Live Biotherapeutic Products & Microbiome CDMO Market
Live Biotherapeutic Products & Microbiome CDMO Market Size and Share Forecast Outlook 2025 to 2035
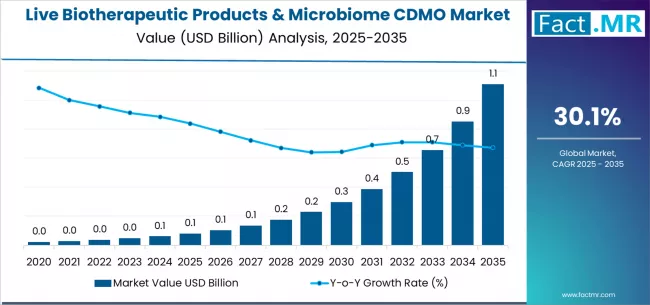
Live biotherapeutic products & microbiome CDMO market is projected to grow from USD 0.1 billion in 2025 to USD 1.1 billion by 2035, at a CAGR of 30.1%. C. Difficile will dominate with a 87.0% market share, while anaerobic lbp manufacturing will lead the manufacturing capability segment with a 42.5% share.
Live Biotherapeutic Products & Microbiome CDMO Market Forecast and Outlook 2025 to 2035
The global live biotherapeutic products & microbiome CDMO market is projected to grow from USD 0.08 billion in 2025 to approximately USD 1.11 billion by 2035, expanding at a compound annual growth rate (CAGR) of 30.1% between 2025 and 2035.
Quick Stats on Live Biotherapeutic Products & Microbiome CDMO Market
- Live Biotherapeutic Products & Microbiome CDMO Market Value (2025): USD 0.08 billion
- Live Biotherapeutic Products & Microbiome CDMO Market Forecast Value (2035): USD 1.11 billion
- Live Biotherapeutic Products & Microbiome CDMO Market Forecast CAGR (2025 to 2035): 30.1%
- Leading Application in Live Biotherapeutic Products & Microbiome CDMO Market: C. Difficile (87.0%)
- Leading Region in Live Biotherapeutic Products & Microbiome CDMO Market: North America (76.9%)
- Leading Manufacturing Capability in Live Biotherapeutic Products & Microbiome CDMO Market: Anaerobic LBP Manufacturing (42.5%)
- Key Growth Regions in Live Biotherapeutic Products & Microbiome CDMO Market: North America, Europe, and Asia Pacific
- Key Players in Live Biotherapeutic Products & Microbiome CDMO Market: Lonza, Arrant Bio, 4D Pharma, Cerbios, Biose Industrie, Assembly Biosciences Inc., Wacker Chemie AG, Quay Pharmaceuticals, NIZO, Inpac Probiotics
The market is positioned for extraordinary expansion, driven by accelerating live biotherapeutic product development, expanding microbiome therapeutics pipeline, and increasing demand for specialized anaerobic manufacturing services across pharmaceutical companies, biotechnology organizations, and clinical-stage microbiome developers globally.
The market demonstrates exceptional fundamentals supported by growing deployment of GMP-compliant anaerobic manufacturing systems, pharmaceutical sponsors' focus on outsourced development optimization and rising recognition of specialized CDMO capabilities as critical enablers in achieving successful clinical translation, regulatory compliance, and commercial manufacturing readiness within complex live biotherapeutic architectures across diverse therapeutic applications.
Market growth is underpinned by technological innovations in anaerobic bioprocessing, particularly oxygen-free fermentation systems and specialized fill-finish technologies, which offer enhanced microbial viability preservation, stringent quality control capabilities, and superior product stability compared to conventional biomanufacturing platforms prevalent in traditional biologics production.
Pharmaceutical organizations and microbiome therapeutic developers increasingly prioritize CDMO partners that deliver optimal balance between anaerobic manufacturing expertise, regulatory compliance capabilities, and development timeline efficiency while adhering to increasingly sophisticated live biotherapeutic product requirements and specialized handling protocols across global pharmaceutical outsourcing markets.
The convergence of C. difficile infection therapeutic development in North American pharmaceutical pipelines, inflammatory bowel disease program expansion in clinical-stage biotechnology, and metabolic disease microbiome intervention exploration in precision medicine corridors creates multifaceted growth opportunities for specialized CDMO service providers and live biotherapeutic manufacturing experts.
Advanced live biotherapeutic manufacturing technologies incorporating automated anaerobic handling systems, real-time viability monitoring platforms, and validated stability preservation protocols are improving product quality, manufacturing consistency, and regulatory success rates across microbiome therapeutic development.
The market's trajectory reflects the pharmaceutical industry's recognition of outsourcing imperative for highly specialized anaerobic manufacturing, the expanding clinical pipeline of live biotherapeutic candidates requiring commercial-scale manufacturing, and the growing regulatory clarity enabling advanced development stage progression.
Recurrent C. difficile infection treatment programs and microbiome modulation therapy networks are establishing comprehensive frameworks for therapeutic validation, while specialized CDMO infrastructure development enables pharmaceutical companies to access essential manufacturing capabilities without substantial capital investment across global live biotherapeutic product development operations.
Between 2020 and 2025, the live biotherapeutic products & microbiome CDMO market expanded from USD 0.02 billion to USD 0.08 billion, demonstrating explosive foundational growth driven by pioneering clinical trial initiations, breakthrough regulatory guidance publications, and initial establishment of specialized GMP anaerobic manufacturing infrastructure supporting emerging microbiome therapeutics sector. This growth phase validated live biotherapeutic development paradigm, established specialized CDMO service offerings, and created foundational manufacturing networks supporting nascent pharmaceutical microbiome programs.
From 2025 to 2030, the market is projected to experience extraordinary acceleration, reaching USD 0.4 billion, representing a pivotal inflection point as multiple live biotherapeutic candidates advance through late-stage clinical development and as pharmaceutical companies establish outsourcing partnerships for specialized manufacturing requirements. This period will be characterized by commercial-scale CDMO capacity expansion, enhanced regulatory pathway clarity, and widespread adoption of live biotherapeutic development programs across major pharmaceutical organizations addressing unmet medical needs.
From 2030 to 2035, the market is forecast to reach USD 1.11 billion, driven by anticipated commercial launches of breakthrough live biotherapeutic products, expansion beyond C. difficile into inflammatory and metabolic disease applications, and comprehensive pharmaceutical industry integration of microbiome therapeutics into development portfolios. The growing adoption of consortium-based live biotherapeutic products, personalized microbiome interventions, and next-generation anaerobic manufacturing platforms will drive demand for sophisticated CDMO services with proven regulatory track records and comprehensive manufacturing capability integration.
Live Biotherapeutic Products & Microbiome CDMO Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value (2025E) | USD 0.08 billion |
| Forecast Value (2035F) | USD 1.11 billion |
| Forecast CAGR (2025 to 2035) | 30.1% |
Why is the Live Biotherapeutic Products & Microbiome CDMO Market Growing?
Market expansion is being supported by the extraordinary surge in live biotherapeutic product development and the corresponding need for highly specialized anaerobic manufacturing services unavailable through conventional CDMO infrastructure. Modern pharmaceutical organizations recognize that live biotherapeutic products, containing living microorganisms as active pharmaceutical ingredients, require unprecedented manufacturing expertise encompassing strict anaerobic conditions, complex consortium formulations, and specialized stability protocols that conventional biologics CDMOs cannot provide.
The proven clinical efficacy of live biotherapeutic candidates in addressing recurrent C. difficile infection and emerging applications in inflammatory bowel disease makes specialized CDMO partnerships essential components of successful therapeutic development strategies. The growing recognition of microbiome therapeutics as breakthrough treatment modality and the practical impossibility of internal manufacturing capability development is driving pharmaceutical companies toward specialized CDMO partnerships that provide immediate access to validated anaerobic manufacturing infrastructure and regulatory expertise.
Pharmaceutical sponsors' requirement for partners with demonstrated live biotherapeutic manufacturing track records, established regulatory relationships, and proven product stability capabilities is creating opportunities for early-mover CDMOs establishing market-leading positions. The rising clarity of regulatory pathways through FDA guidance documents and successful regulatory submissions is accelerating development timelines and expanding addressable therapeutic applications beyond initial C. difficile focus.
Opportunity Pathways - Live Biotherapeutic Products & Microbiome CDMO Market
The live biotherapeutic products & microbiome CDMO market represents an unprecedented growth opportunity, expanding from USD 0.08 billion in 2025 to USD 1.11 billion by 2035 at a 30.1% CAGR. As pharmaceutical organizations recognize microbiome modulation potential and confront manufacturing complexity barriers, specialized CDMO services have evolved from experimental capability to essential pharmaceutical outsourcing requirement enabling oxygen-sensitive bacterial cultivation, multi-strain consortium manufacturing, and stability-challenged formulation development across recurrent C. difficile infection programs and expanding inflammatory disease applications.
The convergence of clinical validation acceleration, regulatory pathway maturation, specialized manufacturing capacity scarcity, and pharmaceutical portfolio diversification creates explosive demand momentum. Comprehensive anaerobic manufacturing platforms offering validated GMP infrastructure, established C. difficile application expertise serving dominant current demand, and emerging consortium manufacturing capabilities addressing next-generation products will capture market leadership, while strategic capacity expansion in cost-competitive Asian manufacturing regions and therapeutic application diversification will enable market participation. Pharmaceutical industry recognition of outsourcing necessity and breakthrough therapeutic potential provides unprecedented growth foundation.
- Pathway A - C. Difficile Application Dominance: Leading with 87.0% market share, recurrent C. difficile infection applications drive overwhelming demand through advanced clinical programs requiring specialized anaerobic manufacturing for spore-forming consortium products. Established CDMO expertise in Clostridioides difficile-targeted live biotherapeutics addressing substantial unmet medical need commands premium service pricing from pharmaceutical sponsors pursuing breakthrough therapy designations and accelerated regulatory pathways. Expected revenue pool: USD 67.9-961.5 million.
- Pathway B - North American Regional Leadership: Dominating with 76.9% market share through concentration of pharmaceutical sponsors, regulatory leadership, and pioneering clinical development, North American market serves primary demand while establishing global manufacturing standards. This regional concentration addresses both pharmaceutical industry presence and regulatory pathway advancement, making it the epicenter for CDMO service demand and therapeutic innovation. Opportunity: USD 60.1-850.0 million.
- Pathway C - Asian Manufacturing Emergence: China (44.9% CAGR) and India (42.7% CAGR) lead global growth through cost-competitive manufacturing infrastructure development, expanding pharmaceutical outsourcing relationships, and government biopharmaceutical capability investments. Strategic CDMO capacity establishment in Asian markets, leveraging cost advantages and technical expertise, enables market entry supporting pharmaceutical cost optimization and capacity diversification. Geographic expansion upside: USD 23.4-331.6 million.
- Pathway D - Anaerobic LBP Manufacturing Capability: Anaerobic manufacturing with 42.5% capability share serves foundational requirement for live biotherapeutic production requiring strict oxygen-free conditions throughout cultivation and processing. Specialized anaerobic infrastructure representing substantial capital investment and technical expertise maintains essential capability moat from conventional CDMO competitors and pharmaceutical internal capabilities. Capability revenue potential: USD 33.2-469.7 million.
- Pathway E - Advanced Manufacturing Technology & Automation: Companies investing in sophisticated automated anaerobic handling systems, real-time viability monitoring platforms, and predictive stability modeling gain competitive advantages through superior product quality and manufacturing consistency. Advanced capabilities enabling complex consortium manufacturing and extended stability profiles capture premium positioning and regulatory confidence. Technology differentiation premium: USD 15.6-221.0 million.
- Pathway F - Regulatory Expertise & Submission Support: Specialized regulatory consulting, comprehensive CMC documentation support, and established FDA/EMA relationship management create competitive differentiation in highly regulated markets requiring unprecedented live biotherapeutic product submissions. Companies offering integrated regulatory services enabling successful IND filings and BLA approvals gain preferred partner status with pharmaceutical sponsors navigating novel regulatory territory. Regulatory service value: USD 10.9-154.7 million.
- Pathway G - Therapeutic Expansion & Novel Applications: Beyond recurrent C. difficile infection, live biotherapeutic applications in Crohn's disease, irritable bowel syndrome, metabolic diseases, and oncology represent emerging opportunities as clinical evidence expands. CDMO partners supporting innovative therapeutic development, establishing specialized manufacturing protocols, and enabling novel consortium formulations capture first-mover advantages in expanding applications. Therapeutic diversification opportunity: USD 9.4-132.6 million.
Segmental Analysis
The market is segmented by application, manufacturing capability, region, and service type. By application, the market is divided into C. difficile, Crohn's disease, irritable bowel syndrome (IBS), diabetes, and others.
Based on manufacturing capability, the market is categorized into anaerobic LBP manufacturing, fermentation & scale-up, fill-finish & stability, and analytical & QC services. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and mea.
How does C. Difficile Application Drive Market Dominance?
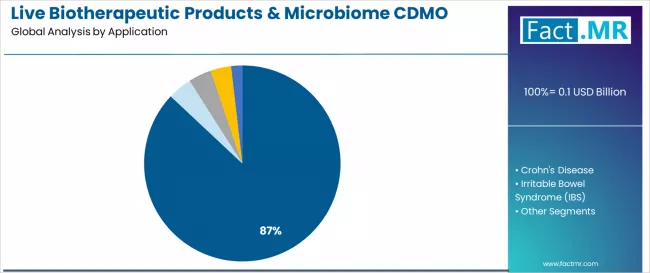
The C. difficile application is projected to account for 87.0% of the live biotherapeutic products & microbiome CDMO market in 2025, establishing overwhelming dominance as the therapeutic indication driving current manufacturing demand. Pharmaceutical companies recognize that recurrent C. difficile infection represents the most clinically validated and commercially attractive initial application for live biotherapeutic products, supported by compelling clinical trial data, substantial unmet medical need affecting hundreds of thousands of patients annually, and favorable regulatory reception including breakthrough therapy designations.
This application addresses both immediate commercial opportunity and technical feasibility as relatively well-characterized microbial consortia demonstrate reproducible manufacturing characteristics. This segment forms the foundation of essentially all current commercial-scale live biotherapeutic manufacturing activity, as multiple pharmaceutical sponsors including leading companies advance C. difficile-targeted products through late-stage clinical development toward anticipated regulatory approvals.
Clinical success rates and favorable benefit-risk profiles continue strengthening pharmaceutical investment confidence in C. difficile live biotherapeutics among both established pharmaceutical companies and specialized microbiome biotechnology organizations. With multiple products potentially reaching market approval within the forecast period and substantial patient populations representing multi-billion dollar market opportunities, C. difficile applications will maintain their central position driving specialized CDMO demand throughout the coming decade.
What drives Anaerobic LBP Manufacturing Capability Prominence?
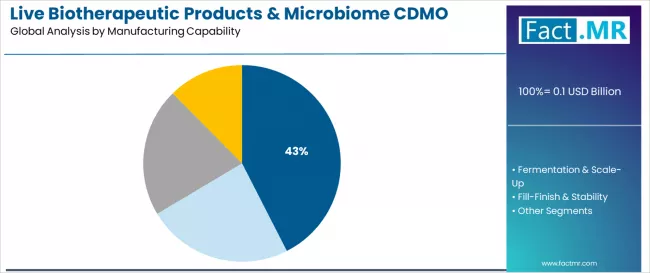
The anaerobic LBP manufacturing capability is projected to account for 42.5% of manufacturing service demand in 2025, establishing its position as the foundational and most technically challenging service requirement. CDMO organizations recognize that anaerobic cultivation infrastructure, encompassing oxygen-free fermentation systems, specialized anaerobic handling chambers, and validated environmental controls, represents the most capital-intensive and expertise-dependent capability distinguishing live biotherapeutic manufacturing from conventional biologics production.
This capability addresses the fundamental biological requirement that many therapeutically relevant gut microbiome bacteria are obligate anaerobes unable to survive oxygen exposure. The segment represents the primary barrier to entry for conventional CDMOs and the essential technical moat protecting specialized live biotherapeutic manufacturing providers from competitive encroachment.
Successful anaerobic manufacturing requires integrated expertise spanning microbiology, process engineering, and quality systems specifically adapted to oxygen-sensitive organisms. As live biotherapeutic development expands into increasingly complex multi-strain consortium products and therapeutic applications requiring diverse microbial species, anaerobic manufacturing expertise will maintain its essential role as the capability foundation enabling successful product development and commercial manufacturing within the global live biotherapeutic products ecosystem.
Why does North America Command Overwhelming Regional Share?
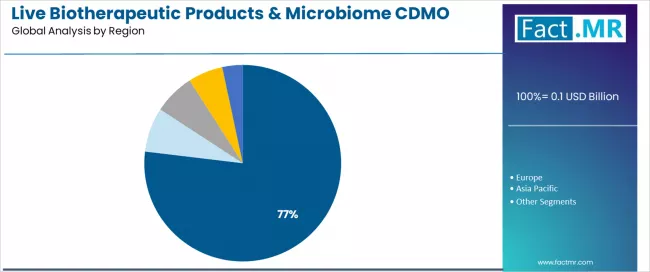
North America is projected to represent 76.9% of live biotherapeutic products & microbiome CDMO demand in 2025, demonstrating extraordinary regional concentration reflecting pharmaceutical industry geographic distribution and regulatory leadership.
The region's dominance stems from concentration of innovative pharmaceutical companies and biotechnology organizations developing live biotherapeutic products, FDA's pioneering regulatory framework development for this novel therapeutic class and established specialized CDMO infrastructure within proximity to pharmaceutical sponsors.
North American pharmaceutical sponsors conducting the vast majority of global live biotherapeutic clinical trials require regional manufacturing capacity supporting clinical supply and commercial preparation. The segment reflects the nascent nature of live biotherapeutic development concentrated in innovation-leading markets with regulatory sophistication and risk capital availability supporting breakthrough therapeutic modalities.
FDA guidance documents specifically addressing live biotherapeutic products and successful regulatory interactions establish North America as the primary development geography, while specialized CDMO capabilities emerged first in this region responding to pharmaceutical sponsor demand.
As the therapeutic class matures and global regulatory harmonization progresses, geographic diversification will gradually occur, but North American dominance will persist throughout the forecast period reflecting pharmaceutical industry concentration and regulatory leadership position.
What are the Drivers, Restraints, and Key Trends of the Live Biotherapeutic Products & Microbiome CDMO Market?
The live biotherapeutic products & microbiome CDMO market is experiencing unprecedented growth due to revolutionary recognition of microbiome therapeutic potential and pharmaceutical industry's confrontation with manufacturing complexity requiring specialized outsourcing.
However, the market faces challenges, including limited global manufacturing capacity creating potential supply bottlenecks for multiple concurrent programs, technical complexity of multi-strain consortium products exceeding current manufacturing capabilities, and regulatory pathway uncertainties for novel therapeutic mechanisms despite recent guidance progress. Continuous innovation in anaerobic bioprocessing technologies and evolving regulatory frameworks continues influencing market development and pharmaceutical investment patterns.
Clinical Validation Breakthrough and Regulatory Pathway Maturation
The accelerating clinical success of live biotherapeutic candidates in Phase 3 trials is validating therapeutic approach and driving pharmaceutical investment in specialized manufacturing infrastructure partnerships. Pharmaceutical sponsors achieving compelling clinical endpoints in recurrent C. difficile infection trials and receiving breakthrough therapy designations require immediate access to commercial-scale anaerobic manufacturing capabilities unavailable internally.
FDA's publication of comprehensive guidance documents specifically addressing live biotherapeutic products provides regulatory clarity enabling confident pharmaceutical investment and accelerated development timelines supporting market expansion.
Pharmaceutical Portfolio Diversification and Microbiome Therapeutic Integration
Major pharmaceutical organizations are systematically integrating microbiome therapeutics into development portfolios recognizing breakthrough potential across inflammatory diseases, metabolic disorders, and oncology applications. This strategic shift drives demand for specialized CDMO partnerships enabling pharmaceutical companies to access live biotherapeutic manufacturing expertise without prohibitive internal capability development investments.
Pharmaceutical recognition that microbiome modulation represents fundamentally new therapeutic modality with potential across multiple disease areas creates sustained demand growth as organizations establish platforms supporting multiple development programs simultaneously.
Analysis of the Live Biotherapeutic Products & Microbiome CDMO Market by Key Countries
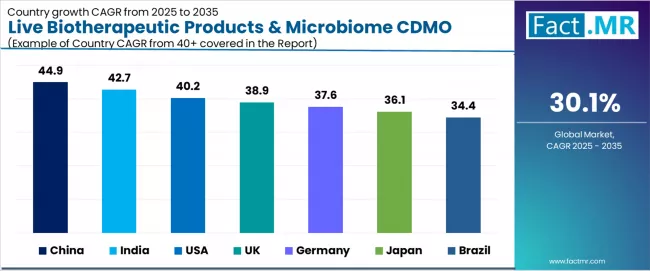
| Country | CAGR (2025-2035) |
|---|---|
| China | 44.9% |
| India | 42.7% |
| USA | 40.2% |
| UK | 38.9% |
| Germany | 37.6% |
| Japan | 36.1% |
| Brazil | 34.4% |
The live biotherapeutic products & microbiome CDMO market is experiencing extraordinary growth globally, with China leading at a 44.9% CAGR through 2035, driven by fastest Asia Pacific capacity scaling, high microbiome R&D funding, and strategic government support for advanced biomanufacturing capabilities.
India follows at 42.7%, supported by growing CDMO outsourcing demand, lower-cost biologics infrastructure advantages, and expanding pharmaceutical service expertise. The USA records 40.2% growth, benefiting from dominant position in early-stage live biotherapeutic trials and extensive GMP capacity expansions. UK demonstrates 38.9% growth, emphasizing leading academic microbiome programs and specialized CDMO development.
Germany shows 37.6% growth, representing large pharmaceutical R&D base and bioprocessing excellence. Japan records 36.1% growth with high volume of microbiome clinical trials and precision fermentation expertise, while Brazil shows 34.4% growth, representing growing investments in microbial therapeutics and regional CDMO infrastructure development.
How Does China Demonstrate Exceptional Market Potential with Rapid Capacity Scaling?
Revenue from live biotherapeutic products & microbiome CDMO services in China is projected to exhibit exceptional growth with a CAGR of 44.9% through 2035, driven by fastest Asia Pacific manufacturing capacity scaling and increasing recognition of cost-competitive anaerobic manufacturing capabilities attracting global pharmaceutical partnerships.
The country's rapidly developing biopharmaceutical CDMO sector and substantial government microbiome research funding are creating significant opportunities for specialized live biotherapeutic manufacturing infrastructure deployment serving both domestic pharmaceutical programs and international outsourcing demand.
International pharmaceutical sponsors and Chinese CDMO providers are establishing anaerobic manufacturing facilities to serve the emerging global demand for cost-effective live biotherapeutic production across clinical development and potential commercial manufacturing applications. The government's strategic emphasis on advanced biomanufacturing capability development and microbiome research prioritization is driving substantial investments in specialized infrastructure and technical expertise development.
This policy support, combined with the country's established CDMO industry leadership and cost-competitive manufacturing advantages, creates favorable environment for live biotherapeutic CDMO market development. Chinese CDMO organizations are increasingly investing in specialized anaerobic manufacturing infrastructure, with live biotherapeutic capabilities representing strategic differentiation in competitive global pharmaceutical outsourcing markets.
- Government biomanufacturing initiatives and microbiome research funding driving investments in specialized anaerobic manufacturing infrastructure
- CDMO industry expansion and pharmaceutical outsourcing growth supporting development of live biotherapeutic manufacturing capabilities nationwide
- Pharmaceutical partnerships and technology transfer agreements increasingly enabling Chinese CDMOs to access specialized live biotherapeutic manufacturing expertise
- Cost advantage positioning and capacity scaling capabilities accelerating Chinese CDMO adoption for live biotherapeutic manufacturing programs
What Makes India Demonstrate Market Leadership with CDMO Outsourcing Growth?
Revenue from live biotherapeutic products & microbiome CDMO services in India is expanding at a CAGR of 42.7%, supported by growing pharmaceutical outsourcing demand, lower-cost biologics infrastructure advantages, and expanding specialized manufacturing expertise. The country's established pharmaceutical services industry and increasing technical sophistication are driving adoption of specialized live biotherapeutic capabilities in both existing CDMO facilities and purpose-built anaerobic manufacturing centers.
International pharmaceutical companies and Indian CDMO providers are establishing partnerships to develop cost-competitive live biotherapeutic manufacturing capacity serving global pharmaceutical development programs. India's pharmaceutical CDMO sector continues to benefit from established international client relationships, cost-competitive operational advantages, and growing technical expertise supporting complex biologics manufacturing.
The country's focus on advanced biomanufacturing capability development is driving investments in specialized technologies including anaerobic fermentation systems and live biotherapeutic product handling infrastructure. This development enables Indian CDMOs to participate in emerging live biotherapeutic manufacturing markets while leveraging established cost advantages and pharmaceutical industry relationships.
- Pharmaceutical outsourcing relationships and cost advantages creating opportunities for Indian CDMO participation in live biotherapeutic manufacturing
- Technical capability development and specialized infrastructure investments supporting deployment of anaerobic manufacturing systems
- International partnerships and technology transfer enabling Indian CDMOs to acquire live biotherapeutic manufacturing expertise and regulatory knowledge
- CDMO industry expansion and government support accelerating specialized capability development and market participation
Why Does USA Maintain Early-Stage Development and Manufacturing Leadership?
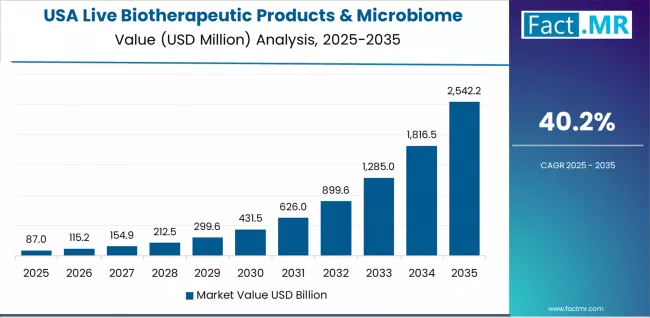
Revenue from live biotherapeutic products & microbiome CDMO services in USA is projected to exhibit extraordinary growth with a CAGR of 40.2% through 2035, driven by dominant position in early stage live biotherapeutic clinical trials and extensive GMP anaerobic manufacturing capacity expansions. The country's concentration of innovative pharmaceutical companies and biotechnology organizations developing live biotherapeutic products creates sustained demand for proximate specialized CDMO services supporting clinical development and commercial preparation.
American specialized CDMOs leverage pioneering anaerobic manufacturing expertise and established FDA regulatory relationships to serve pharmaceutical sponsors navigating unprecedented regulatory submissions. The USA market benefits from first-mover advantages in live biotherapeutic CDMO infrastructure development, comprehensive regulatory expertise specific to this novel therapeutic class, and proximity to pharmaceutical sponsor decision-makers prioritizing development speed and regulatory success over manufacturing cost considerations. This market positioning supports premium pricing for specialized services while establishing technical standards and regulatory precedents shaping global market development.
Strategic Market Considerations:
- Pharmaceutical biotechnology concentration and clinical trial dominance driving sustained demand for specialized anaerobic manufacturing services
- Regulatory expertise advantages and FDA relationship establishment supporting pharmaceutical sponsor preference for USA-based CDMO partnerships
- Technical innovation leadership and manufacturing capability advancement maintaining competitive positioning despite cost premium relative to emerging markets
- Capacity expansion investments and pharmaceutical partnership agreements accelerating market growth and service capability enhancement
How Does UK Maintain Academic Excellence and Specialized CDMO Development?
UK's advanced research ecosystem demonstrates sophisticated live biotherapeutic development activity supporting a 38.9% CAGR through 2035. The country's leading academic microbiome programs and established pharmaceutical industry presence are creating demand for specialized CDMO services supporting both academic research translation and commercial pharmaceutical development. UK-based specialized CDMOs and research institutions leverage microbiome research excellence to establish differentiated service offerings supporting novel therapeutic development programs.
The UK life sciences sector benefits from academic research leadership in microbiome science, government support for biotechnology innovation, and established pharmaceutical industry relationships supporting specialized manufacturing capability development. The market reflects integration of academic research excellence with commercial CDMO services enabling seamless research translation and pharmaceutical partnership development.
Strategic Market Considerations:
- Academic microbiome programs and research excellence creating pipeline of innovative live biotherapeutic candidates requiring specialized manufacturing
- Pharmaceutical industry presence and biotechnology ecosystem supporting demand for proximate specialized CDMO services
- Technical expertise development supported by research institution collaboration and government innovation funding
- Regulatory expertise and European market access positioning supporting pharmaceutical partnership development and service expansion
What drives Germany Market Growth with Pharmaceutical R&D Excellence?
Germany's established pharmaceutical market demonstrates strong live biotherapeutic CDMO adoption with a 37.6% CAGR through 2035, driven by large pharmaceutical R&D base, bioprocessing technology leadership, and comprehensive manufacturing quality standards.
The country's pharmaceutical industry concentration and precision engineering expertise are creating opportunities for specialized CDMO development supporting both domestic pharmaceutical programs and international client partnerships. German CDMOs leverage established bioprocessing excellence and quality system sophistication to develop differentiated live biotherapeutic manufacturing capabilities.
Market dynamics reflect integration of pharmaceutical industry demand with specialized CDMO capability development important to German manufacturing excellence and quality leadership reputation. Established bioprocessing infrastructure and technical expertise create foundation for live biotherapeutic manufacturing adoption and service development.
Strategic Market Considerations:
- Pharmaceutical R&D concentration and biotechnology presence driving demand for specialized live biotherapeutic manufacturing services
- Bioprocessing excellence and quality system sophistication supporting development of advanced anaerobic manufacturing capabilities
- Technical expertise and precision engineering capabilities enabling sophisticated live biotherapeutic product manufacturing and handling
- Regulatory compliance excellence and pharmaceutical industry relationships supporting CDMO partnership development and service expansion
How Does Japan Demonstrate Microbiome Clinical Research Strength?
Japan's sophisticated pharmaceutical ecosystem demonstrates substantial live biotherapeutic activity with a 36.1% CAGR through 2035 driven by high volume of microbiome clinical trials and precision fermentation expertise. The country's pharmaceutical industry investment in microbiome research and established bioprocessing capabilities are creating demand for specialized CDMO services supporting clinical development and potential commercial manufacturing. Japanese pharmaceutical companies and specialized CDMOs leverage fermentation technology excellence to develop live biotherapeutic manufacturing capabilities.
The Japanese market benefits from pharmaceutical industry microbiome research investment, precision fermentation technology leadership, and comprehensive quality management systems supporting specialized biomanufacturing capability development. This foundation enables participation in emerging live biotherapeutic manufacturing markets while maintaining quality standards and technical sophistication.
Strategic Market Considerations:
- Microbiome clinical trial activity and pharmaceutical research investment driving demand for specialized manufacturing services
- Fermentation technology expertise and bioprocessing capabilities supporting development of anaerobic manufacturing systems
- Quality management excellence and regulatory compliance supporting pharmaceutical partnership development and service adoption
- Pharmaceutical industry relationships and technical collaboration enabling CDMO capability development and market participation
Why does Brazil Show Emerging Microbial Therapeutics Investment?
Revenue from live biotherapeutic products & microbiome CDMO services in Brazil is projected to grow at a CAGR of 34.4% through 2035, driven by growing investments in microbial therapeutics and regional CDMO infrastructure development. The country's expanding pharmaceutical sector and increasing biotechnology investment are creating opportunities for specialized manufacturing capability development supporting both domestic therapeutic programs and potential regional service hub positioning. Brazilian pharmaceutical organizations and CDMO providers are exploring live biotherapeutic manufacturing capabilities to participate in emerging therapeutic markets.
The Brazilian market benefits from pharmaceutical industry development, government support for biotechnology innovation, and strategic positioning as potential Latin American manufacturing hub. This development creates emerging demand for specialized live biotherapeutic capabilities as pharmaceutical investment increases and therapeutic development programs advance.
Strategic Market Considerations:
- Pharmaceutical investment growth and biotechnology sector development creating emerging demand for specialized manufacturing services
- CDMO infrastructure expansion and capability development supporting potential live biotherapeutic manufacturing adoption
- Regional market positioning and Latin American hub potential supporting strategic infrastructure investment
- Government support programs and biotechnology funding beginning to influence capability development and market participation
Europe Market Split by Country
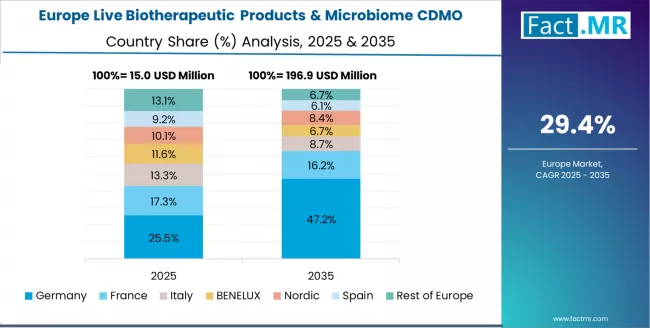
The live biotherapeutic products & microbiome CDMO market in Europe is projected to grow from USD 10.0 million in 2025 to USD 141.5 million by 2035, registering a CAGR of 30.2% over the forecast period. UK is expected to maintain its leadership position with a 34.2% market share in 2025, rising to 36.8% by 2035, supported by its leading academic microbiome research programs, specialized CDMO development, and pharmaceutical industry innovation capabilities throughout established life sciences operations.
Germany follows with a 28.6% share in 2025, projected to reach 29.4% by 2035, driven by pharmaceutical R&D excellence, bioprocessing technology leadership, and comprehensive manufacturing quality infrastructure serving European pharmaceutical markets. France holds a 16.8% share in 2025, expected to increase to 17.2% by 2035, supported by pharmaceutical industry presence and specialized fermentation expertise.
Switzerland commands a 12.4% share in 2025, projected to reach 11.6% by 2035, while Netherlands accounts for 5.2% in 2025, expected to reach 3.8% by 2035. The Rest of Europe region, including Nordic countries with biotechnology innovation, Eastern European emerging pharmaceutical markets, and smaller Western European research centers, is anticipated to hold 2.8% in 2025, declining slightly to 1.2% by 2035, attributed to market consolidation toward larger core markets with established pharmaceutical infrastructure and specialized live biotherapeutic manufacturing capabilities.
Competitive Landscape of the Live Biotherapeutic Products & Microbiome CDMO Market
The live biotherapeutic products & microbiome CDMO market is characterized by pioneering competition among specialized contract manufacturers, established CDMO organizations developing novel capabilities, and integrated pharmaceutical companies building internal expertise focused on delivering validated, regulatory-compliant, and technically sophisticated anaerobic manufacturing solutions.
Companies are investing in anaerobic infrastructure expansion, regulatory expertise development, strategic pharmaceutical partnership cultivation, and comprehensive technical support integration to deliver effective, reliable, and commercially viable manufacturing solutions meeting unprecedented live biotherapeutic product requirements and pharmaceutical sponsor expectations.
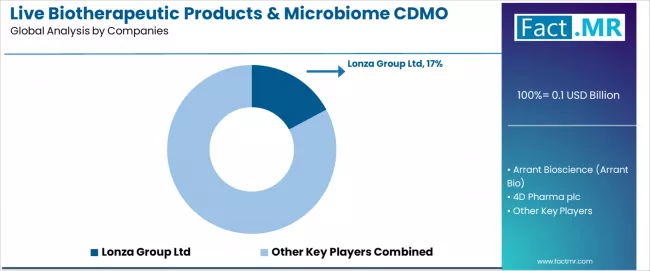
First-mover advantage establishment, regulatory track record development, and technical capability differentiation are central to strengthening competitive position and pharmaceutical client relationships. Lonza leads the market with a 17.2% market share, offering comprehensive live biotherapeutic manufacturing services with focus on GMP-compliant anaerobic manufacturing infrastructure and established pharmaceutical partnership support for clinical and commercial programs.
Arrant Bio provides specialized microbiome CDMO services with emphasis on consortium product manufacturing and technical expertise specific to complex live biotherapeutic formulations. 4D Pharma focuses on integrated pharmaceutical development and manufacturing capabilities supporting proprietary programs while offering selective CDMO services.
Cerbios delivers specialized small-molecule and biologic CDMO services expanding into live biotherapeutic manufacturing capabilities. Biose Industrie operates with focus on probiotic and live biotherapeutic manufacturing expertise serving European pharmaceutical markets. Assembly Biosciences Inc. specializes in microbiome therapeutic development with manufacturing capabilities supporting internal programs.
Wacker Chemie AG provides bioprocessing technologies and fermentation expertise applicable to microbial therapeutic production. Quay Pharmaceuticals delivers specialized pharmaceutical development and manufacturing services including oral solid dose and liquid formulations for live biotherapeutic products.
NIZO and Inpac Pharma Co. Ltd. focus on fermentation technology expertise and probiotic manufacturing capabilities translating to live biotherapeutic product applications, emphasizing anaerobic processing excellence and quality system compliance through specialized service strategies.
Key Players in the Live Biotherapeutic Products & Microbiome CDMO Market
- Lonza Group Ltd
- Arrant Bioscience (Arrant Bio)
- 4D Pharma plc
- Cerbios-Pharma SA
- Biose Industrie
- Assembly Biosciences, Inc.
- Wacker Chemie AG
- Quay Pharmaceuticals Ltd.
- NIZO Food Research B.V.
- Inpac Pharma Co., Ltd.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 0.08 billion |
| Application | C. Difficile, Crohn's Disease, Irritable Bowel Syndrome (IBS), Diabetes, Others |
| Manufacturing Capability | Anaerobic LBP Manufacturing, Fermentation & Scale-Up, Fill-Finish & Stability, Analytical & QC Services |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, mea |
| Countries Covered | USA, Germany, UK, Japan, India, China, Brazil and 40+ countries |
| Key Companies Profiled | Lonza, Arrant Bio, 4D Pharma, Cerbios, Biose Industrie, Assembly Biosciences Inc., Wacker Chemie AG, Quay Pharmaceuticals, NIZO, Inpac Probiotics |
| Additional Attributes | Dollar sales by application, manufacturing capability, regional demand trends, competitive landscape, pharmaceutical sponsor preferences for specialized CDMO services, integration with regulatory submission support, innovations in anaerobic bioprocessing technologies, consortium manufacturing advancement, and product stability optimization capabilities |
Live Biotherapeutic Products & Microbiome CDMO Market by Segments
-
Application :
- C. Difficile
- Crohn's Disease
- Irritable Bowel Syndrome (IBS)
- Diabetes
- Others
-
Manufacturing Capability :
- Anaerobic LBP Manufacturing
- Fermentation & Scale-Up
- Fill-Finish & Stability
- Analytical & QC Services
-
Region :
-
North America
- USA
- Canada
- Mexico
-
Europe
- Germany
- France
- UK
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
-
Asia Pacific
- China
- India
- Japan
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
-
Latin America
- Brazil
- Argentina
- Chile
- Rest of Latin America
-
Mea
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Countries
- Rest of mea
-
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- C. Difficile
- Crohn's Disease
- Irritable Bowel Syndrome (IBS)
- Diabetes
- Others
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Manufacturing Capability
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Manufacturing Capability, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Manufacturing Capability, 2025 to 2035
- Anaerobic LBP Manufacturing
- Fermentation & Scale-Up
- Fill-Finish & Stability
- Analytical & QC Services
- Y to o to Y Growth Trend Analysis By Manufacturing Capability, 2020 to 2024
- Absolute $ Opportunity Analysis By Manufacturing Capability, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Europe
- Asia Pacific
- Latin America
- mea
- Y to o to Y Growth Trend Analysis By Region, 2020 to 2024
- Absolute $ Opportunity Analysis By Region, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Application
- By Manufacturing Capability
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Application
- By Manufacturing Capability
- By Region
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Application
- By Manufacturing Capability
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Application
- By Manufacturing Capability
- By Region
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Application
- By Manufacturing Capability
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Application
- By Manufacturing Capability
- By Region
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Application
- By Manufacturing Capability
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Application
- By Manufacturing Capability
- By Region
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Application
- By Manufacturing Capability
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Application
- By Manufacturing Capability
- By Region
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Application
- By Manufacturing Capability
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Application
- By Manufacturing Capability
- By Region
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Application
- By Manufacturing Capability
- By Region
- By Country
- Market Attractiveness Analysis
- By Country
- By Application
- By Manufacturing Capability
- By Region
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Application
- By Manufacturing Capability
- By Region
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Application
- By Manufacturing Capability
- By Region
- Competition Analysis
- Competition Deep Dive
- Lonza Group Ltd
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Arrant Bioscience (Arrant Bio)
- 4D Pharma plc
- Cerbios-Pharma SA
- Biose Industrie
- Assembly Biosciences, Inc.
- Wacker Chemie AG
- Quay Pharmaceuticals Ltd.
- NIZO Food Research B.V.
- Inpac Pharma Co., Ltd.
- Lonza Group Ltd
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Manufacturing Capability, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Manufacturing Capability, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Manufacturing Capability, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Manufacturing Capability, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Manufacturing Capability, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Manufacturing Capability, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Manufacturing Capability, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Manufacturing Capability, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by Region, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Application
- Figure 6: Global Market Value Share and BPS Analysis by Manufacturing Capability, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Manufacturing Capability, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Manufacturing Capability
- Figure 9: Global Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Application
- Figure 26: North America Market Value Share and BPS Analysis by Manufacturing Capability, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Manufacturing Capability, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Manufacturing Capability
- Figure 29: North America Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Region
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Application
- Figure 36: Latin America Market Value Share and BPS Analysis by Manufacturing Capability, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Manufacturing Capability, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Manufacturing Capability
- Figure 39: Latin America Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Region
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Application
- Figure 46: Western Europe Market Value Share and BPS Analysis by Manufacturing Capability, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Manufacturing Capability, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Manufacturing Capability
- Figure 49: Western Europe Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by Region
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Application
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Manufacturing Capability, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Manufacturing Capability, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Manufacturing Capability
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by Region
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Application
- Figure 66: East Asia Market Value Share and BPS Analysis by Manufacturing Capability, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Manufacturing Capability, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Manufacturing Capability
- Figure 69: East Asia Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by Region
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Manufacturing Capability, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Manufacturing Capability, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Manufacturing Capability
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by Region
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Manufacturing Capability, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Manufacturing Capability, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Manufacturing Capability
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by Region
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the live biotherapeutic products & microbiome CDMO market in 2025?
The global live biotherapeutic products & microbiome CDMO market is estimated to be valued at USD 0.1 billion in 2025.
What will be the size of live biotherapeutic products & microbiome CDMO market in 2035?
The market size for the live biotherapeutic products & microbiome CDMO market is projected to reach USD 1.1 billion by 2035.
How much will be the live biotherapeutic products & microbiome CDMO market growth between 2025 and 2035?
The live biotherapeutic products & microbiome CDMO market is expected to grow at a 30.1% CAGR between 2025 and 2035.
What are the key product types in the live biotherapeutic products & microbiome CDMO market?
The key product types in live biotherapeutic products & microbiome CDMO market are c. difficile, crohn's disease, irritable bowel syndrome (ibs), diabetes and others.
Which manufacturing capability segment to contribute significant share in the live biotherapeutic products & microbiome CDMO market in 2025?
In terms of manufacturing capability, anaerobic lbp manufacturing segment to command 42.5% share in the live biotherapeutic products & microbiome CDMO market in 2025.