular applications across comprehensive medical centers.
The country maintains solid growth momentum with a CAGR of 7.6% through 2035, driven by demographic trends and advanced healthcare system capabilities emphasizing quality-focused treatment methodologies that align with patient safety priorities applied to Japanese medical practice.
Major metropolitan regions, including Tokyo, Osaka, Nagoya, and Fukuoka, showcase sophisticated interventional capabilities where liquid embolic agents integrate systematically with multidisciplinary neurovascular programs and comprehensive stroke management protocols.
Key market characteristics:
- University hospitals and advanced treatment centers driving liquid embolic utilization with emphasis on technical excellence
- Aging population demographics creating increasing neurovascular disease burden requiring interventional management
- Quality-focused medical culture supporting meticulous technique and comprehensive outcome documentation
- Conservative adoption patterns emphasizing proven technologies and extensive clinical evidence before widespread implementation
What Characterizes Brazil's Emerging Market Development?
In major metropolitan centers including São Paulo, Rio de Janeiro, Brasília, and Belo Horizonte, the adoption of liquid embolic agents serves expanding neurovascular intervention capabilities and comprehensive stroke center development, driven by healthcare system modernization and increasing access to advanced interventional treatments. The market demonstrates robust growth potential with a CAGR of 10.5% through 2035, linked to fastest LATAM growth trajectory and increasing physician training in interventional techniques supporting treatment availability.
Brazilian interventional physicians are implementing liquid embolic approaches capable of addressing complex vascular pathologies to improve patient outcomes while meeting expectations in tertiary care environments. The country's expanding private healthcare sector and medical tourism growth create ongoing demand for advanced neurovascular treatments, while increasing emphasis on stroke care quality drives adoption of comprehensive interventional capabilities.
Key development areas:
- Private hospitals and specialized neuroscience centers leading liquid embolic agent adoption with emphasis on comprehensive neurovascular services
- Physician training programs including international fellowships and visiting professor programs building interventional expertise
- Healthcare infrastructure investment supporting catheterization laboratory development and imaging technology availability
- Integration of international clinical protocols and treatment guidelines supporting evidence-based practice adoption
Europe Market Split by Country
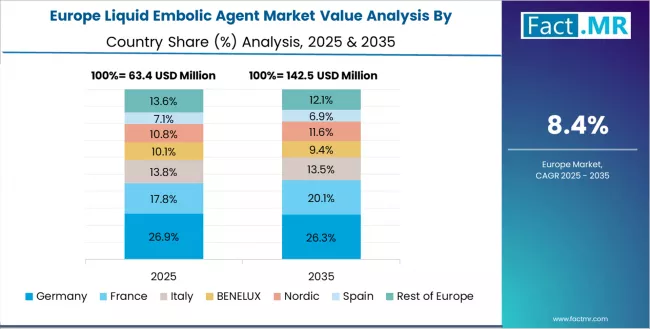
The liquid embolic agent market in Europe is projected to grow from USD 74.8 million in 2025 to USD 168.3 million by 2035, registering a CAGR of 8.4% over the forecast period. Germany is expected to maintain its leadership position with a 28.9% market share in 2025, adjusting to 27.6% by 2035, supported by its extensive neurovascular research programs, advanced interventional radiology infrastructure, and comprehensive university hospital networks serving major European patient populations.
France follows with a 22.1% share in 2025, projected to reach 22.8% by 2035, driven by comprehensive stroke center development and established neurointerventional expertise in major academic hospitals implementing advanced embolization protocols. UK holds a 19.7% share in 2025, expected to reach 20.3% by 2035 through National Health Service integration and regional neuroscience center expansion.
Italy commands a 14.2% share, while Spain accounts for 9.8% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 5.3% to 6.7% by 2035, attributed to increasing liquid embolic agent adoption in Nordic comprehensive stroke centers and emerging Eastern European neurovascular programs implementing modern interventional practices.
Competitive Landscape of the Liquid Embolic Agent Market
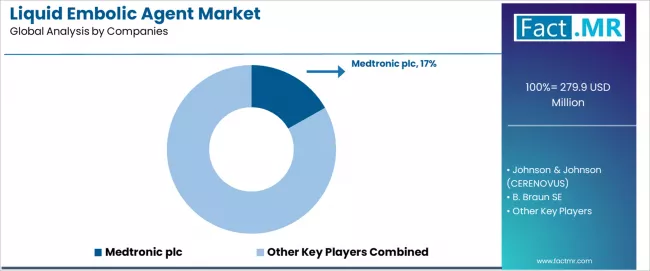
The liquid embolic agent market features approximately 10-15 meaningful players with moderate concentration, where the top three companies control roughly 40-50% of global market share through established product portfolios, comprehensive clinical evidence, and strong physician relationships. Competition centers on clinical performance, ease of use, and safety profiles rather than pricing competition alone.
Market leaders include Medtronic plc., maintaining a 16.8% market share through its Onyx liquid embolic system, Johnson & Johnson (CERENOVUS), and B. Braun SE, which maintain competitive advantages through proven clinical track records, extensive physician training programs, and comprehensive technical support networks, creating strong brand loyalty among neurointerventional specialists seeking reliable embolization solutions.
These companies leverage global commercial infrastructure and ongoing clinical research investments to defend market positions while developing next-generation formulations with enhanced handling characteristics and improved delivery systems.
Challengers encompass established medical device companies including Terumo, Boston Scientific, and specialized neurovascular players including Balt and Gem srl, which compete through innovative product designs and regional market expertise supporting specific clinical applications.
Emerging manufacturers, including Meril, Sirtex / BlackSwan, and INVAMED, focus on developing markets or novel formulations, offering differentiated capabilities in cost-competitive products, ready-to-use systems, and alternative polymer chemistries.
Emerging regional manufacturers and technology innovators create competitive pressure through simplified preparation protocols and value-based positioning, particularly in regions including Asia Pacific and Latin America, where price sensitivity and local manufacturing provide market access advantages.
Market dynamics favor companies that combine clinical evidence with comprehensive physician education that addresses diverse customer requirements from academic neurovascular centers through community-based interventional practices.
Strategic emphasis on ease-of-use features, enhanced visualization technologies, and safety innovations enables differentiation in increasingly outcomes-focused and evidence-driven liquid embolic agent market segments across neurovascular, oncologic, and trauma applications.
Global Liquid Embolic Agent Market - Stakeholder Contribution Framework
Liquid embolic agents represent a critical interventional technology that enables physicians and neurosurgeons to achieve definitive vascular occlusion and treat complex neurovascular pathologies while minimizing procedural invasiveness and improving patient outcomes without extensive surgical exposure and traditional treatment complications, typically providing essential performance characteristics including controlled flow dynamics, permanent vessel occlusion, and minimal inflammatory response compared to conventional embolic materials alone while ensuring improved treatment efficacy and comprehensive patient safety outcomes.
With the market projected to grow from USD 279.9 million in 2025 to USD 744.3 million by 2035 at a 10.3% CAGR, these products offer compelling advantages for AVM applications, EVOH formulations, and diverse neurovascular conditions seeking definitive embolization. Scaling market adoption and clinical utilization requires coordinated action across clinical evidence generation, physician training programs, medical device manufacturers, healthcare providers, and reimbursement systems.
How Could Governments Spur Local Development and Adoption?
- Stroke Care Programs: Include comprehensive embolization capabilities in stroke center certification requirements, providing clear treatment protocols and quality metrics supporting advanced interventional services and supporting training programs through fellowship funding and educational grants.
- Reimbursement & Coverage Policy: Establish adequate procedural reimbursement for liquid embolic agent utilization, provide coverage determinations for appropriate clinical indications and evidence-based applications, and eliminate administrative barriers that impede treatment access.
- Regulatory Framework Development: Create streamlined approval pathways for liquid embolic products across neurovascular applications, establish clear safety standards and clinical evidence requirements for market authorization, and develop international harmonization protocols that facilitate cross-border product availability.
- Healthcare Infrastructure Investment: Fund interventional suite development and advanced imaging capabilities, invest in comprehensive stroke center networks providing 24/7 neurovascular coverage, and establish regional hub models ensuring treatment access across geographic regions.
- Medical Education Support: Establish fellowship programs for neurointerventional training incorporating liquid embolic techniques, support continuing medical education initiatives for practicing interventionalists, and create simulation facilities enabling safe technique development and competency assessment.
How Could Industry Bodies Support Market Development?
- Clinical Guidelines & Standards: Define evidence-based recommendations for liquid embolic agent utilization across clinical indications, establish quality metrics and outcome benchmarks for embolization procedures, and create training curricula that physician programs can implement.
- Market Education & Awareness: Lead initiatives demonstrating liquid embolic advantages, emphasizing clinical outcomes, safety profiles, and appropriate patient selection compared to alternative embolization approaches.
- Safety & Quality Programs: Develop frameworks for adverse event reporting, complication tracking, and quality improvement initiatives, ensuring patient safety across clinical adoption and procedural implementation.
- Professional Certification: Run credentialing programs for interventional physicians on liquid embolic techniques, safety protocols, and complication management approaches in diverse clinical scenarios and anatomical locations.
How Could Medical Device Manufacturers Strengthen the Ecosystem?
- Advanced Product Development: Develop next-generation liquid embolic formulations with enhanced radiopacity, improved flow control, and ready-to-use preparations that reduce procedural complexity while improving safety profiles and clinical outcomes.
- Clinical Evidence Generation: Provide comprehensive clinical trial data, registry studies, and real-world evidence supporting appropriate utilization, comparative effectiveness, and long-term outcome validation across diverse patient populations.
- Physician Education Programs: Offer comprehensive training curricula, hands-on workshops, and proctoring services that support physician competency development and safe clinical implementation.
- Research & Development Networks: Build collaborative research programs, investigator-initiated study support, and innovation partnerships that advance liquid embolic technology and enable new clinical applications.
How Could Healthcare Providers and Physicians Navigate the Market?
- Clinical Protocol Development: Establish evidence-based treatment algorithms incorporating liquid embolic agents through multidisciplinary consensus, with particular focus on appropriate patient selection, technique standardization, and outcome measurement for quality improvement.
- Competency Assessment Programs: Implement systematic physician training requirements addressing minimum case volumes, supervised experience, and ongoing proficiency maintenance through optimized credentialing and privileging frameworks.
- Multidisciplinary Care Models: Develop integrated neurovascular teams combining interventional radiologists, neurosurgeons, and neurologists providing comprehensive treatment planning and coordinated patient management.
- Outcome Registry Participation: Establish systematic data collection addressing procedural success rates, complication tracking, and long-term clinical outcomes supporting continuous quality improvement and evidence generation.
How Could Investors and Financial Enablers Unlock Value?
- Innovation Investment: Provide growth capital for established manufacturers like Medtronic, Johnson & Johnson, and emerging companies to fund next-generation product development and clinical trial programs supporting market expansion.
- Technology Development Financing: Back companies developing novel polymer formulations, enhanced delivery systems, and complementary technologies that improve procedural efficiency and clinical outcomes.
- Market Access Funding: Finance commercial infrastructure expansion for medical device companies establishing operations in high-growth regions including China and India, supporting physician training programs and clinical evidence generation.
- Clinical Research Support: Enable investigator-initiated studies, registry development, and comparative effectiveness research providing evidence base supporting appropriate utilization and reimbursement optimization.
Key Players in the Liquid Embolic Agent Market
- Medtronic plc
- Johnson & Johnson (CERENOVUS)
- B. Braun SE
- Terumo Corporation
- Boston Scientific Corporation
- Meril Life Sciences Pvt. Ltd.
- GEM Srl
- Balt Group
- Sirtex Medical (BlackSwan Vascular, Inc.)
- INVAMED Research & Development Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 279.9 Million |
| Product Type | EVOH, Cyanoacrylates, N-BCA, N-HCA, Others |
| Application | AVM, Hypervascular Tumors, Peripheral Vasculature Hemorrhage, Others |
| End-use | Hospitals, ASCs, Specialty Clinics, Others |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | India, China, Brazil, USA, UK, Germany, Japan, and 40+ countries |
| Key Companies Profiled | Medtronic, Johnson & Johnson (CERENOVUS), B. Braun SE, Terumo, Boston Scientific, Meril, Gem srl, Balt, Sirtex / BlackSwan, INVAMED |
| Additional Attributes | Dollar sales by product type and application categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with medical device manufacturers and specialty companies, product specifications and clinical performance requirements, integration with interventional procedures and neurovascular treatment protocols, innovations in polymer formulations and delivery systems, and development of specialized applications with enhanced safety profiles and procedural efficiency capabilities. |
Liquid Embolic Agent Market by Segments
-
Product Type :
- EVOH
- Cyanoacrylates
- N-BCA
- N-HCA
- Others
-
Application :
- AVM
- Hypervascular Tumors
- Peripheral Vasculature Hemorrhage
- Others
-
End-use :
- Hospitals
- ASCs
- Specialty Clinics
- Others
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- USA Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- USA Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- USA Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product Type, 2025 to 2035
- EVOH
- Cyanoacrylates
- N-BCA
- N-HCA
- Others
- Y to o to Y Growth Trend Analysis By Product Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Product Type, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- AVM
- Hypervascular Tumors
- Peripheral Vasculature Hemorrhage
- Others
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End-use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End-use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End-use, 2025 to 2035
- Hospitals
- ASCs
- Specialty Clinics
- Others
- Y to o to Y Growth Trend Analysis By End-use, 2020 to 2024
- Absolute $ Opportunity Analysis By End-use, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- USA
- Market Attractiveness Analysis By Region
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- By Product Type
- By Application
- By End-use
- Market Attractiveness Analysis
- By Country
- By Product Type
- By Application
- By End-use
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product Type
- By Application
- By End-use
- Competition Analysis
- Competition Deep Dive
- Medtronic plc
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Johnson & Johnson (CERENOVUS)
- B. Braun SE
- Terumo Corporation
- Boston Scientific Corporation
- Meril Life Sciences Pvt. Ltd.
- GEM Srl
- Balt Group
- Sirtex Medical (BlackSwan Vascular, Inc.)
- INVAMED Research & Development Inc.
- Medtronic plc
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: USA Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 3: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: USA Market Value (USD Million) Forecast by End-use, 2020 to 2035
- Table 5: USA Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: USA Market Value (USD Million) Forecast by Product Type, 2020 to 2035
- Table 7: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 8: USA Market Value (USD Million) Forecast by End-use, 2020 to 2035
List Of Figures
- Figure 1: USA Market Pricing Analysis
- Figure 2: USA Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: USA Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 4: USA Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 5: USA Market Attractiveness Analysis by Product Type
- Figure 6: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: USA Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 8: USA Market Attractiveness Analysis by Application
- Figure 9: USA Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 10: USA Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 11: USA Market Attractiveness Analysis by End-use
- Figure 12: USA Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: USA Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: USA Market Attractiveness Analysis by Region
- Figure 15: USA Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: USA Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 17: USA Market Value Share and BPS Analysis by Product Type, 2025 and 2035
- Figure 18: USA Market Y to o to Y Growth Comparison by Product Type, 2025 to 2035
- Figure 19: USA Market Attractiveness Analysis by Product Type
- Figure 20: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 21: USA Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 22: USA Market Attractiveness Analysis by Application
- Figure 23: USA Market Value Share and BPS Analysis by End-use, 2025 and 2035
- Figure 24: USA Market Y to o to Y Growth Comparison by End-use, 2025 to 2035
- Figure 25: USA Market Attractiveness Analysis by End-use
- Figure 26: USA Market - Tier Structure Analysis
- Figure 27: USA Market - Company Share Analysis
- FAQs -
How big is the liquid embolic agent market in 2025?
The global liquid embolic agent market is estimated to be valued at USD 279.9 million in 2025.
What will be the size of liquid embolic agent market in 2035?
The market size for the liquid embolic agent market is projected to reach USD 744.3 million by 2035.
How much will be the liquid embolic agent market growth between 2025 and 2035?
The liquid embolic agent market is expected to grow at a 10.3% CAGR between 2025 and 2035.
What are the key product types in the liquid embolic agent market?
The key product types in liquid embolic agent market are evoh, cyanoacrylates, n-bca, n-hca and others.
Which application segment to contribute significant share in the liquid embolic agent market in 2025?
In terms of application, avm segment to command 46.7% share in the liquid embolic agent market in 2025.