Liver Disease Therapeutics Market
Liver Disease Therapeutics Market Size and Share Forecast Outlook 2025 to 2035
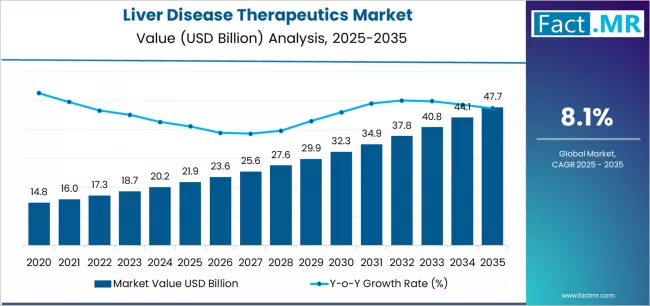
Liver disease therapeutics market is projected to grow from USD 21.9 billion in 2025 to USD 47.7 billion by 2035, at a CAGR of 8.1%. Antiviral Drugs will dominate with a 39.8% market share, while hepatitis will lead the disease segment with a 40.4% share.
Liver Disease Therapeutics Market Forecast and Outlook 2025 to 2035
The global liver disease therapeutics market is projected to reach USD 47.7 billion by 2035, recording an absolute increase of USD 25.83 billion over the forecast period. The market is valued at USD 21.87 billion in 2025 and is set to rise at a CAGR of 8.1% during the assessment period.
The market is expected to grow by approximately 2.2 times during the same period, supported by increasing prevalence of liver diseases worldwide, driving demand for specialized antiviral therapies and increasing investments in novel treatment mechanisms with enhanced efficacy across hepatitis, non-alcoholic fatty liver disease, and hepatocellular carcinoma applications globally.
Quick Stats for Liver Disease Therapeutics Market
- Liver Disease Therapeutics Market Value (2025): USD 21.87 billion
- Liver Disease Therapeutics Market Forecast Value (2035): USD 47.7 billion
- Liver Disease Therapeutics Market Forecast CAGR: 8.1%
- Leading Product Type in Liver Disease Therapeutics Market: Antiviral Drugs (39.8%)
- Key Growth Regions in Liver Disease Therapeutics Market: North America, Europe, and Asia Pacific
- Top Players in Liver Disease Therapeutics Market: Gilead Sciences, AbbVie, Bristol-Myers Squibb, F. Hoffmann-La Roche, Takeda Pharmaceutical, Johnson & Johnson, Merck & Co., Novartis, Pfizer, Sanofi, GSK
Patients with liver disorders face mounting pressure to manage progressive disease and prevent complications while addressing viral infections, metabolic dysfunction, and autoimmune pathology, with modern liver disease therapeutics providing documented clinical benefits including sustained virologic response, reduced fibrosis progression, and improved survival outcomes compared to conventional management approaches alone.
Rising awareness about early diagnosis and expanding access to direct-acting antivirals enabling curative hepatitis treatment create substantial opportunities for pharmaceutical manufacturers and healthcare partners. However, high drug costs and reimbursement complexities across markets may pose obstacles to universal patient access.
The antiviral drugs segment dominates market activity, driven by extensive utilization in hepatitis B and hepatitis C treatment and proven efficacy of direct-acting antiviral regimens achieving high cure rates worldwide. Healthcare providers increasingly recognize the transformative impact of modern antiviral therapies, with typical treatment protocols providing sustained virologic response and viral elimination at established regimens through comprehensive hepatology networks.
The vaccines segment demonstrates meaningful presence, supported by expanding hepatitis A and hepatitis B vaccination programs, while chemotherapy, targeted therapy, and immunosuppressants serve critical roles across hepatocellular carcinoma and autoimmune liver disease management. Hepatitis emerges as the dominant disease indication, reflecting the substantial global burden of viral hepatitis and established therapeutic protocols. North America represents the leading regional market, driven by advanced healthcare infrastructure and comprehensive pharmaceutical access across major treatment centers.
Regional dynamics show North America maintaining market leadership at approximately 40% share, supported by high treatment adoption rates and established reimbursement frameworks across USA healthcare systems. Europe demonstrates substantial presence driven by comprehensive hepatology services and national viral hepatitis elimination programs, while Asia Pacific emphasizes large patient populations and expanding healthcare access initiatives.
USA leads country-level growth through extensive direct-acting antiviral utilization and emerging NASH drug approvals, followed by China supported by massive hepatitis B carrier population and government vaccination initiatives.
The competitive landscape features moderate concentration with Gilead Sciences maintaining market leadership position at a 12.5% market share through pioneering direct-acting antiviral portfolios, while established pharmaceutical companies including AbbVie, Bristol-Myers Squibb, and F. Hoffmann-La Roche compete through comprehensive hepatitis and oncology franchises across diverse liver disease indications.
Liver Disease Therapeutics Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 21.87 billion |
| Market Forecast Value (2035) | USD 47.7 billion |
| Forecast CAGR (2025-2035) | 8.1% |
Why is the Liver Disease Therapeutics Market Growing?
The liver disease therapeutics market grows by enabling patients to achieve disease control and prevent liver failure while addressing diverse etiologies including viral hepatitis, metabolic dysfunction, and autoimmune pathology without exclusive reliance on supportive care interventions.
Patients with liver diseases face mounting pressure to halt disease progression and avoid transplantation while managing symptoms and complications, with modern therapeutic agents typically providing targeted mechanisms including viral eradication, fibrosis regression, and immune modulation compared to conventional treatments alone, making advanced pharmacotherapy essential for comprehensive hepatology management protocols.
Rising non-alcoholic fatty liver disease prevalence linked to obesity and diabetes epidemics creates expanding patient populations requiring therapeutic intervention, with NASH representing a critical unmet medical need driving pharmaceutical development investment and regulatory priority designation.
Growing direct-acting antiviral accessibility and declining hepatitis C treatment costs drive global elimination initiatives, with WHO targets supporting widespread screening and treatment programs demonstrating curative outcomes across diverse patient populations. Increasing hepatocellular carcinoma incidence and expanding targeted therapy options enable improved survival outcomes for liver cancer patients through systemic therapies including tyrosine kinase inhibitors and immune checkpoint inhibitors.
Segmental Analysis
The market is segmented by product, disease, and region. By product, the market is divided into antiviral drugs, vaccines, chemotherapy, targeted therapy, immunosuppressants, and other products.
Based on disease, the market is categorized into hepatitis, autoimmune diseases, non-alcoholic fatty liver disease, cancer, genetic disorders, and other diseases. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and MEA.
What Makes Antiviral Drugs the Leading Product Category?
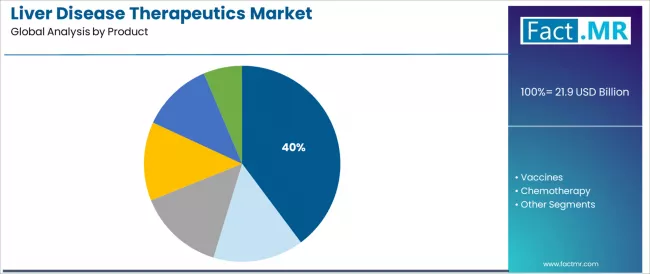
The antiviral drugs segment represents the dominant force in the liver disease therapeutics market, capturing 39.8% of the total market share in 2025. This established product category encompasses direct-acting antivirals for hepatitis C and nucleotide analogs for hepatitis B featuring clinically validated viral suppression and eradication capabilities, including pangenotypic regimens and single-tablet combinations that enable high cure rates and sustained virologic response across diverse patient populations worldwide.
The antiviral drugs segment's market leadership stems from its transformative clinical impact, with therapies capable of achieving hepatitis C cure rates exceeding 95% and hepatitis B viral suppression while maintaining excellent safety profiles and convenient oral administration standards across treatment-naive and experienced patients.
The vaccines segment maintains meaningful market presence at 11.0%, serving preventive applications requiring hepatitis A and hepatitis B immunization programs protecting at-risk populations and supporting disease elimination strategies. These products offer critical primary prevention functionality reducing disease incidence while providing population-level impact through comprehensive vaccination campaigns.
The chemotherapy segment accounts for approximately 9.2% market share, providing systemic cytotoxic agents for hepatocellular carcinoma management, while targeted therapy represents 14.8% serving advanced liver cancer applications through tyrosine kinase inhibitors and immune checkpoint inhibitors. Immunosuppressants account for 7.6% serving autoimmune hepatitis and liver transplant patients, while other products comprise 17.6% encompassing ursodeoxycholic acid, corticosteroids, and supportive therapies.
Key therapeutic advantages driving the antiviral drugs segment include revolutionary hepatitis C cure capabilities with direct-acting antiviral regimens demonstrating sustained virologic response rates exceeding 95% across all genotypes, established hepatitis B suppression through nucleotide analogs enabling long-term viral control and reduced cirrhosis progression, convenient oral administration with short treatment durations supporting patient adherence and treatment completion, and expanding global access through generic competition and pharmaceutical access programs supporting viral hepatitis elimination objectives aligned with WHO targets.
How does Hepatitis Lead Disease Indication Segments?
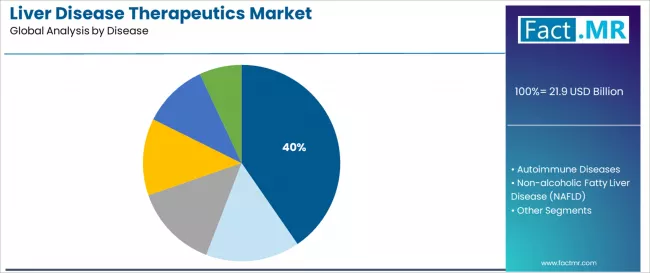
Hepatitis represents the dominant disease indication segment in the liver disease therapeutics market with a 40.4% market share in 2025, reflecting the substantial global burden of viral hepatitis and the availability of effective therapeutic interventions. The hepatitis segment demonstrates robust demand driven by chronic hepatitis B affecting approximately 296 million people globally and ongoing hepatitis C treatment despite declining prevalence through successful cure campaigns.
Non-alcoholic fatty liver disease accounts for 13.5% market share in 2025, demonstrating significant growth potential driven by obesity and diabetes epidemics creating massive patient populations with metabolic liver disease requiring therapeutic intervention. NAFLD/NASH represents the leading cause of chronic liver disease in developed countries and a critical pharmaceutical development focus.
Cancer accounts for an 18.1% market share, serving hepatocellular carcinoma patients requiring systemic therapies including targeted agents and immunotherapies. Autoimmune diseases represent 8.2% serving autoimmune hepatitis, primary biliary cholangitis, and primary sclerosing cholangitis patients, while genetic disorders account for 6.1% including Wilson disease and hemochromatosis. Other diseases comprise 13.5% encompassing alcoholic liver disease, drug-induced liver injury, and other etiologies.
Key disease dynamics include hepatitis dominance driven by large existing patient populations and effective antiviral therapies supporting treatment scale-up, NAFLD/NASH emergence representing future growth driver pending regulatory approvals of novel therapies targeting metabolic dysfunction and fibrosis, cancer applications expanding through targeted therapy and immunotherapy advances improving survival outcomes in advanced disease, and autoimmune disease management requiring chronic immunosuppression and emerging therapeutic mechanisms supporting remission induction.
How Does North America Demonstrate Regional Leadership?
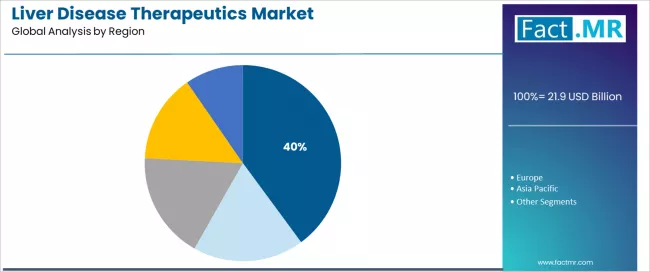
North America represents the dominant regional segment in the liver disease therapeutics market with 39.9% market share in 2025, reflecting advanced healthcare infrastructure, comprehensive insurance coverage, and established hepatology practice networks supporting widespread therapeutic access. The North American segment's market leadership stems from high direct-acting antiviral adoption, extensive hepatitis C elimination programs, and emerging NASH drug development positioning USA as epicenter of metabolic liver disease research and commercialization.
Europe accounts for approximately 27.3% market share in 2025, demonstrating substantial presence through comprehensive national health systems, established viral hepatitis programs, and strong pharmaceutical markets across major countries. The European segment benefits from universal healthcare coverage, hepatology centers of excellence, and national disease elimination strategies.
Asia Pacific represents approximately 22.6% market share, driven by massive hepatitis B endemic populations, expanding healthcare access, and government-supported vaccination and treatment initiatives across China, India, and Southeast Asian countries. Latin America and MEA account for 5.8% and 4.7% respectively, reflecting emerging market dynamics and expanding pharmaceutical access.
Key regional dynamics include North American leadership driven by comprehensive pharmaceutical market development and emerging NASH therapeutic landscape, European strength reflecting universal healthcare systems and viral hepatitis elimination commitment, Asia Pacific potential representing large patient populations and expanding treatment access through government programs and generic competition, and emerging regions demonstrating long-term growth opportunities pending infrastructure development and pharmaceutical accessibility improvements.
What are the Drivers, Restraints, and Key Trends of the Liver Disease Therapeutics Market?
The market is driven by three concrete demand factors tied to patient outcomes. First, rising non-alcoholic fatty liver disease prevalence and increasing NASH with fibrosis cases create expanding patient populations requiring therapeutic intervention, with pharmaceutical companies investing heavily in novel mechanisms targeting metabolic dysfunction and fibrosis progression, demanding regulatory pathways for approval. Second, growing direct-acting antiviral accessibility and declining hepatitis C treatment costs drive global elimination initiatives, with numerous countries implementing national programs achieving significant reductions in chronic hepatitis C prevalence through widespread screening and treatment by 2030. Third, increasing hepatocellular carcinoma incidence and expanding systemic therapy options enable improved survival outcomes through targeted agents including sorafenib, lenvatinib, and immune checkpoint inhibitors demonstrating clinical benefit in advanced disease.
Market restraints include high treatment costs for novel therapies exceeding USD 50,000 for direct-acting antiviral regimens that can challenge patient access in low-resource settings, particularly in regions where healthcare budgets limit pharmaceutical expenditure and generic availability remains limited. Limited approved therapies for NASH and lack of regulatory precedents for non-cirrhotic NASH pose another significant obstacle, as pharmaceutical development faces challenges in endpoint selection and long-term efficacy demonstration, potentially delaying market entry for promising candidates. Diagnosis gaps and limited screening programs create additional barriers for market growth, with substantial proportions of patients with chronic liver disease remaining undiagnosed and untreated globally.
Key trends indicate accelerated NASH drug development in developed markets, particularly USA and Europe, where pharmaceutical companies demonstrate intense focus on metabolic liver disease programs with multiple late-stage candidates pursuing regulatory approval. Combination therapy approaches targeting multiple pathways simultaneously optimize treatment outcomes for complex liver diseases including combination antiviral regimens and multi-target NASH therapies. However, the market thesis could face disruption if significant safety signals emerge from NASH development programs or major prevention breakthroughs through vaccination eliminate primary disease drivers reducing long-term therapeutic demand.
Analysis of the Liver Disease Therapeutics Market by Key Countries
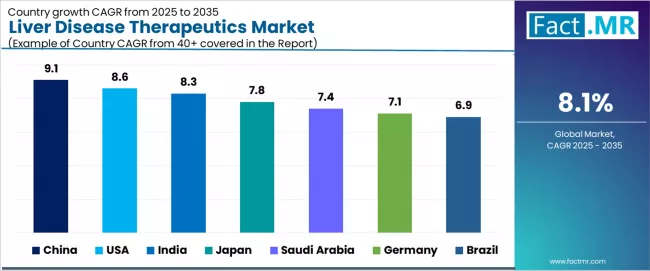 >
>
| Country | CAGR (2025 to 2035) |
|---|---|
| China | 9.1% |
| USA | 8.6% |
| India | 8.3% |
| Japan | 7.8% |
| Saudi Arabia | 7.4% |
| Germany | 7.1% |
| Brazil | 6.9% |
The global liver disease therapeutics market is expanding rapidly, with China leading at a 9.1% CAGR through 2035, driven by high hepatitis B carrier population of approximately 70 million patients, government-supported vaccination programs, and expanding treatment access initiatives. USA follows at 8.6%, supported by strong direct-acting antiviral adoption, newly approved NASH drugs, and comprehensive hepatology infrastructure. India records 8.3%, reflecting rapid NAFLD growth linked to diabetes and obesity epidemics affecting urban populations.
Japan advances at 7.8%, leveraging aging population demographics and strong hepatitis C screening programs. Saudi Arabia posts 7.4%, driven by highest NASH prevalence globally linked to diabetes rates, while Germany grows steadily at 7.1%, emphasizing strong diagnostic infrastructure and clinical trial activity. Brazil demonstrates 6.9% growth, anchored by alcohol-related liver disease prevalence and rising obesity trends.
What Makes China the Fastest Growing Market Globally?
China demonstrates the strongest growth potential in the liver disease therapeutics market with a CAGR of 9.1% through 2035. The country's leadership position stems from massive chronic hepatitis B carrier population estimated at 70 million patients, government-supported viral hepatitis elimination initiatives, and expanding pharmaceutical access through national insurance programs.
Growth is concentrated in major urban centers and provincial hospitals, including specialized infectious disease centers where hepatologists are increasingly prescribing direct-acting antivirals for hepatitis C and nucleotide analogs for hepatitis B suppression. Distribution channels through public hospital pharmacies, specialty drug programs, and expanding private healthcare networks improve medication accessibility across diverse patient populations. The country's comprehensive hepatitis B vaccination program for newborns combined with treatment scale-up for chronic carriers provides strong momentum for antiviral drug utilization, including growing adoption across rural areas through government healthcare initiatives.
Key market factors include massive hepatitis B endemic population concentrated in adult age groups requiring long-term antiviral suppression therapy, government elimination programs through National Health Commission supporting widespread screening, vaccination, and treatment initiatives with budget allocation, expanding pharmaceutical reimbursement through National Reimbursement Drug List inclusions enabling affordable access to branded and generic therapies, and domestic pharmaceutical development featuring Chinese companies developing generic direct-acting antivirals and hepatitis B therapies supporting cost-effective treatment scale-up.
How is USA Driving Market Innovation?
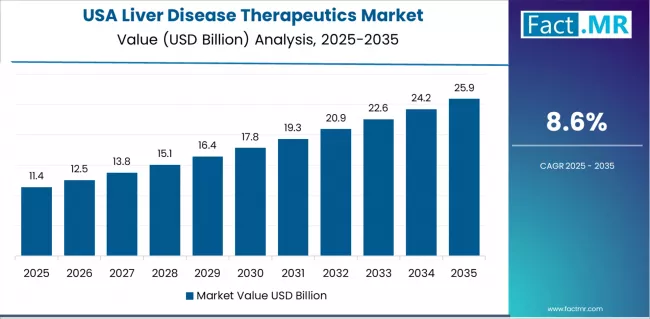
USA market expansion is driven by comprehensive hepatology infrastructure, including academic medical centers, community hepatology practices, and specialized liver transplant programs supporting advanced therapeutic interventions. The country demonstrates strong growth momentum with a CAGR of 8.6% through 2035, linked to extensive direct-acting antiviral utilization achieving hepatitis C elimination targets and emerging NASH therapeutic landscape with multiple regulatory approvals anticipated.
American hepatologists are implementing novel therapies for non-alcoholic steatohepatitis while maintaining established treatment protocols for viral hepatitis and hepatocellular carcinoma. The country's pharmaceutical innovation ecosystem creates ongoing opportunities for next-generation liver disease drugs, while comprehensive insurance coverage through Medicare, Medicaid, and commercial payers supports widespread treatment access.
Key development areas include NASH drug approvals expected through FDA regulatory pathways with multiple late-stage candidates addressing fibrosis and metabolic endpoints, hepatitis C elimination progress through public health programs and screening initiatives reducing chronic infection prevalence, hepatocellular carcinoma treatment advancement with expanding use of immune checkpoint inhibitors and combination systemic therapies, and comprehensive reimbursement frameworks supporting high-cost specialty pharmaceuticals through pharmacy benefit management and patient assistance programs.
What Drives India's Rapid Market Expansion?
India market growth is characterized by rapidly increasing non-alcoholic fatty liver disease prevalence driven by diabetes and obesity epidemics affecting urban populations. The country demonstrates meaningful growth potential with a CAGR of 8.3% through 2035, supported by expanding hepatology expertise, growing awareness about liver disease, and improving pharmaceutical access. Indian patients face rising metabolic liver disease burden alongside persistent viral hepatitis challenges, creating dual therapeutic demands. The country's large population and expanding middle-class healthcare spending support pharmaceutical market growth, while generic drug manufacturing capabilities enable cost-effective treatment access.
Market characteristics include NAFLD emergence as leading liver disease etiology in urban India driven by lifestyle factors and diabetes prevalence, hepatitis B and C persistence requiring ongoing antiviral therapy particularly in high-prevalence regions, generic pharmaceutical availability supporting affordable access to direct-acting antivirals and other liver disease medications, and expanding hepatology services through private hospitals and government medical colleges improving diagnosis and treatment capabilities.
How does Japan Demonstrate Aging Population Impact?
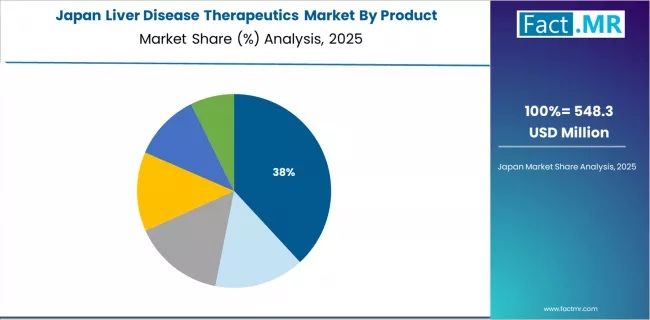
Japan's liver disease therapeutics market demonstrates mature healthcare system characteristics with emphasis on comprehensive screening and treatment programs addressing aging population healthcare needs. The country maintains steady growth momentum with a CAGR of 7.8% through 2035, driven by established viral hepatitis management programs and aging-related liver disease increase.
Japanese hepatologists implement advanced therapeutic protocols including direct-acting antivirals for hepatitis C elimination and comprehensive hepatocellular carcinoma management. The country's universal healthcare coverage and advanced diagnostic infrastructure support high treatment rates and comprehensive disease monitoring.
Key market characteristics include aging population demographics increasing liver disease prevalence including hepatocellular carcinoma in elderly patients, comprehensive hepatitis C screening and treatment programs achieving significant cure rates and reducing cirrhosis complications, established pharmaceutical market with both domestic and international drug access through national health insurance reimbursement, and advanced hepatology practice standards supporting optimal therapeutic outcomes and guideline-based treatment protocols.
What Positions Saudi Arabia for NASH Market Leadership?
Saudi Arabia demonstrates unique market dynamics with highest global NASH prevalence linked to extremely high diabetes rates affecting approximately 24% of adult population. The country shows strong growth potential with a CAGR of 7.4% through 2035, driven by metabolic disease epidemic creating massive non-alcoholic fatty liver disease burden. Saudi healthcare system transformation through Vision 2030 initiatives supports expanding specialty care including hepatology services and advanced therapeutic access. The country's wealthy population and comprehensive national health insurance create favorable conditions for pharmaceutical market development.
Key development factors include highest global NASH prevalence creating substantial patient population requiring therapeutic intervention, diabetes epidemic driving metabolic liver disease with approximately one-quarter of adults affected by diabetes mellitus, healthcare system modernization through government investment in tertiary care hospitals and specialty centers, and pharmaceutical access improvement through national formulary development and international drug registration supporting novel therapy availability.
How does Germany Demonstrate Clinical Excellence?
Germany leads European markets in diagnostic infrastructure and clinical trial activity supporting comprehensive liver disease management. The country shows steady growth potential with a CAGR of 7.1% through 2035, driven by sophisticated healthcare system and strong hepatology expertise across university hospitals and specialized practices.
German hepatologists implement evidence-based treatment protocols with emphasis on clinical guidelines and comprehensive patient monitoring. The country's pharmaceutical innovation ecosystem and clinical research leadership support advanced therapeutic development and rapid adoption of novel agents.
Leading market segments include university hospital hepatology departments implementing comprehensive liver disease management with emphasis on clinical trial participation, statutory health insurance providing universal coverage for approved liver disease therapeutics, clinical research leadership through academic institutions and pharmaceutical companies advancing novel therapy development, and established pharmaceutical market with rapid access to newly approved drugs through reimbursement pathways.
What Characterizes Brazil's Market Development?
Brazil demonstrates emerging market dynamics with liver disease burden driven by alcohol-related liver disease and rising obesity trends. The country shows meaningful growth potential with a CAGR of 6.9% through 2035, linked to expanding healthcare access through SUS public system and growing private insurance coverage.
Brazilian hepatologists are implementing modern treatment protocols while addressing significant alcohol-related liver disease prevalence. The country's pharmaceutical market development and domestic generic manufacturing support expanding therapeutic access across diverse patient populations.
Key development areas include alcohol-related liver disease representing significant disease burden in adult populations, obesity epidemic driving NAFLD growth in urban centers and higher socioeconomic groups, viral hepatitis programs through Ministry of Health supporting free direct-acting antiviral access for hepatitis C patients, and pharmaceutical market expansion through both domestic generic production and international brand availability supporting treatment accessibility.
Europe Market Split by Country
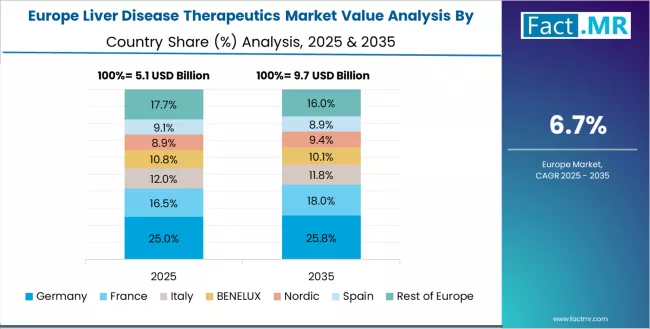
The liver disease therapeutics market in Europe is projected to grow from USD 5.97 billion in 2025 to USD 13.02 billion by 2035, registering a CAGR of 8.1% over the forecast period. Germany is expected to maintain its leadership position with a 28.5% market share in 2025, adjusting to 28.2% by 2035, supported by its extensive hepatology expertise, comprehensive university hospital networks, and strong pharmaceutical market infrastructure serving major European populations.
France follows with a 22.8% share in 2025, projected to reach 23.0% by 2035, driven by comprehensive viral hepatitis elimination programs and established hepatology services through academic medical centers implementing advanced treatment protocols. UK holds a 19.5% share in 2025, expected to maintain 19.7% by 2035 through NHS specialized hepatology commissioning and national viral hepatitis programs.
Italy commands a 15.2% share, while Spain accounts for 14.0% in 2025. The rest of Europe region is anticipated to maintain stable presence, with collective share remaining steady by 2035, attributed to consistent liver disease therapeutic adoption across Nordic countries and smaller European markets implementing evidence-based hepatology management programs.
Competitive Landscape of the Liver Disease Therapeutics Market
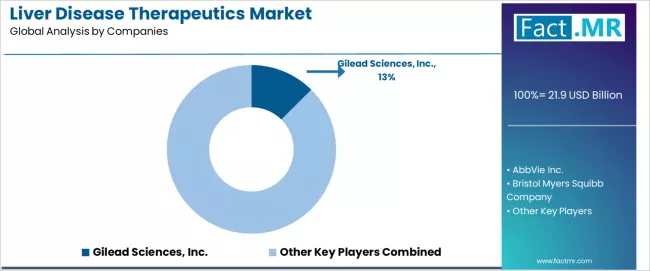
The liver disease therapeutics market features approximately 15-20 meaningful players with moderate concentration, where Gilead Sciences Inc. maintains 12.5% of the global market share through pioneering direct-acting antiviral portfolios, established hepatitis franchise, and comprehensive liver disease therapeutic development programs. Competition centers on therapeutic innovation, clinical evidence generation, and indication expansion rather than price competition alone in branded markets, with generic competition intensifying in off-patent segments.
Market leaders include Gilead Sciences, AbbVie, and Bristol-Myers Squibb, which maintain competitive advantages through comprehensive hepatitis C and hepatitis B franchises, established hepatology relationships, and deep expertise in antiviral drug development, creating high prescriber confidence among hepatologists seeking effective treatment solutions.
These companies leverage regulatory approval successes including pangenotypic direct-acting antiviral regimens and ongoing clinical development programs to defend market positions while expanding into adjacent indications including NASH and hepatocellular carcinoma applications.
Major pharmaceutical companies encompass F. Hoffmann-La Roche, Takeda Pharmaceutical, Johnson & Johnson, Merck & Co., Novartis, Pfizer, Sanofi, and GSK, which compete through diverse portfolios spanning viral hepatitis, hepatocellular carcinoma, and emerging NASH programs. These established players leverage global commercial infrastructure, comprehensive development capabilities, and broad therapeutic expertise across multiple liver disease indications.
Emerging biotechnology companies and specialty pharmaceutical developers create competitive pressure through novel mechanisms targeting NASH, primary biliary cholangitis, and hepatocellular carcinoma, particularly in high-growth therapeutic areas where unmet medical needs support premium pricing and favorable reimbursement.
Market dynamics favor companies combining clinical efficacy demonstration with favorable safety profiles addressing diverse liver disease etiologies and severity stages. Strategic emphasis on combination regimens, biomarker-guided patient selection, and real-world evidence generation enables differentiation in increasingly competitive hepatology pharmaceutical segments across developed and emerging markets.
Key Players in the Liver Disease Therapeutics Market
- Gilead Sciences, Inc.
- AbbVie Inc.
- Bristol Myers Squibb Company
- F. Hoffmann-La Roche Ltd.
- Takeda Pharmaceutical Company Limited
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- GSK plc
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 21.87 Billion |
| Product | Antiviral Drugs, Vaccines, Chemotherapy, Targeted Therapy, Immunosuppressants, Other Products |
| Disease | Hepatitis, Autoimmune Diseases, Non-alcoholic Fatty Liver Disease, Cancer, Genetic Disorders, Other Diseases |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, MEA |
| Country Covered | USA, China, India, Japan, Germany, Brazil, Saudi Arabia, and 40+ countries |
| Key Companies Profiled | Gilead Sciences, AbbVie, Bristol-Myers Squibb Company, F. Hoffmann-La Roche, Takeda Pharmaceutical Company, Johnson & Johnson, Merck & Co., Novartis, Pfizer, Sanofi, GSK |
| Additional Attributes | Dollar sales by product and disease categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with pharmaceutical manufacturers and biotechnology companies, therapeutic protocols and clinical guideline requirements, integration with hepatology practice networks and specialty pharmacy infrastructure, innovations in direct-acting antivirals and NASH therapeutics, and development of specialized applications with clinical validation and real-world evidence generation capabilities. |
Liver Disease Therapeutics Market by Segments
-
Product :
- Antiviral Drugs
- Vaccines
- Chemotherapy
- Targeted Therapy
- Immunosuppressants
- Other Products
-
Disease :
- Hepatitis
- Autoimmune Diseases
- Non-alcoholic Fatty Liver Disease (NAFLD)
- Cancer
- Genetic Disorders
- Other Diseases
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- South Korea
- Australia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- MEA
- Saudi Arabia
- UAE
- South Africa
- Rest of MEA
- North America
Table of Content
- Executive Summary
- USA Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- USA Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- USA Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2025 to 2035
- Antiviral Drugs
- Vaccines
- Chemotherapy
- Targeted Therapy
- Immunosuppressants
- Other Products
- Y to o to Y Growth Trend Analysis By Product, 2020 to 2024
- Absolute $ Opportunity Analysis By Product, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Disease
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Disease, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Disease, 2025 to 2035
- Hepatitis
- Autoimmune Diseases
- Non-alcoholic Fatty Liver Disease (NAFLD)
- Cancer
- Genetic Disorders
- Other Diseases
- Y to o to Y Growth Trend Analysis By Disease, 2020 to 2024
- Absolute $ Opportunity Analysis By Disease, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Europe
- Asia Pacific
- Latin America
- MEA
- Y to o to Y Growth Trend Analysis By Region, 2020 to 2024
- Absolute $ Opportunity Analysis By Region, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- USA
- Market Attractiveness Analysis By Region
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- By Product
- By Disease
- By Region
- Market Attractiveness Analysis
- By Country
- By Product
- By Disease
- By Region
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product
- By Disease
- By Region
- Competition Analysis
- Competition Deep Dive
- Gilead Sciences, Inc.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- AbbVie Inc.
- Bristol Myers Squibb Company
- F. Hoffmann-La Roche Ltd.
- Takeda Pharmaceutical Company Limited
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- GSK plc
- Gilead Sciences, Inc.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: USA Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 3: USA Market Value (USD Million) Forecast by Disease, 2020 to 2035
- Table 4: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 5: USA Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: USA Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 7: USA Market Value (USD Million) Forecast by Disease, 2020 to 2035
- Table 8: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
List Of Figures
- Figure 1: USA Market Pricing Analysis
- Figure 2: USA Market Value (USD Million) Forecast 2020-2035
- Figure 3: USA Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 4: USA Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 5: USA Market Attractiveness Analysis by Product
- Figure 6: USA Market Value Share and BPS Analysis by Disease, 2025 and 2035
- Figure 7: USA Market Y to o to Y Growth Comparison by Disease, 2025 to 2035
- Figure 8: USA Market Attractiveness Analysis by Disease
- Figure 9: USA Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: USA Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 11: USA Market Attractiveness Analysis by Region
- Figure 12: USA Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: USA Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: USA Market Attractiveness Analysis by Region
- Figure 15: USA Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: USA Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 17: USA Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 18: USA Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 19: USA Market Attractiveness Analysis by Product
- Figure 20: USA Market Value Share and BPS Analysis by Disease, 2025 and 2035
- Figure 21: USA Market Y to o to Y Growth Comparison by Disease, 2025 to 2035
- Figure 22: USA Market Attractiveness Analysis by Disease
- Figure 23: USA Market Value Share and BPS Analysis by Region, 2025 and 2035
- Figure 24: USA Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 25: USA Market Attractiveness Analysis by Region
- Figure 26: USA Market - Tier Structure Analysis
- Figure 27: USA Market - Company Share Analysis
- FAQs -
How big is the liver disease therapeutics market in 2025?
The global liver disease therapeutics market is estimated to be valued at USD 21.9 billion in 2025.
What will be the size of liver disease therapeutics market in 2035?
The market size for the liver disease therapeutics market is projected to reach USD 47.7 billion by 2035.
How much will be the liver disease therapeutics market growth between 2025 and 2035?
The liver disease therapeutics market is expected to grow at a 8.1% CAGR between 2025 and 2035.
What are the key product types in the liver disease therapeutics market?
The key product types in liver disease therapeutics market are antiviral drugs, vaccines, chemotherapy, targeted therapy, immunosuppressants and other products.
Which disease segment to contribute significant share in the liver disease therapeutics market in 2025?
In terms of disease, hepatitis segment to command 40.4% share in the liver disease therapeutics market in 2025.