Drug Delivery Systems Market
Drug Delivery Systems Market Size and Share Forecast Outlook 2025 to 2035
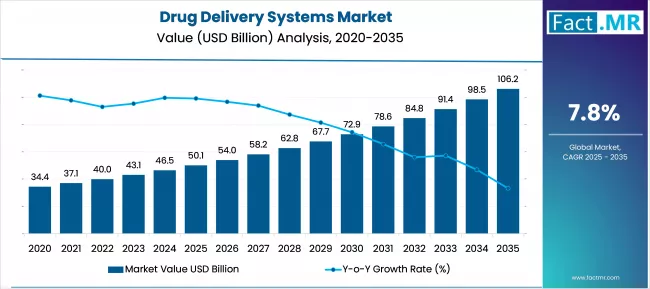
The Global Drug Delivery Systems Market Is Forecasted To Reach USD 50.1 Billion In 2025, And Further To USD 105.9 Billion By 2035, Expanding At A 7.8% CAGR. Drug Delivery Systems Inhalation Will Be The Leading Delivery Route Segment, While Hospitals Will Be The Key End-User Segment.
Drug Delivery Systems Market Size and Share Forecast Outlook 2025 to 2035
The global drug delivery systems market is projected to increase from USD 50.1 billion in 2025 to USD 105.9 billion by 2035, with a CAGR of 7.8% during the forecast period. The increase in prevalence of chronic diseases and the need for specific treatment promote its growth.
The development of nanotechnology and biotechnology opens the possibilities of precise and sustained drug delivery and improved results. Smart and non-invasive delivery systems and other patient-centric innovations enhance adherence and mitigate the side effects.
Quick Stats of Drug Delivery Systems Market
- Drug Delivery Systems Market Size (2025): USD 50.1 billion
- Projected Drug Delivery Systems Market Size (2035): USD 105.9 billion
- Forecast CAGR of Drug Delivery Systems Market (2025 to 2035): 7.8%
- Leading Delivery Route Segment of Drug Delivery Systems Market: Inhalation
- Leading End-User Segment of Drug Delivery Systems Market: Hospitals
- Key Growth Regions of Drug Delivery Systems Market: U.K., Germany, India, Canada
- Prominent Players in the Drug Delivery Systems Market: Johnson & Johnson, Pfizer, Novartis, BD (Becton, Dickinson and Company), 3M Healthcare, Others.
| Metric | Value |
|---|---|
| Industry Size (2025E) | USD 50.1 billion |
| Industry Size (2035F) | USD 105.9 billion |
| CAGR (2025-2035) | 7.8% |
The global Drug Delivery Systems market is forecast to expand from USD 50.1 billion in 2025 to USD 105.9 billion by 2035 at a CAGR of 7.3 percent. Initial growth in 2025-2026 will be shaped by early adoption of advanced delivery platforms and evaluation of smart, patient-centric systems.
The global drug delivery systems market is growing as chronic and infectious diseases remain leading health concerns. The World Health Organization (2023) states that nearly 74% of deaths worldwide are noncommunicable diseases, such as cardiovascular disorders, diabetes, and cancer, which pose a stable demand for an accurate and efficient method of drug delivery. This demand is strongest in aging populations and emerging economies, where treatment access is limited.
The development of nanotechnology and biotechnology is facilitating the targeted delivery and release of drugs. The Food and Drug Administration reported progress in nanoparticle-based drugs and inhalable delivery systems for respiratory diseases. These innovations improve patient convenience, reduce drug wastage, and enhance therapeutic efficiency, particularly for costly biologics and specialty medicines.
Regulators in the U.S. are issuing new guidance for combination products. The FDA’s “Essential Drug Delivery Outputs” (EDDO) guidance (2024) recommends what data and performance design outputs must be submitted for drug-device and biologic-device delivery systems. Also, the FDA’s Office of Combination Products has updated user fee rules and safety reporting requirements specifically for combination products.
The future development relies on the balance between innovation and affordability. The cost of the R&D and the complexity of the regulations can reduce the adoption in countries with low and middle incomes. But initiatives such as the WHO prequalification program and regional health innovation funds are forging equity of access and making it possible to integrate drug delivery with digital health and tailored medicine by 2035.
Key Drug Delivery Systems Market Dynamics
The Drug Delivery Systems Market is increasing because medical professionals and pharmaceutical companies are implementing new advanced systems to enhance treatment results and patient compliance.
The increasing prevalence of chronic illnesses, such as diabetes, cancer, and cardiovascular diseases, is driving the rise of the market of targeted and controlled release solutions. The use of smart injectables, transdermal patches, and nanoparticle-based formulations to optimize therapy is growing.
Adoption is being hastened by regulatory encouragement, whereby drug-device combination product guidance is being provided by the FDA, and the requirements under MDR, as stipulated by EMA, are facilitating safer approvals. However, the intense cost of development, rigorous regulations of compliance, and manufacturing complexities in low- and middle-income areas still restrict wider access.
Rising Burden of Chronic Diseases
The increase in chronic diseases is growing worldwide, and noncommunicable diseases (NCDs) cause 75% of non-pandemic-related mortality in 2021. The leading causes of death were cardiovascular diseases (more than 19 million), cancers (10 million), chronic respiratory diseases (4 million), and diabetes (1.6 million).
73% of the total NCD deaths and 82% of premature NCD deaths (under 70) are in low- and middle-income countries. This gap highlights the necessity of the availability of healthcare solutions.
The rising use of chronic conditions is necessitating the need for new drug delivery systems. These systems are expected to improve the therapeutic effect and patient compliance in light of the increasing demands on effective management of chronic diseases.
Advancements in Nanotechnology and Smart Delivery Platforms
Nanotechnology has transformed drug delivery in the sense that it has made possible the creation of nanoparticles capable of encapsulating therapeutic agents, thereby improving their stability and bioavailability. These nanoparticles can be as small as 10 to 1000 nanometers and help in targeted delivery, which helps them to avoid systemic side effects as well as enhance therapeutic efficacy.
Smart drug delivery systems (SDDS) combine stimuli-reactive materials, which discharge drugs when a particular physiological condition (pH variations or enzyme action) is encountered. This method enables the controlled availability of the drug and its ability to maximize its effects and reduce its side effects.
Multifunctional carriers with the capability of imaging, targeting, and therapy have been developed as a result of the combination of nanotechnology and smart delivery platforms. Such developed systems are especially promising in the treatment of complicated illnesses such as cancer, where they are able to deliver drugs to cancer centers, increasing effectiveness and decreasing the side effects on healthy tissues.
Regulatory Support for Drug-Device Combination Approvals
Regulatory authorities have developed systems through which drug-device combination products can be approved. The U.S. Food and Drug Administration (FDA) provides thorough advice under the Office of Combination Products (OCP), explaining the classification, jurisdiction, and premarket expectations of such products.
The European Medicines Agency (EMA) provides scientific guidelines in the area of quality documentation of medicinal products used alongside medical devices in the European Union. Such standards guarantee that the component of the device is safe and meets the performance standards, and it is consistent with the EU Medical Devices Regulation.
These regulatory frameworks are designed to facilitate these combination products to be approved so that they would comply with the required safety, efficacy, and quality standards. Agencies enable the development and introduction of new therapeutic solutions that combine drugs and device components by providing clear-cut guidelines.
High Development and Manufacturing Costs
The process of developing sophisticated drug delivery systems is a capital-intensive process. The average research and development (R&D) expenditure on a new drug has been put at about 1.3 billion, with both successful and unsuccessful experiments and capital expenditure factored in. This number highlights the huge financial resources that are necessary to take novel drug delivery technologies into the market.
There is an added complexity and cost in the manufacturing of such systems. Drug-device combinations require specialized equipment, high-purity materials, and high-quality control measures to provide safety and efficacy. Such requirements have the potential to raise the cost of production, especially of small-scale or customized therapies.
Smaller pharmaceutical companies may find it difficult to enter this market because of the financial difficulties related to the creation and production of sophisticated drug delivery systems. Moreover, the expensive nature might restrict the availability of these new treatment options in the low- and middle-income nations, but the effective solutions to drug delivery are increasingly demanded worldwide.
Stringent Regulatory and Compliance Requirements
The processes of the drug delivery system are highly regulated to guarantee safety, efficacy, and quality. Combinations of drugs and devices must have detailed submissions in premarket, risk evaluation, and performance testing to satisfy the standards of national safety.
The Medical Devices Regulation (MDR 2017/745) in the European Union requires conformity assessments of devices that are used in connection with medicinal products. In the case of combination products, the manufacturers are required to submit documents showing the safety of the device and the integration with the drug, which further complicates the regulatory submissions.
Compliance is not restricted to approvals. Continued post-market observation, adverse event reporting, and compliance with good manufacturing practices (GMP) must be undertaken all over the world. These strict criteria add to the length of development cycles, costs, and resources, and compliance is a major challenge facing the pharmaceutical firms that seek to commercially introduce sophisticated drug delivery models.
Limited Access in Low- and Middle-Income Regions
In low- and middle-income countries, there is limited access to the use of advanced drug delivery systems because of the excessive costs of their development and production. Inadequate healthcare facilities and a lack of trained staff also further limit the use of new treatments in these areas.
Based on the World Health Organization (WHO, 2023), three-quarters of the global NCD deaths (32 million) are found in low- and middle-income nations, which implies that the need to find solutions to these problems on a global scale is crucial.
There are also supply chain issues, such as the popularity of high-quality active pharmaceutical ingredients and delivery devices, that consistently restrict access. These together contribute to slow adoption so that populations in resource-constrained areas cannot enjoy the full benefits of the improvements in drug delivery technology.
Analyzing the Drug Delivery Systems Market by Key Regions
The drug delivery systems market is dominated by North America, which is backed up by high R&D investment rates, good healthcare infrastructure, as well as the level of chronic diseases. The drug-device combination regulatory system at the U.S. FDA has expedited approval of novel delivery systems and has increased uptake in the fields of oncology and diabetes care.
Europe comes in with strong programs of public health and investment in precision medicine. The European Medicines Agency (EMA) promotes advanced therapy medicinal products, which is why the combination of targeted drug delivery and biologics is encouraged to enhance patient outcomes.
Asia-Pacific is also undergoing a swift development, which is supported by the increasing healthcare access and growing production of pharmaceuticals in China and India. Government programs like the Production Linked Incentive (PLI) scheme by the Indian government on pharmaceuticals are boosting the domestic production of advanced delivery devices.
Latin America, the Middle East, and Africa are emerging markets with demand but have challenges, such as a lack of infrastructure and high costs. The key to increasing availability in these areas is public-private collaborations and programs led by the WHO.
Country-Wise Outlook
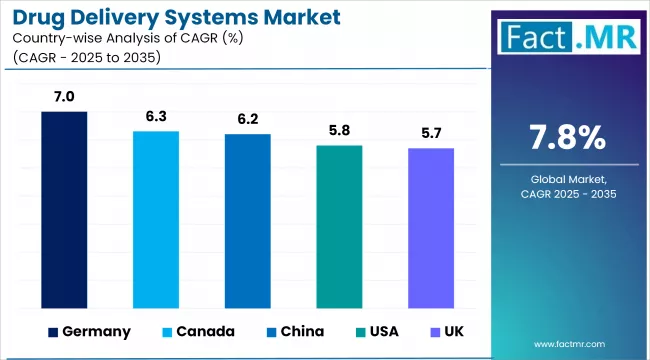
| Country | CAGR (2025-2035) |
|---|---|
| Germany | 7.0% |
| Canada | 6.3% |
| China | 6.2% |
| U.K. | 5.7% |
Germany Strengthens Drug Delivery Market with High Healthcare Spending and Innovation Support
The healthcare industry of Germany is one of the most developed in Europe, having spent over €494.65 billion in 2023, which is approximately 12.8% of GDP. Such intense government funding as Statutory Health Insurance stimulates the demand for an innovative drug delivery system. There is a strong pharmaceutical production base that facilitates the use of targeted therapies and advanced platforms of delivery.
Investment in research and development is another major growth driver. In 2022, the pharmaceutical sector incurred over 9.6 billion on research and development, and its pace of research is about 11.1%, which is the highest in the EU countries. There are also over 30 biotechnology clusters in Germany that support the capacity to collaborate with academia, start-ups, and industry leaders, among which is an ecosystem that is best suited to creating precision medicine and smart delivery systems.
There is the R&D tax credit (Forschungszulage) that was introduced in 2020, and it offers incentives, whereby companies are allowed to claim credits on the research costs that are eligible. There will be updates under the Growth Opportunities Act in 2024 that incorporate material costs, reducing the financial risks associated with the development of drug-device combination products.
The National Pharmaceutical Strategy of Germany was announced in December 2023, and it is aimed at increasing national production and the reduction of regulatory routes. There are such programs as BioPharma -the strategy competition of the medicine of the future, which awards €100 million to work out the process of research translation into production.
- High expenditure on healthcare (12.8% of GDP) is a very strong demand factor for the advanced drug delivery solutions.
- Pharma R&D investment of over €9.6 billion annually and 30 or more biotech clusters promote innovation.
- Government initiatives like the National Pharmaceutical Strategy boost domestic production and streamline regulations.
Canada Strengthens Drug Delivery Market with Rising Health Spending and National Drug Policy
Health care spending in Canada is on the rise, which contributes to the need for superior drug delivery systems. Health expenditure is estimated to rise to CAD $372 billion in 2024 or about 12.4 percent of GDP. In 2022, the amount spent by public drug programs was CAD $17.2 billion due to a rise in the consumption of specialty drugs such as Trikafta in the treatment of cystic fibrosis.
Aging and increased prevalence of chronic diseases, such as diabetes and cardiovascular disease, are generating high demand for targeted and sustained drug delivery systems. The trends are driving healthcare providers to embrace solutions that will enhance adherence and therapeutic outcomes and manage the costs.
The federal government is undertaking measures to enhance the affordability and access to prescription medicines. In December 2023, the Minister of Health announced the establishment of the Canadian Drug Agency to take on the coordination of drug policy and management of national formularies, in order to maximize their benefits.
In Health Canada's 2024-2025 plan, the promotion of universal pharmacare by proposing a Pharmacare Act and collaboration with provinces to increase the coverage are also part of the plan. The Pediatric Drug Action Plan also places more focus on access to the necessary medications in children and enhances the regulatory routes of the pediatric medicines.
Such projects open avenues for drug delivery apparatus producers to deliver solutions based on the objectives of affordability and availability. The domain of smart injectables, controlled-release systems, and devices that are easy to use by patients can be on the rise due to the emphasis on adherence and cost-effectiveness of the public health programs.
- In 2022, the CAD 17.2 billion amount of the public drug spending increased due to the specialty drugs.
- The establishment of the Canadian Drug Agency is meant to facilitate ease of service and reduce the cost of control.
- The prospects of advanced delivery systems are open due to federal pharmacare and pediatric drug access plans.
China Expands Drug Delivery Innovation with Strong Government Support and R&D Investments
The increased prevalence of chronic diseases and an aging population is increasing the need for sophisticated drug delivery systems in the healthcare sector of China. A recent study by CEIC Data has shown that the level of healthcare expenditure was as high as RMB 9 trillion in 2023, which is evidence of the immense governmental attention paid to patient-centric solutions and medical innovation.
The Pharmaceutical industry in the country is also part of the advantageous impact of the Healthy China 2030 plan that encourages the development of biopharmaceuticals and innovative methods of delivery. Nanotechnology-based drug carriers and sustained-release formulations are being invested in by domestic firms in order to enhance the effectiveness of treatment and minimize the number of hospital visits.
The National Medical Products Administration (NMPA) has been able to expedite the drug approval process, which has been a welcome move to global pharma companies to introduce new delivery platforms within China. This will create collaboration between the local biotech companies and the multinational players to increase access to innovative treatments for patients.
There is an opportunity in the digital health integration where the use of telemedicine promotes the use of individual dosing and remote monitoring. The further development of AI and big data analytics through government support also establishes a good climate for research on precision drug delivery.
- In 2023, China recorded a record spending of RMB 8.06 trillion in healthcare, which enhanced drug innovation.
- The regulatory reforms by NMPA encourage the world pharma use of new delivery systems.
- The Healthy China 2030 plan motivates health care to concentrate on biopharma research and development, as well as on patient-centered solutions.
U.K. Strengthens Drug Delivery Market with Robust Healthcare Funding and Regulatory Reforms
The U.K. spends approximately 10.9-11.1% of its GDP on healthcare, which depicts a very high level of investment in the health of its people. In 2023, healthcare expenditure increased by 5.6%, contributing to the improvement of services in the face of inflationary pressure. As one of the largest publicly funded systems in the world, the NHS still provokes the need for innovative drug delivery solutions and further patient access.
Life sciences continue to be driven by research and development. Recently, the U.K. revised its Health R&D spending to 0.15% of GDP, which is better than its estimates, and this increases expenditure on medical sciences. Infrastructure in clinical trials is also receiving growing interest, as well as expedited testing of new treatments by Regulatory Agencies.
The MHRA International Recognition Procedure enables the U.K. to trust the approval of other reputable regulators, such as the U.S. FDA and EMA, and accelerate the entry of new therapies into the market. The VPAS scheme is a scheme that connects the prices of drugs with the NHS, enhancing affordability and accessibility for patients.
Pharmaceutical expenditure constitutes only 9% of all healthcare expenditure, and there is room to enlarge innovative drug delivery solutions. Rapid approvals and international presence opportunities can promote an earlier introduction of a new sophisticated delivery platform in the U.K. market.
- The U.K.’s pharmaceutical spending (≈9% of health spending) is lower than peers, highlighting growth potential for innovative drug delivery adoption.
- Healthcare expenditure forms ~11% of GDP, reflecting strong demand and resources to support advanced therapies.
- Higher health R&D spending (0.15% of GDP) and MHRA’s recognition procedure enable faster introduction of drug-device innovations.
Category-wise Analysis
Injectable Drug Delivery Emerges as the Dominant Route with Rising Demand for Biologics
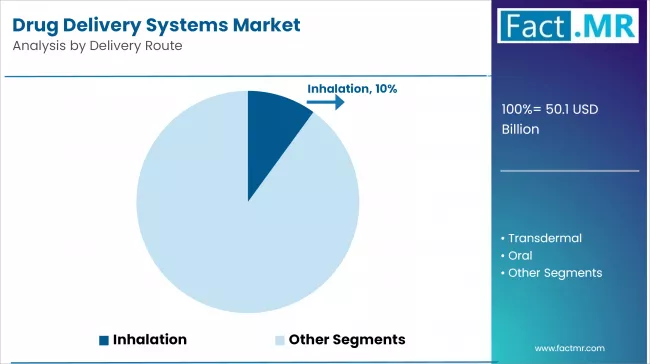
Injectable drug delivery is the largest segment as it provides the capability to administer accurate doses, quick therapeutic efficacy, and is compatible with biologics and vaccines. The increased rate of chronic diseases, such as diabetes and cancer, contributes to the high levels of insulin pens, prefilled syringes, and auto-injectors, which are becoming in high demand.
The World Health Organization (WHO) states that more than 422 million individuals around the world are diabetic, and injectables are becoming more independent. Technologies like wearable injectors and needle-free equipment are making patients more convenient and reducing less time spent in hospitals. The increased use of biosimilars and custom medicines intensifies the supremacy of this sector in the global drug delivery market.
- Growing diabetes burden fuels demand for insulin delivery devices and self-injection systems.
- Wearable and connected injectors enhance treatment adherence and remote monitoring capabilities.
- Biologics and biosimilars adoption expands the need for advanced injectable platforms globally.
Hospitals Lead End-User Segment with Strong Infrastructure and High Patient Inflow
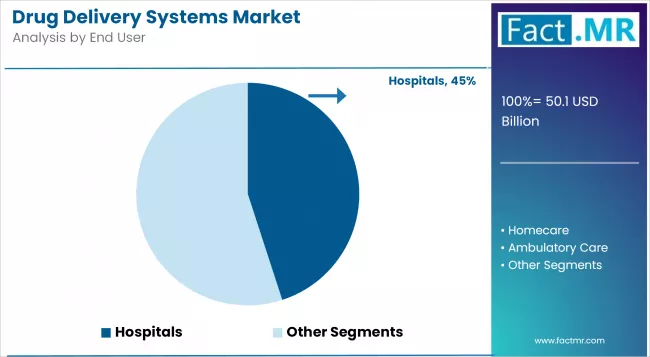
The drug delivery systems market is the largest in terms of hospitals because they are still the most critical institutions where complex therapies, biologics, and intravenous treatment must be carried out. Large inflow of patients, availability of qualified health workers, and access to sophisticated infusion systems facilitate growth.
Such public health measures as better investment in hospital infrastructure as part of the NHS Long Term Plan and the U.S. HHS modernization campaigns boost hospital-based adoption. Another factor in this segment is the growing surgical volume and cancer treatments, which demand controlled and precise drug delivery systems.
- Hospitals drive demand for infusion pumps, auto-injectors, and prefilled syringes.
- Government investments in hospital infrastructure boost access to advanced delivery platforms.
- Rising cancer and chronic disease treatments make hospitals the key drug delivery hub.
Competitive Analysis
The Drug Delivery Systems market is characterized by competition determined by the innovation of targeted, sustained, and patient-friendly methods of drug administration. Major competitors like Johnson and Johnson, Pfizer, Novartis, BD, and 3M Healthcare are concentrating on growing portfolios of advanced injectables, wearable delivery, and inhalation systems to satisfy the demand for precision therapies.
Such firms are capitalizing on the joint ventures with biotech companies and research centers in developing products at a faster rate. As an example, the efforts of Pfizer and BioNTech in developing mRNA-drug delivery platforms have established precedence in terms of scale and global distribution, which has forced other competitors to reinforce R&D pipelines of comparable modalities.
Digital health integration is now emerging as a major differentiator, and companies are spending on connected delivery devices facilitating real-time adherence monitoring. BD has become the first to launch linked drug delivery systems, and 3M Healthcare is promoting technologies of transdermal delivery, which enhance the comfort and adherence of patients.
Competition is also characterised by geographic expansion and alignment of regulations, whereby players are targeting emerging markets where the prevalence of chronic diseases is high. The acquisition of companies, alliances, and technology licensing deals is aiding organizations to expand their market horizons and establish end-to-end drug-device chains.
Key Players in the Market
- Johnson & Johnson
- Pfizer
- Novartis
- BD (Becton, Dickinson and Company)
- 3M Healthcare
- Roche
- AstraZeneca
- GSK (GlaxoSmithKline)
- Eli Lilly and Company
- Sanofi
Recent Developments
- In May 2025, researchers from Andhra Pradesh, India, in collaboration with scientists from Saudi Arabia and the UAE, developed a colon-targeted drug delivery system using cross-linked mastic gum nanoparticles to deliver 5-fluorouracil directly to the colon, achieving ~83.5% encapsulation efficiency and 95.2% colon-specific release.
- In January 2025, BD (Becton, Dickinson and Company) showcased new drug delivery innovations at Pharmapack, including prefillable syringes, wearable injectors, and nasal spray systems. They emphasized biologics, patient self-care, and home-based treatments while also hosting learning labs on device design and needle technologies.
Segmentation of Drug Delivery Systems Market
-
By Delivery Route :
- Inhalation
- Transdermal
- Oral
- Injectable
- Others
-
By End User :
- Hospitals
- Homecare
- Ambulatory Care
- Retail Pharmacies
- Others
-
By Region :
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia & Pacific
- Middle East & Africa
Table of Content
- Executive Summary
- Global Market Outlook
- Demand-side Trends
- Supply-side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020-2024 and Forecast, 2025-2035
- Historical Market Size Value (USD Billion) & Units Analysis, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Projections, 2025-2035
- Y-o-Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020-2024 and Forecast 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Delivery Route
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By Delivery Route, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By Delivery Route, 2025-2035
- Inhalation
- Transdermal
- Oral
- Injectable
- Others
- Y-o-Y Growth Trend Analysis By Delivery Route, 2020-2024
- Absolute $ Opportunity Analysis By Delivery Route, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By End User
- Introduction / Key Findings
- Historical Market Size Value (USD Billion) & Units Analysis By End User, 2020-2024
- Current and Future Market Size Value (USD Billion) & Units Analysis and Forecast By End User, 2025-2035
- Hospitals
- Homecare
- Ambulatory Centre
- Retail Pharmacies
- Others
- Y-o-Y Growth Trend Analysis By End User, 2020-2024
- Absolute $ Opportunity Analysis By End User, 2025-2035
- Global Market Analysis 2020-2024 and Forecast 2025-2035, By Region
- Introduction
- Historical Market Size Value (USD Billion) & Units Analysis By Region, 2020-2024
- Current Market Size Value (USD Billion) & Units Analysis and Forecast By Region, 2025-2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia & Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- U.S.
- Canada
- Mexico
- By Delivery Route
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Delivery Route
- By End User
- Key Takeaways
- Latin America Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Delivery Route
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Delivery Route
- By End User
- Key Takeaways
- Western Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Germany
- U.K.
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Europe
- By Delivery Route
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Delivery Route
- By End User
- Key Takeaways
- Eastern Europe Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltics
- Rest of Eastern Europe
- By Delivery Route
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Delivery Route
- By End User
- Key Takeaways
- East Asia Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- China
- Japan
- South Korea
- By Delivery Route
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Delivery Route
- By End User
- Key Takeaways
- South Asia & Pacific Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia & Pacific
- By Delivery Route
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Delivery Route
- By End User
- Key Takeaways
- Middle East & Africa Market Analysis 2020-2024 and Forecast 2025-2035, By Country
- Historical Market Size Value (USD Billion) & Units Trend Analysis By Market Taxonomy, 2020-2024
- Market Size Value (USD Billion) & Units Forecast By Market Taxonomy, 2025-2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Delivery Route
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Delivery Route
- By End User
- Key Takeaways
- Key Countries Market Analysis
- U.S.
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- U.K.
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Nordic
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- BENELUX
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Balkan & Baltics
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Delivery Route
- By End User
- U.S.
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Delivery Route
- By End User
- Competition Analysis
- Competition Deep Dive
- Johnson & Johnson
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Pfizer
- Novartis
- BD (Becton, Dickinson and Company)
- 3M Healthcare
- Roche
- AstraZeneca
- GSK (GlaxoSmithKline)
- Eli Lilly and Company
- Sanofi
- Johnson & Johnson
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Billion) Forecast by Region, 2020 to 2035
- Table 2: Global Market Units Forecast by Region, 2020 to 2035
- Table 3: Global Market Value (USD Billion) Forecast by Delivery Route, 2020 to 2035
- Table 4: Global Market Units Forecast by Delivery Route, 2020 to 2035
- Table 5: Global Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 6: Global Market Units Forecast by End User, 2020 to 2035
- Table 7: North America Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 8: North America Market Units Forecast by Country, 2020 to 2035
- Table 9: North America Market Value (USD Billion) Forecast by Delivery Route, 2020 to 2035
- Table 10: North America Market Units Forecast by Delivery Route, 2020 to 2035
- Table 11: North America Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 12: North America Market Units Forecast by End User, 2020 to 2035
- Table 13: Latin America Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 14: Latin America Market Units Forecast by Country, 2020 to 2035
- Table 15: Latin America Market Value (USD Billion) Forecast by Delivery Route, 2020 to 2035
- Table 16: Latin America Market Units Forecast by Delivery Route, 2020 to 2035
- Table 17: Latin America Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 18: Latin America Market Units Forecast by End User, 2020 to 2035
- Table 19: Western Europe Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 20: Western Europe Market Units Forecast by Country, 2020 to 2035
- Table 21: Western Europe Market Value (USD Billion) Forecast by Delivery Route, 2020 to 2035
- Table 22: Western Europe Market Units Forecast by Delivery Route, 2020 to 2035
- Table 23: Western Europe Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 24: Western Europe Market Units Forecast by End User, 2020 to 2035
- Table 25: Eastern Europe Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 26: Eastern Europe Market Units Forecast by Country, 2020 to 2035
- Table 27: Eastern Europe Market Value (USD Billion) Forecast by Delivery Route, 2020 to 2035
- Table 28: Eastern Europe Market Units Forecast by Delivery Route, 2020 to 2035
- Table 29: Eastern Europe Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 30: Eastern Europe Market Units Forecast by End User, 2020 to 2035
- Table 31: East Asia Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 32: East Asia Market Units Forecast by Country, 2020 to 2035
- Table 33: East Asia Market Value (USD Billion) Forecast by Delivery Route, 2020 to 2035
- Table 34: East Asia Market Units Forecast by Delivery Route, 2020 to 2035
- Table 35: East Asia Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 36: East Asia Market Units Forecast by End User, 2020 to 2035
- Table 37: South Asia & Pacific Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 38: South Asia & Pacific Market Units Forecast by Country, 2020 to 2035
- Table 39: South Asia & Pacific Market Value (USD Billion) Forecast by Delivery Route, 2020 to 2035
- Table 40: South Asia & Pacific Market Units Forecast by Delivery Route, 2020 to 2035
- Table 41: South Asia & Pacific Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 42: South Asia & Pacific Market Units Forecast by End User, 2020 to 2035
- Table 43: Middle East & Africa Market Value (USD Billion) Forecast by Country, 2020 to 2035
- Table 44: Middle East & Africa Market Units Forecast by Country, 2020 to 2035
- Table 45: Middle East & Africa Market Value (USD Billion) Forecast by Delivery Route, 2020 to 2035
- Table 46: Middle East & Africa Market Units Forecast by Delivery Route, 2020 to 2035
- Table 47: Middle East & Africa Market Value (USD Billion) Forecast by End User, 2020 to 2035
- Table 48: Middle East & Africa Market Units Forecast by End User, 2020 to 2035
List Of Figures
- Figure 1: Global Market Units Forecast 2020 to 2035
- Figure 2: Global Market Pricing Analysis
- Figure 3: Global Market Value (USD Billion) Forecast 2020 to 2035
- Figure 4: Global Market Value Share and BPS Analysis by Delivery Route, 2025 and 2035
- Figure 5: Global Market Y-o-Y Growth Comparison by Delivery Route, 2025 to 2035
- Figure 6: Global Market Attractiveness Analysis by Delivery Route
- Figure 7: Global Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 8: Global Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 9: Global Market Attractiveness Analysis by End User
- Figure 10: Global Market Value (USD Billion) Share and BPS Analysis by Region, 2025 and 2035
- Figure 11: Global Market Y-o-Y Growth Comparison by Region, 2025 to 2035
- Figure 12: Global Market Attractiveness Analysis by Region
- Figure 13: North America Market Incremental $ Opportunity, 2025 to 2035
- Figure 14: Latin America Market Incremental $ Opportunity, 2025 to 2035
- Figure 15: Western Europe Market Incremental $ Opportunity, 2025 to 2035
- Figure 16: Eastern Europe Market Incremental $ Opportunity, 2025 to 2035
- Figure 17: East Asia Market Incremental $ Opportunity, 2025 to 2035
- Figure 18: South Asia & Pacific Market Incremental $ Opportunity, 2025 to 2035
- Figure 19: Middle East & Africa Market Incremental $ Opportunity, 2025 to 2035
- Figure 20: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 21: North America Market Value Share and BPS Analysis by Delivery Route, 2025 and 2035
- Figure 22: North America Market Y-o-Y Growth Comparison by Delivery Route, 2025 to 2035
- Figure 23: North America Market Attractiveness Analysis by Delivery Route
- Figure 24: North America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 25: North America Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 26: North America Market Attractiveness Analysis by End User
- Figure 27: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 28: Latin America Market Value Share and BPS Analysis by Delivery Route, 2025 and 2035
- Figure 29: Latin America Market Y-o-Y Growth Comparison by Delivery Route, 2025 to 2035
- Figure 30: Latin America Market Attractiveness Analysis by Delivery Route
- Figure 31: Latin America Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 32: Latin America Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 33: Latin America Market Attractiveness Analysis by End User
- Figure 34: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 35: Western Europe Market Value Share and BPS Analysis by Delivery Route, 2025 and 2035
- Figure 36: Western Europe Market Y-o-Y Growth Comparison by Delivery Route, 2025 to 2035
- Figure 37: Western Europe Market Attractiveness Analysis by Delivery Route
- Figure 38: Western Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 39: Western Europe Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 40: Western Europe Market Attractiveness Analysis by End User
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 42: Eastern Europe Market Value Share and BPS Analysis by Delivery Route, 2025 and 2035
- Figure 43: Eastern Europe Market Y-o-Y Growth Comparison by Delivery Route, 2025 to 2035
- Figure 44: Eastern Europe Market Attractiveness Analysis by Delivery Route
- Figure 45: Eastern Europe Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 46: Eastern Europe Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 47: Eastern Europe Market Attractiveness Analysis by End User
- Figure 48: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 49: East Asia Market Value Share and BPS Analysis by Delivery Route, 2025 and 2035
- Figure 50: East Asia Market Y-o-Y Growth Comparison by Delivery Route, 2025 to 2035
- Figure 51: East Asia Market Attractiveness Analysis by Delivery Route
- Figure 52: East Asia Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 53: East Asia Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 54: East Asia Market Attractiveness Analysis by End User
- Figure 55: South Asia & Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 56: South Asia & Pacific Market Value Share and BPS Analysis by Delivery Route, 2025 and 2035
- Figure 57: South Asia & Pacific Market Y-o-Y Growth Comparison by Delivery Route, 2025 to 2035
- Figure 58: South Asia & Pacific Market Attractiveness Analysis by Delivery Route
- Figure 59: South Asia & Pacific Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 60: South Asia & Pacific Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 61: South Asia & Pacific Market Attractiveness Analysis by End User
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: Middle East & Africa Market Value Share and BPS Analysis by Delivery Route, 2025 and 2035
- Figure 64: Middle East & Africa Market Y-o-Y Growth Comparison by Delivery Route, 2025 to 2035
- Figure 65: Middle East & Africa Market Attractiveness Analysis by Delivery Route
- Figure 66: Middle East & Africa Market Value Share and BPS Analysis by End User, 2025 and 2035
- Figure 67: Middle East & Africa Market Y-o-Y Growth Comparison by End User, 2025 to 2035
- Figure 68: Middle East & Africa Market Attractiveness Analysis by End User
- Figure 69: Global Market - Tier Structure Analysis
- Figure 70: Global Market - Company Share Analysis
- FAQs -
What is the Global Drug Delivery Systems Market size in 2025?
The drug delivery systems market is valued at USD 50.1 billion in 2025.
Who are the Major Players Operating in the Drug Delivery Systems Market?
Prominent players in the market include Johnson & Johnson, Pfizer, Novartis, BD (Becton, Dickinson and Company), 3M Healthcare, and Roche.
What is the Estimated Valuation of the Drug Delivery Systems Market by 2035?
The market is expected to reach a valuation of USD 105.9 billion by 2035.
What Value CAGR Did the Drug Delivery Systems Market Exhibit over the Last Five Years?
The historic growth rate of the drug delivery systems market is 7.8% from 2020-2024.