Peptide Synthesis Market
Peptide Synthesis Market Size and Share Forecast Outlook 2025 to 2035
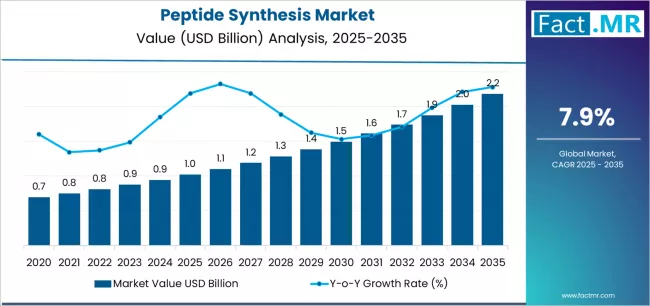
Peptide synthesis market is projected to grow from USD 1.0 billion in 2025 to USD 2.2 billion by 2035, at a CAGR of 7.9%. Reagents & Consumables will dominate with a 48.0% market share, while therapeutics will lead the application segment with a 70.4% share.
Peptide Synthesis Market Forecast and Outlook 2025 to 2035
The global peptide synthesis market is projected to reach USD 2.18 billion by 2035, recording an absolute increase of USD 1.16 billion over the forecast period. The market is valued at USD 1.02 billion in 2025 and is set to rise at a CAGR of 7.9% during the assessment period.
The market is expected to grow by approximately 2.1 times during the same period, supported by increasing demand for peptide-based therapeutics worldwide, driving consumption of specialized synthesis reagents and increasing investments in automated peptide manufacturing technologies with enhanced purity and yield across pharmaceutical, diagnostic, and research applications globally.
Quick Stats for Peptide Synthesis Market
- Peptide Synthesis Market Value (2025): USD 1.02 billion
- Peptide Synthesis Market Forecast Value (2035): USD 2.18 billion
- Peptide Synthesis Market Forecast CAGR: 7.9%
- Leading Product Type in Peptide Synthesis Market: Reagents & Consumables (48.0%)
- Key Growth Regions in Peptide Synthesis Market: Asia Pacific, North America, and Europe
- Top Players in Peptide Synthesis Market: Thermo Fisher Scientific, Merck KGaA, GenScript, Bachem Holding, Biotage, Creative Diagnostics, PolyPeptide Group, Syngene International, Puresynth Research Chemicals, Lonza
Biopharmaceutical companies face mounting pressure to develop novel peptide drugs and optimize manufacturing efficiency while addressing scalability challenges and regulatory quality requirements, with modern peptide synthesis products providing documented performance benefits including improved coupling efficiency, reduced synthesis time, and enhanced peptide purity compared to conventional chemical synthesis approaches alone.
Rising awareness about peptide therapeutics advantages including target specificity and expanding contract development and manufacturing organization services enabling broader access create substantial opportunities for reagent suppliers and technology providers. However, high synthesis costs and technical complexity across markets may pose obstacles to widespread adoption in cost-sensitive applications.
The reagents & consumables segment dominates market activity, driven by essential utilization in both solid-phase and liquid-phase peptide synthesis processes and recurring consumption patterns across pharmaceutical manufacturing worldwide. Research institutions and biopharmaceutical companies increasingly recognize the critical importance of high-quality synthesis reagents, with typical product offerings providing reliable peptide assembly and purification capabilities at competitive price points through established chemical supply networks.
The resins segment demonstrates robust presence, supported by fundamental role as solid supports in SPPS methodologies requiring specialized functionalized polymers. Therapeutics emerge as the dominant application segment, reflecting the substantial growth of peptide-based drugs including GLP-1 receptor agonists, oncology peptides, and metabolic disorder treatments. Liquid phase peptide synthesis represents the leading technology approach, driven by industrial-scale manufacturing advantages and cost-effectiveness for large peptide production.
Regional dynamics show North America maintaining market leadership, supported by extensive pharmaceutical R&D infrastructure and established peptide drug development pipelines across major biopharmaceutical hubs. Asia Pacific demonstrates the fastest growth trajectory driven by rapidly expanding contract manufacturing capabilities and increasing biosimilar peptide production, while Europe emphasizes advanced biotechnology research and regulatory excellence in peptide therapeutics. India leads country-level growth through expanding biopharma manufacturing infrastructure and peptide CDMO sector development, followed by China supported by automation investments and industrial peptide scale-up initiatives.
The competitive landscape features moderate concentration with Thermo Fisher Scientific maintaining market leadership position at approximately 11.2% market share, while specialized players including Merck KGaA, GenScript, and Bachem Holding compete through targeted technology innovations and comprehensive peptide synthesis solutions across diverse application portfolios.
Peptide Synthesis Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 1.02 billion |
| Market Forecast Value (2035) | USD 2.18 billion |
| Forecast CAGR (2025-2035) | 7.9% |
Why is the Peptide Synthesis Market Growing?
The peptide synthesis market grows by enabling pharmaceutical companies and research institutions to develop and manufacture peptide-based therapeutics while addressing drug development timelines and manufacturing scalability without exclusive reliance on biological expression systems.
Biopharmaceutical organizations face mounting pressure to advance peptide drug candidates and meet commercial production requirements while managing synthesis complexity and quality standards, with modern peptide synthesis technologies typically providing targeted capabilities including automated assembly, high-purity production, and scalable manufacturing compared to traditional recombinant approaches alone, making chemical synthesis essential for comprehensive peptide drug development and production protocols.
Rising peptide therapeutics approvals and increasing clinical pipeline activity create expanding consumption opportunities for synthesis reagents and services, with peptide drugs representing critical therapeutic modalities across oncology, metabolic disorders, and infectious diseases requiring reliable manufacturing capabilities.
Growing demand for GLP-1 receptor agonists and other blockbuster peptide drugs drives large-scale synthesis requirements, with pharmaceutical companies demonstrating significant investment in peptide manufacturing capacity and technology advancement. Increasing contract manufacturing adoption and outsourcing of peptide synthesis enables market expansion for CDMO providers offering specialized capabilities that support pharmaceutical development while reducing capital investment requirements for drug developers.
Segmental Analysis
The market is segmented by product, application, and technology. By product, the market is divided into reagents & consumables, resins, amino acids, coupling reagents, and others. Based on application, the market is categorized into therapeutics, diagnosis, research, and others. By technology, the market includes liquid phase peptide synthesis, solid phase peptide synthesis, and hybrid technology.
What Makes Reagents & Consumables the Leading Product Category?
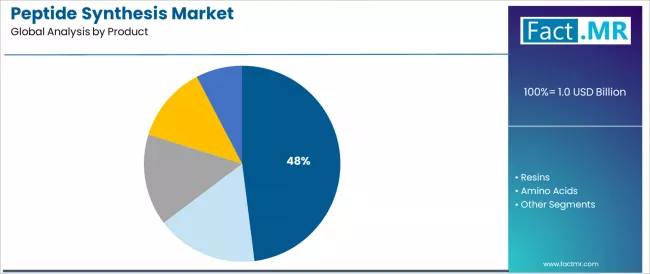
The reagents & consumables segment represents the dominant force in the peptide synthesis market, capturing 48.0% of the total market share in 2025. This established product category encompasses essential chemical reagents and consumable materials featuring critical synthesis functionality and recurring consumption patterns, including coupling reagents, deprotection agents, cleavage cocktails, and purification consumables that enable reliable peptide assembly and isolation across both solid-phase and liquid-phase synthesis methodologies worldwide.
The reagents & consumables segment's market leadership stems from its indispensable role in every peptide synthesis workflow, with products capable of providing efficient amino acid coupling, selective protecting group removal, and high-purity peptide recovery while maintaining consistent quality standards and broad compatibility across diverse synthesis scales and peptide sequences.
The resins segment maintains substantial market presence at approximately 18.0%, serving solid-phase peptide synthesis applications requiring specialized polymer supports including polystyrene, polyethylene glycol, and controlled pore glass resins functionalized with various linker chemistries for peptide assembly and cleavage.
These products offer critical solid support functionality enabling sequential amino acid addition while providing mechanical stability and chemical compatibility. The resins segment demonstrates steady demand driven by SPPS methodology prevalence and ongoing resin technology advancement.
The amino acids segment accounts for approximately 14.5% market share, providing protected amino acid building blocks essential for peptide chain assembly, while coupling reagents represent 9.3% serving critical activation functions in peptide bond formation. Others comprise 10.2%, encompassing specialty reagents, solvents, and auxiliary materials.
Key commercial advantages driving the reagents & consumables segment include essential functionality across all peptide synthesis methodologies with demonstrated performance in coupling efficiency and purity outcomes, recurring consumption patterns providing stable revenue streams and customer retention across pharmaceutical and research applications, broad product portfolios enabling comprehensive synthesis workflow support from assembly through purification stages, and continuous innovation in reagent chemistry supporting improved synthesis efficiency and reducing side reactions while maintaining cost-effectiveness standards.
Which is the Dominant Application Area for Peptide Synthesis?
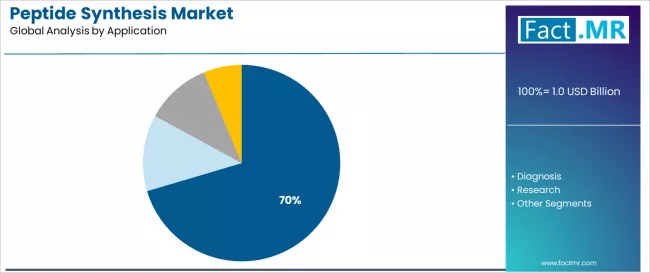
Therapeutics represent the dominant application segment in the peptide synthesis market with a 70.4% market share in 2025, reflecting the fundamental importance of peptide-based drugs in modern pharmaceutical development and the substantial synthesis requirements for clinical and commercial manufacturing.
The therapeutics segment demonstrates robust demand driven by expanding peptide drug approvals, growing clinical pipelines across multiple therapeutic areas, and blockbuster peptide medications including GLP-1 receptor agonists, oncology peptides, and hormonal therapies requiring large-scale manufacturing capabilities.
The diagnosis segment emerges as an important application category with approximately 15.0% market share, demonstrating growth potential driven by peptide-based diagnostic assays, biomarker detection applications, and point-of-care testing platforms requiring synthetic peptide antigens and calibrators. Diagnostic peptides serve critical roles in immunoassays, mass spectrometry standards, and molecular diagnostic applications.
The research segment accounts for approximately 8.2% market share, serving academic institutions, biotechnology companies, and pharmaceutical research laboratories requiring custom peptides for drug discovery, target validation, and biological studies. Others represent 6.5%, encompassing cosmetic peptides, agricultural applications, and industrial uses.
Key application dynamics include therapeutics requirements accelerating across expanding peptide drug development with emphasis on complex sequences and modified peptides requiring advanced synthesis capabilities, diagnostic applications driving demand for high-purity synthetic antigens and standardized peptide reagents supporting clinical laboratory testing, research utilization prioritizing custom synthesis services and diverse peptide libraries enabling drug discovery and biological investigation, and emerging applications expanding through peptide-drug conjugates, peptide vaccines, and novel therapeutic modalities requiring specialized synthesis expertise.
What Positions Liquid Phase Peptide Synthesis as the Leading Technology?
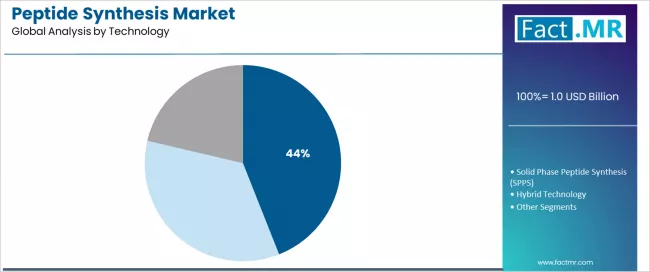
Liquid phase peptide synthesis represents the dominant technology segment in the peptide synthesis market with a 44.0% market share in 2025, reflecting the critical advantages of solution-phase chemistry for industrial-scale peptide manufacturing and cost-effectiveness considerations. The LPPS segment demonstrates leadership through proven scalability, economical reagent utilization, and suitability for large peptide and small protein production where manufacturing economics favor liquid-phase methodologies.
Solid phase peptide synthesis maintains substantial market presence at approximately 32.2%, serving applications requiring high-purity peptides, complex sequences, and automated synthesis workflows where resin-bound chemistry provides advantages in purification and process control. SPPS technology benefits from automation compatibility, simplified purification workflows, and versatility across diverse peptide sequences.
Hybrid technology accounts for approximately 23.8% market share, representing convergent synthesis strategies combining both SPPS and LPPS methodologies to optimize manufacturing efficiency, purity outcomes, and cost structures for specific peptide targets.
What are the Drivers, Restraints, and Key Trends of the Peptide Synthesis Market?
The market is driven by three concrete demand factors tied to pharmaceutical outcomes. First, rising peptide therapeutics development and increasing FDA approvals create expanding consumption opportunities for synthesis reagents and manufacturing services, with peptide drugs representing rapidly growing pharmaceutical segments including metabolic disorders, oncology, and rare diseases requiring reliable synthesis capabilities, demanding widespread technology availability. Second, growing blockbuster peptide drug commercialization including GLP-1 receptor agonists drives large-scale manufacturing requirements, with pharmaceutical companies demonstrating significant investment in peptide production capacity and advanced synthesis technologies supporting multi-ton annual production by 2030. Third, increasing contract manufacturing adoption and pharmaceutical outsourcing enable market expansion for peptide CDMO providers offering specialized synthesis expertise that reduces drug development timelines while optimizing manufacturing costs across diverse peptide drug candidates.
Market restraints include high synthesis costs and reagent expenses that can challenge economic viability for complex peptide sequences, particularly for long peptides where cumulative reagent consumption and multiple synthesis steps significantly impact manufacturing economics and commercial feasibility. Technical complexity of peptide synthesis and specialized expertise requirements pose another significant obstacle, as efficient peptide manufacturing depends on experienced chemists, optimized protocols, and sophisticated purification technologies, potentially limiting production capacity and quality consistency. Regulatory compliance complexity and stringent quality requirements for pharmaceutical peptides create additional barriers for market expansion, demanding comprehensive analytical characterization, impurity profiling, and validation documentation supporting regulatory submissions.
Key trends indicate accelerated automation adoption in developed markets, particularly North America and Europe, where pharmaceutical companies demonstrate willingness to invest in advanced peptide synthesizers and robotic purification systems that optimize throughput and reproducibility. Continuous manufacturing integration trends toward flow chemistry and automated synthesis platforms enable real-time monitoring and process control optimizing synthesis efficiency and product quality. However, the market thesis could face disruption if significant breakthrough technologies in biological peptide production or major advances in recombinant expression systems provide cost-effective alternatives to chemical synthesis for specific peptide drug classes.
Analysis of the Peptide Synthesis Market by Key Countries
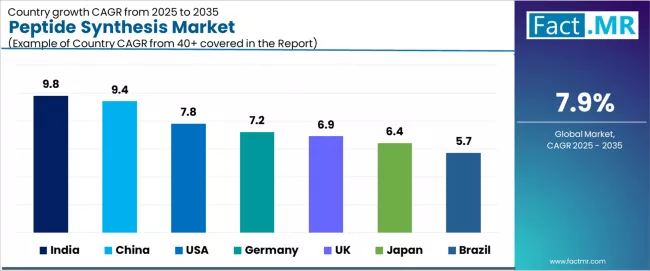
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 9.8% |
| China | 9.4% |
| USA | 7.8% |
| Germany | 7.2% |
| UK | 6.9% |
| Japan | 6.4% |
| Brazil | 5.7% |
The global peptide synthesis market is expanding steadily, with India leading at a 9.8% CAGR through 2035, driven by expanding biopharma manufacturing infrastructure, peptide CDMO sector growth, and increasing pharmaceutical outsourcing to Indian contract manufacturers. China follows at 9.4%, supported by automation investments in peptide production, industrial peptide scale-up capabilities, and government support for biopharmaceutical manufacturing. USA records 7.8%, reflecting strong pharma R&D ecosystem, GLP-1 drug expansion, and established peptide therapeutics development pipelines.
Germany advances at 7.2%, leveraging leading biotech research infrastructure and regulatory support for peptide drug development. UK posts 6.9%, focusing on increased peptide-focused venture funding and academic-industry collaboration, while Japan grows steadily at 6.4%, emphasizing aging population driving peptide therapeutics demand. Brazil demonstrates 5.7% growth, anchored by regional expansion of peptide diagnostic markets and pharmaceutical manufacturing development.
What Makes India the Fastest Growing Market Globally?
India demonstrates the strongest growth potential in the peptide synthesis market with a CAGR of 9.8% through 2035. The country's leadership position stems from rapidly expanding biopharma manufacturing infrastructure, growing peptide CDMO sector, and increasing pharmaceutical outsourcing from Western companies seeking cost-effective synthesis capabilities.
Growth is concentrated in major pharmaceutical clusters including Hyderabad, Ahmedabad, Bangalore, and Mumbai, where contract manufacturing organizations are increasingly implementing advanced peptide synthesis technologies supporting global pharmaceutical supply chains. Distribution channels through specialized chemical suppliers, direct manufacturer relationships, and international trading networks expand reagent accessibility across pharmaceutical manufacturers and research institutions.
The country's growing emphasis on biosimilar peptide development and API manufacturing provides strong momentum for peptide synthesis capability building, including comprehensive adoption across generic pharmaceutical companies entering peptide drug markets.
Key market factors include expanding peptide CDMO infrastructure concentrated in pharmaceutical manufacturing zones with rising capabilities in custom synthesis and commercial production, pharmaceutical outsourcing growth through Western companies partnering with Indian manufacturers for cost-effective peptide API production, regulatory advancement through improved GMP compliance and international certification supporting pharmaceutical export capabilities, and domestic pharmaceutical development featuring Indian companies including Syngene International and Puresynth Research Chemicals establishing peptide synthesis expertise while serving global customers.
How is China Emerging as a High-Growth Market?
In major biopharmaceutical hubs including Shanghai, Beijing, Suzhou, and Wuxi, the adoption of advanced peptide synthesis technologies is accelerating across contract manufacturing organizations and pharmaceutical companies, driven by automation investments and emphasis on industrial-scale peptide production capabilities. The market demonstrates strong growth momentum with a CAGR of 9.4% through 2035, linked to comprehensive biopharmaceutical sector development and government support for pharmaceutical manufacturing modernization.
Peptide manufacturers are implementing automated synthesis platforms and continuous manufacturing technologies to enhance production efficiency while meeting international pharmaceutical quality standards. The country's strategic focus on biopharmaceutical self-sufficiency creates ongoing demand for peptide synthesis capability expansion, while increasing technology development drives adoption of domestically produced synthesis equipment and reagents.
Key development areas include contract manufacturing organizations leading peptide synthesis capacity expansion with emphasis on large-scale production and pharmaceutical outsourcing capture, automation technology investment through advanced peptide synthesizers and robotic purification systems enabling high-throughput manufacturing, quality system advancement supporting international regulatory compliance and pharmaceutical customer requirements, and domestic market growth alongside export orientation serving both Chinese pharmaceutical development and global CDMO customers requiring cost-competitive synthesis services.
What drives USA’s Market Leadership?
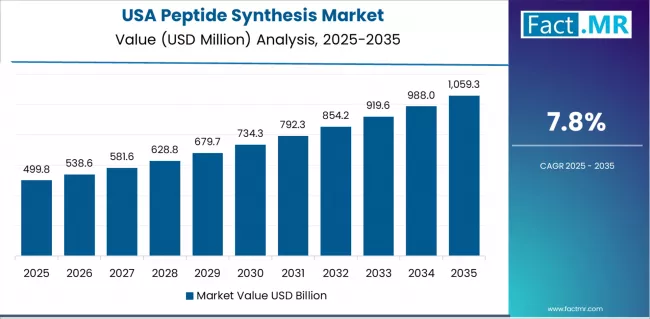
USA’s market expansion is driven by robust pharmaceutical R&D ecosystem, including extensive peptide drug development pipelines in biotechnology companies and academic research institutions. The country demonstrates steady growth potential with a CAGR of 7.8% through 2035, supported by continuous innovation from established pharmaceutical companies and specialized peptide therapeutics developers.
American pharmaceutical industry faces favorable conditions related to strong venture capital funding for peptide drug development and expanding commercial manufacturing requirements for blockbuster peptide medications including GLP-1 receptor agonists. Established peptide synthesis expertise and comprehensive reagent supply infrastructure create stable baseline demand, particularly in pharmaceutical development applications where custom synthesis services and high-purity reagents drive primary purchasing decisions.
Market characteristics include pharmaceutical and biotechnology companies showing robust peptide synthesis reagent consumption with substantial R&D expenditure across therapeutic development, regional concentration in major biopharma clusters including Boston-Cambridge, San Francisco Bay Area, and San Diego supporting dense networks of peptide synthesis service providers, future projections indicate continued innovation emphasis on novel peptide modalities including peptide-drug conjugates and constrained peptides requiring advanced synthesis capabilities, and growing commercial manufacturing scale supporting large-volume reagent consumption for approved peptide therapeutics production.
How Does Germany Demonstrate Biotechnology Research Leadership?
The Germany market leads in advanced biotech research and peptide drug development based on integration with university research institutions and pharmaceutical companies emphasizing innovation excellence. The country shows strong potential with a CAGR of 7.2% through 2035, driven by sophisticated biotechnology infrastructure and government support for life sciences research and development.
German research institutions and pharmaceutical companies are adopting peptide synthesis technologies through comprehensive academic-industry collaboration and regulatory frameworks supporting peptide therapeutics development, particularly in specialized research centers and biotechnology companies demanding rigorous quality standards. Distribution channels through established chemical suppliers and direct manufacturer relationships expand reagent coverage across research institutions and pharmaceutical development organizations.
Leading market segments include academic research institutions implementing peptide synthesis for drug discovery and biological studies with emphasis on custom peptide libraries, pharmaceutical companies partnering with peptide synthesis providers achieving integrated development capabilities from discovery through clinical manufacturing, biotechnology startups focusing on novel peptide therapeutics driving demand for specialized synthesis services and advanced technologies, and regulatory excellence supporting peptide drug development through comprehensive quality frameworks and scientific expertise.
What Positions UK for Venture-Funded Innovation Leadership?
UK market expansion is characterized by increased peptide-focused venture funding and growing biotechnology sector activity supporting peptide therapeutics development. The country shows steady growth potential with a CAGR of 6.9% through 2035, linked to strong academic research base and emerging peptide therapeutics companies attracting investment capital.
The research institutions and biotechnology startups are implementing peptide synthesis for novel drug development while meeting growing expectations for innovative therapeutic modalities. The country's established pharmaceutical industry and specialty chemical sector create ongoing opportunities for peptide synthesis reagent supply and contract manufacturing services supporting drug development pipelines.
Market development factors include venture capital investment supporting peptide therapeutics startups with emphasis on novel drug candidates and technology platforms, academic research excellence through universities including Cambridge, Oxford, and Imperial College driving peptide science advancement, contract research organizations providing peptide synthesis services supporting pharmaceutical development outsourcing, and pharmaceutical industry presence including major companies maintaining UK-based peptide research and development operations.
What Characterizes Japan's Market Development?
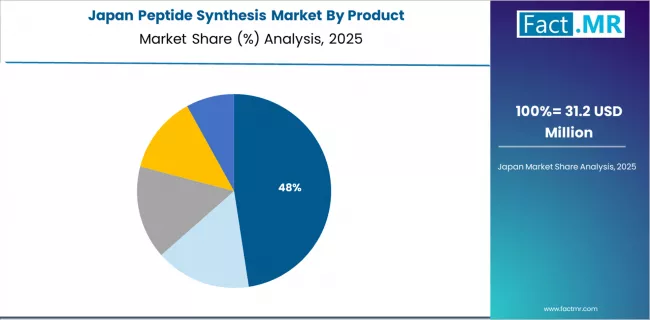
Japan's peptide synthesis market demonstrates mature pharmaceutical infrastructure focused on therapeutic development for aging population healthcare needs, with documented peptide drug utilization across metabolic disorders, oncology, and age-related conditions.
The country maintains steady growth momentum with a CAGR of 6.4% through 2035, driven by established pharmaceutical industry and emphasis on peptide therapeutics addressing demographic healthcare challenges. Major pharmaceutical companies in Tokyo, Osaka, and other regions showcase peptide synthesis integration where internal capabilities combine with contract manufacturing partnerships supporting drug development and commercial production requirements.
Key market characteristics include pharmaceutical companies driving peptide synthesis reagent demand for internal R&D and manufacturing operations, aging population creating therapeutic needs addressable through peptide drugs including metabolic disorder treatments and cancer therapies, quality-focused market emphasizing high-purity reagents and rigorous synthesis standards aligned with Japanese pharmaceutical manufacturing requirements, and technology adoption in automated synthesis platforms and advanced purification systems supporting pharmaceutical quality objectives.
What drives Brazil's Regional Market Expansion?
Brazil demonstrates meaningful growth potential with a CAGR of 5.7% through 2035, driven by regional expansion of peptide diagnostic markets and growing pharmaceutical manufacturing sector. Brazilian research institutions and pharmaceutical companies are implementing peptide synthesis capabilities for diagnostic reagent production and pharmaceutical development applications.
The country's largest Latin American market position creates strategic importance for peptide synthesis reagent supply and service provider expansion. Market development requires addressing infrastructure challenges and expanding technical expertise supporting peptide synthesis adoption across research and commercial applications.
Key development areas include diagnostic market expansion driving peptide antigen and calibrator synthesis requirements, pharmaceutical sector growth including biosimilar development and generic peptide drug opportunities, research institution adoption supporting academic peptide science and drug discovery programs, and regional supply chain development through local reagent distributors and contract manufacturing service providers establishing peptide synthesis capabilities.
Europe Market Split by Country
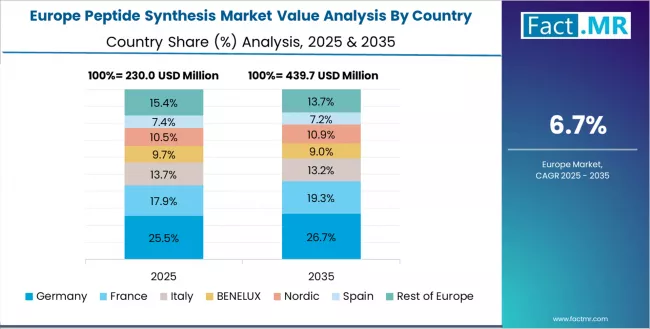
The peptide synthesis market in Europe is projected to grow from USD 0.26 billion in 2025 to USD 0.54 billion by 2035, registering a CAGR of 7.5% over the forecast period. Germany is expected to maintain its leadership position with a 29.8% market share in 2025, adjusting to 29.5% by 2035, supported by its extensive biotechnology research infrastructure, pharmaceutical industry integration, and comprehensive peptide therapeutics development capabilities serving major European markets.
UK follows with a 22.5% share in 2025, projected to reach 22.8% by 2035, driven by venture capital-funded peptide therapeutics companies and academic research excellence in major university centers implementing innovative peptide drug discovery programs. France holds a 19.2% share in 2025, expected to maintain 19.5% by 2035 through ongoing pharmaceutical industry development and expanding biotechnology sector activity.
Italy commands a 14.8% share, while Spain accounts for 13.7% in 2025. The rest of Europe region is anticipated to maintain stable presence, with collective share from 0% to 0% by 2035, attributed to steady peptide synthesis adoption in Nordic countries and smaller European markets implementing peptide research and pharmaceutical development programs.
Competitive Landscape of the Peptide Synthesis Market
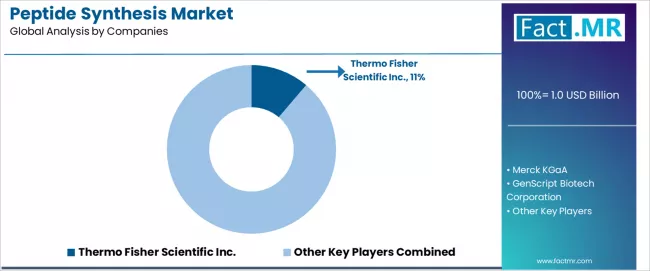
The peptide synthesis market features approximately 15-20 meaningful players with moderate concentration, where Thermo Fisher Scientific Inc. maintains an 11.2% of global market share through extensive product portfolios, global distribution networks, and comprehensive peptide synthesis reagent offerings. Competition centers on product quality, technical support capabilities, and reagent innovation rather than price competition alone.
Market leaders include Thermo Fisher Scientific, Merck KGaA, and GenScript, which maintain competitive advantages through broad peptide synthesis product ranges, established pharmaceutical customer relationships, and deep expertise in peptide chemistry and manufacturing support, creating high customer confidence among pharmaceutical companies and research institutions seeking reliable reagent supply and technical expertise.
These companies leverage global supply chains and ongoing product development initiatives to defend market positions while expanding service offerings including custom peptide synthesis and contract manufacturing capabilities.
Specialized peptide companies encompass Bachem Holding, PolyPeptide Group, and Lonza, which compete through dedicated peptide focus, comprehensive CDMO services, and pharmaceutical-grade manufacturing capabilities serving peptide drug development and commercial production.
Technology providers including Biotage focus on automated synthesis equipment and purification systems, while regional players including Syngene International, Puresynth Research Chemicals, and Creative Diagnostics offer specialized capabilities in contract synthesis, custom reagents, and diagnostic peptides.
Emerging biotechnology companies and specialized reagent manufacturers create competitive pressure through innovative synthesis technologies and application-specific product development, particularly in high-growth markets including India and China, where expanding pharmaceutical manufacturing provides advantages in market access and cost positioning.
Market dynamics favor companies that combine product quality with comprehensive technical support and flexible manufacturing capabilities addressing complete workflows from research-scale synthesis through commercial pharmaceutical production. Strategic emphasis on automation solutions, novel reagent chemistry, and integrated service offerings enables differentiation in increasingly competitive peptide synthesis markets across pharmaceutical development and manufacturing applications.
Key Players in the Peptide Synthesis Market
- Thermo Fisher Scientific Inc.
- Merck KGaA
- GenScript Biotech Corporation
- Bachem Holding AG
- Biotage AB
- Creative Diagnostics LLC
- PolyPeptide Group AG
- Syngene International Ltd.
- Puresynth Research Chemicals Pvt. Ltd.
- Lonza Group Ltd.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 1.02 Billion |
| Product | Reagents & Consumables, Resins, Amino Acids, Coupling Reagents, Others |
| Application | Therapeutics, Diagnosis, Research, Others |
| Technology | Liquid Phase Peptide Synthesis, Solid Phase Peptide Synthesis, Hybrid Technology |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Country Covered | India, China, USA, Germany, UK, Japan, Brazil, and 40+ countries |
| Key Companies Profiled | Thermo Fisher Scientific, Merck KGaA, GenScript, Bachem Holding, Biotage, Creative Diagnostics, PolyPeptide Group, Syngene International, Puresynth Research Chemicals, Lonza |
| Additional Attributes | Dollar sales by product and application categories, regional adoption trends across Asia Pacific, North America, and Europe, competitive landscape with chemical reagent suppliers and contract manufacturing organizations, product specifications and synthesis protocol requirements, integration with pharmaceutical development and commercial manufacturing, innovations in automated synthesis and purification technologies, and development of specialized applications with quality assurance and regulatory compliance capabilities. |
Peptide Synthesis Market by Segments
-
Product :
- Reagents & Consumables
- Resins
- Amino Acids
- Coupling Reagents
- Others
-
Application :
- Therapeutics
- Diagnosis
- Research
- Others
-
Technology :
- Liquid Phase Peptide Synthesis (LPPS)
- Solid Phase Peptide Synthesis (SPPS)
- Hybrid Technology
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- India
- China
- Japan
- South Korea
- Australia
- Rest of Asia Pacific
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- USA Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- USA Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- USA Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Product
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Product, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Product, 2025 to 2035
- Reagents & Consumables
- Resins
- Amino Acids
- Coupling Reagents
- Others
- Y to o to Y Growth Trend Analysis By Product, 2020 to 2024
- Absolute $ Opportunity Analysis By Product, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- Therapeutics
- Diagnosis
- Research
- Others
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Technology
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Technology, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Technology, 2025 to 2035
- Liquid Phase Peptide Synthesis (LPPS)
- Solid Phase Peptide Synthesis (SPPS)
- Hybrid Technology
- Y to o to Y Growth Trend Analysis By Technology, 2020 to 2024
- Absolute $ Opportunity Analysis By Technology, 2025 to 2035
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- USA
- Market Attractiveness Analysis By Region
- USA Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- By Product
- By Application
- By Technology
- Market Attractiveness Analysis
- By Country
- By Product
- By Application
- By Technology
- Key Takeaways
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Product
- By Application
- By Technology
- Competition Analysis
- Competition Deep Dive
- Thermo Fisher Scientific Inc.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Merck KGaA
- GenScript Biotech Corporation
- Bachem Holding AG
- Biotage AB
- Creative Diagnostics LLC
- PolyPeptide Group AG
- Syngene International Ltd.
- Puresynth Research Chemicals Pvt. Ltd.
- Lonza Group Ltd.
- Thermo Fisher Scientific Inc.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: USA Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: USA Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 3: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: USA Market Value (USD Million) Forecast by Technology, 2020 to 2035
- Table 5: USA Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: USA Market Value (USD Million) Forecast by Product, 2020 to 2035
- Table 7: USA Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 8: USA Market Value (USD Million) Forecast by Technology, 2020 to 2035
List Of Figures
- Figure 1: USA Market Pricing Analysis
- Figure 2: USA Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: USA Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 4: USA Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 5: USA Market Attractiveness Analysis by Product
- Figure 6: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: USA Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 8: USA Market Attractiveness Analysis by Application
- Figure 9: USA Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 10: USA Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 11: USA Market Attractiveness Analysis by Technology
- Figure 12: USA Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: USA Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: USA Market Attractiveness Analysis by Region
- Figure 15: USA Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: USA Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 17: USA Market Value Share and BPS Analysis by Product, 2025 and 2035
- Figure 18: USA Market Y to o to Y Growth Comparison by Product, 2025 to 2035
- Figure 19: USA Market Attractiveness Analysis by Product
- Figure 20: USA Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 21: USA Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 22: USA Market Attractiveness Analysis by Application
- Figure 23: USA Market Value Share and BPS Analysis by Technology, 2025 and 2035
- Figure 24: USA Market Y to o to Y Growth Comparison by Technology, 2025 to 2035
- Figure 25: USA Market Attractiveness Analysis by Technology
- Figure 26: USA Market - Tier Structure Analysis
- Figure 27: USA Market - Company Share Analysis
- FAQs -
How big is the peptide synthesis market in 2025?
The global peptide synthesis market is estimated to be valued at USD 1.0 billion in 2025.
What will be the size of peptide synthesis market in 2035?
The market size for the peptide synthesis market is projected to reach USD 2.2 billion by 2035.
How much will be the peptide synthesis market growth between 2025 and 2035?
The peptide synthesis market is expected to grow at a 7.9% CAGR between 2025 and 2035.
What are the key product types in the peptide synthesis market?
The key product types in peptide synthesis market are reagents & consumables, resins, amino acids, coupling reagents and others.
Which application segment to contribute significant share in the peptide synthesis market in 2025?
In terms of application, therapeutics segment to command 70.4% share in the peptide synthesis market in 2025.