Traumatic Brain Injury Biomarkers Market
Traumatic Brain Injury Biomarkers Market Size and Share Forecast Outlook 2026 to 2036
Traumatic brain injury biomarkers market is projected to grow from USD 1.2 billion in 2026 to USD 6.7 billion by 2036, at a CAGR of 18.9%. Protein Biomarkers will dominate with a 55.7% market share, while blood-based will lead the sample type segment with a 63.7% share.
Traumatic Brain Injury Biomarkers Market Forecast and Outlook 2026 to 2036
The global traumatic brain injury biomarkers market is projected to grow from USD 1.19 billion in 2026 to approximately USD 6.7 billion by 2036, recording an extraordinary absolute increase of USD 5.51 billion over the forecast period. This translates into a total growth of 463.0%, with the market forecast to expand at a compound annual growth rate (CAGR) of 18.9% between 2026 and 2036.
Quick Stats on Traumatic Brain Injury Biomarkers Market
- Traumatic Brain Injury Biomarkers Market Value (2026): USD 1.19 billion
- Traumatic Brain Injury Biomarkers Market Forecast Value (2036): USD 6.7 billion
- Traumatic Brain Injury Biomarkers Market Forecast CAGR (2026 to 2036): 18.9%
- Leading Type in Traumatic Brain Injury Biomarkers Market: Protein Biomarkers (55.73%)
- Leading Application in Traumatic Brain Injury Biomarkers Market: Diagnosis (56.26%)
- Leading Sample Type in Traumatic Brain Injury Biomarkers Market: Blood-based (63.70%)
- Leading End Use in Traumatic Brain Injury Biomarkers Market: Research Institutes (38.70%)
- Key Growth Regions in Traumatic Brain Injury Biomarkers Market: North America, Europe, and Asia Pacific
- Key Players in Traumatic Brain Injury Biomarkers Market: Quanterix, Abbott, Siemens Healthineers, GE Healthcare, Koninklijke Philips N.V., Thermo Fisher Scientific, BioMérieux, Banyan Biomarkers, Oculogica, Randox

Demand for traumatic brain injury biomarkers is expected to grow by over 5.6X during this period, supported by exponential demand for rapid diagnostic solutions, rising adoption of blood-based biomarker testing protocols, and growing emphasis on concussion management and neurological assessment optimization across global healthcare operations.
The traumatic brain injury biomarkers market is positioned for substantial expansion, driven by increasing recognition of objective brain injury assessment importance, growing sports-related concussion concerns with enhanced safety standards, and rising adoption of point-of-care testing technologies across emergency departments and clinical settings globally.
The traumatic brain injury biomarkers market demonstrates robust fundamentals supported by expanding trauma care networks, neurological healthcare professionals' focus on evidence-based diagnostic protocols, and rising recognition of TBI biomarkers as critical diagnostic components in achieving enhanced patient triage outcomes, CT scan reduction capabilities, and neurological injury management effectiveness within modern healthcare architectures across diverse medical care applications.
Growth is underpinned by technological innovations in biomarker detection methodologies, particularly advanced protein biomarker assays and blood-based testing system integration, which offer enhanced diagnostic accuracy, improved clinical utility, and superior compatibility with comprehensive emergency care protocols prevalent in contemporary medical practices.
Healthcare providers increasingly prioritize TBI biomarker tests that deliver optimal balance between diagnostic sensitivity, operational efficiency, and cost-effectiveness while adhering to increasingly stringent regulatory standards and clinical validation requirements across global healthcare markets.
The convergence of emergency care expansion in trauma centers, sports medicine growth in developed economies, and specialized neurological diagnostic development in emerging markets creates multifaceted growth opportunities for TBI biomarker providers and healthcare facility operators.
The traumatic brain injury biomarkers landscape is experiencing transformative changes as healthcare providers adopt sophisticated diagnostic platforms including FDA-cleared blood-based tests, advanced protein detection systems, and point-of-care analyzers that enable rapid brain injury assessment and objective clinical decision-making.
These technological advancements are complemented by evolving biomarker capabilities encompassing GFAP and UCH-L1 protein detection for mild TBI diagnosis, neurofilament light chain measurement for injury severity assessment, and tau protein quantification for neurological outcome prediction that significantly improve patient management and healthcare resource utilization.
The integration of digital health platforms and telemedicine consultation capabilities further enhances access to TBI diagnostic expertise, particularly benefiting rural emergency departments and underserved healthcare regions where neurological specialist availability remains limited.
Between 2026 and 2030, the traumatic brain injury biomarkers market is projected to expand from USD 1.19 billion to USD 2.86 billion, demonstrating strong foundational growth driven by global regulatory approvals expansion, increasing clinical adoption of blood-based testing, and initial deployment of point-of-care diagnostic technologies across emergency care and sports medicine platforms.
This growth phase establishes market infrastructure, validates biomarker clinical utility, and creates comprehensive diagnostic networks supporting global neurological healthcare operations.
From 2030 to 2036, the market is forecast to reach USD 6.7 billion, driven by mature biomarker testing penetration, next-generation multiplex assays requiring sophisticated analytical capabilities, and comprehensive integration of TBI screening programs demanding enhanced accessibility.
The growing adoption of concussion protocols in sports medicine, specialized military healthcare initiatives, and expanded emergency department utilization will drive demand for comprehensive TBI biomarker solutions with enhanced diagnostic performance and seamless healthcare workflow integration functionality.
Traumatic Brain Injury Biomarkers Market Key Takeaways
| Metric | Value |
|---|---|
| Estimated Value (2026E) | USD 1.19 billion |
| Forecast Value (2036F) | USD 6.7 billion |
| Forecast CAGR (2026 to 2036) | 18.9% |
Why is the Traumatic Brain Injury Biomarkers Market Growing?
The traumatic brain injury biomarkers market’s growth is supported by the exponential increase in traumatic brain injury incidence and the corresponding need for objective diagnostic tools in neurological assessment applications across global healthcare operations. Healthcare providers are increasingly focused on blood-based biomarker tests that can improve diagnostic accuracy, reduce unnecessary CT scan exposure, and optimize patient triage protocols while meeting stringent regulatory requirements.
The proven clinical utility of TBI biomarkers in various medical applications makes them an essential component of comprehensive emergency care strategies and neurological injury management programs. The growing emphasis on concussion awareness and evidence-based medicine integration is driving demand for validated TBI biomarkers that meet stringent performance specifications and clinical utility requirements for neurological applications.
Emergency department operators' preference for rapid, objective diagnostic tools that can ensure consistent clinical decision-making is creating opportunities for innovative protein biomarker assays and point-of-care testing solutions. The rising influence of sports safety standards and military healthcare protocols is also contributing to increased adoption of FDA-cleared TBI biomarker tests across different healthcare settings and trauma management systems requiring advanced neurological diagnostic technology.
Opportunity Pathways - Traumatic Brain Injury Biomarkers Market
The traumatic brain injury biomarkers market represents a transformative growth opportunity, expanding from USD 1.19 billion in 2026 to USD 6.7 billion by 2036 at a 18.9% CAGR. As healthcare providers prioritize patient safety optimization, objective neurological assessment, and clinical excellence in complex emergency care environments, TBI biomarkers have evolved from experimental research tools to essential diagnostic components enabling precise brain injury detection, comprehensive CT scan reduction strategies, and evidence-based triage operations across emergency department platforms and sports medicine applications.
The convergence of concussion awareness acceleration, increasing regulatory approval penetration, advanced protein detection technology integration, and stringent healthcare cost containment mandates creates momentum in demand. High-sensitivity blood-based assays offering rapid diagnostic turnaround, cost-effective point-of-care solutions balancing performance with accessibility, and specialized tests for mild TBI detection will capture market premiums, while geographic expansion into high-growth European trauma care markets and emerging Asian healthcare ecosystems will drive volume leadership. Healthcare provider emphasis on clinical evidence and diagnostic reliability provides structural support.
- Pathway A - Protein Biomarkers Type Dominance: Leading with 55.73% market share, protein biomarker applications drive primary demand through validated clinical workflows requiring comprehensive blood-based testing for neurological injury assessment. FDA-cleared GFAP and UCH-L1 assays enabling improved diagnostic accuracy, reduced CT utilization, and enhanced patient management outcomes command premium reimbursement from healthcare systems requiring stringent clinical validation and regulatory compliance. Expected revenue pool: USD 0.66-3.73 billion.
- Pathway B - Diagnosis Application Leadership: Dominating with 56.26% market share through optimal balance of clinical necessity and immediate diagnostic requirements, diagnosis applications serve most TBI biomarker utilization while meeting diverse emergency care demands. This application type addresses both mild TBI detection needs and CT scan reduction expectations, making it the preferred category for emergency physicians and trauma centers seeking objective diagnostic capabilities. Opportunity: USD 0.67-3.77 billion.
- Pathway C - North American Market Leadership: USA leads global adoption through comprehensive FDA regulatory framework, established trauma care infrastructure, and widespread concussion protocol implementation. Strategic partnerships with emergency departments, sports medicine integration, and military healthcare programs enable the expansion of TBI biomarker testing in major trauma centers and athletic facilities. Geographic leadership position: USD 0.54-3.02 billion.
- Pathway D - Blood-based Sample Type Segment: Blood-based applications with 63.70% market share serve critical point-of-care requirements across diverse healthcare settings. Optimized serum and plasma assay protocols supporting emergency department workflows, minimal invasiveness, and proven diagnostic effectiveness maintain significant volumes from trauma centers and urgent care facilities. Revenue potential: USD 0.76-4.27 billion.
- Pathway E - Advanced Detection Technologies & Assay Integration: Companies investing in ultrasensitive protein detection platforms, multiplex biomarker panels, and automated analyzer systems gain competitive advantages through consistent diagnostic delivery and clinical validation. Advanced capabilities enabling rapid turnaround specifications and comprehensive biomarker profiling capture premium healthcare system partnerships. Technology premium: USD 0.36-2.01 billion.
- Pathway F - Healthcare Network Optimization & Clinical Adoption: Specialized diagnostic companies, strategic hospital system integration, and validated clinical outcome systems create competitive differentiation in healthcare markets requiring consistent biomarker test availability. Companies offering comprehensive clinical evidence, reimbursement support services, and physician education programs gain preferred provider status with quality-focused healthcare operators. Healthcare network value: USD 0.30-1.68 billion.
- Pathway G - Emerging Applications & Market Development: Beyond traditional emergency care applications, TBI biomarkers in chronic traumatic encephalopathy monitoring, pediatric concussion management, and therapeutic trial enrichment represent growth opportunities. Companies developing new biomarker candidates, supporting research initiatives, and expanding into adjacent neurological diagnostic markets capture incremental demand while diversifying revenue streams. Emerging opportunity: USD 0.24-1.34 billion.
Segmental Analysis
The market is segmented by type, sample type, application, end use, and region. By type, the market is divided into protein biomarkers (GFAP, UCH-L1, NF-L, tau protein), genetic biomarkers, and metabolomic biomarkers. Based on sample type, the market is categorized into blood-based (serum-based assays, plasma-based assays), cerebrospinal fluid (CSF)-based, and urine-based.
By application, the market is segmented into diagnosis (mild TBI detection, moderate-to-severe TBI, CT-scan reduction biomarker tests), prognosis, and monitoring treatment response. By end use, the market is divided into research institutes (academic centers, government research programs, private research labs), hospitals & clinics, and diagnostic laboratories. Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa.
Which Type Category Dominates in the Traumatic Brain Injury Biomarkers Market?
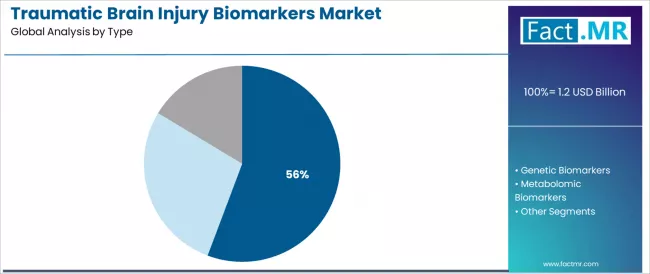
Protein biomarkers are projected to account for 55.73% of the traumatic brain injury biomarkers market in 2026, reaffirming its position as the category's dominant biomarker specification. Healthcare providers increasingly recognize the optimal balance of diagnostic accuracy and clinical validation offered by protein biomarkers for TBI assessment applications, particularly in emergency department triage and mild concussion evaluation environments.
This biomarker category addresses both objective injury detection requirements and regulatory approval demands while providing reliable diagnostic outcomes across diverse clinical operations. This segment forms the foundation of most FDA-cleared TBI diagnostic tests and clinical validation studies, as it represents the most extensively studied and clinically validated biomarker category in the TBI diagnostics industry.
Which is the Leading Category in the Traumatic Brain Injury Biomarkers Market by Application?
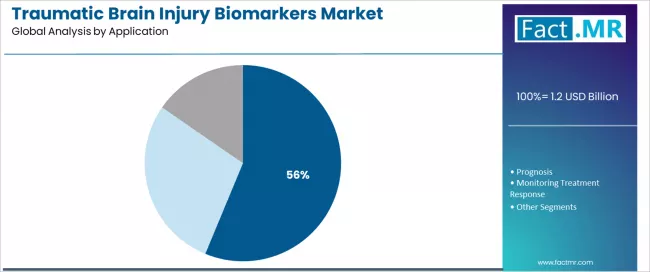
Diagnosis applications are projected to account for 56.26% of the traumatic brain injury biomarkers market in 2026, establishing its position as the dominant clinical use category. Healthcare providers increasingly recognize that diagnostic applications, encompassing emergency department triage, concussion assessment, and CT scan decision-making, represent the most clinically urgent and healthcare resource-impacting application requiring objective TBI biomarkers due to immediate patient management demands and imaging cost considerations.
This application addresses both clinical diagnostic accuracy requirements and healthcare efficiency expectations while providing essential decision-making capabilities across emergency care operations. The segment is supported by the expanding nature of emergency department TBI presentations and mild concussion recognition, driven by sports safety awareness, fall-related injury increases, and motor vehicle accident evaluations requiring immediate clinical assessment.
Why are Blood-based Traumatic Brain Injury Biomarkers Preferred the Most?
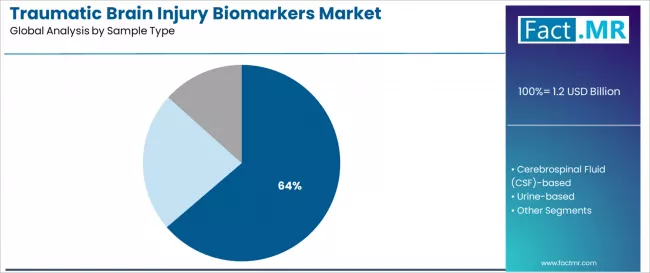
Blood-based samples will account for 63.70% of the traumatic brain injury biomarkers market in 2026, establishing its position as the leading specimen category. Healthcare providers increasingly recognize that blood-based testing, encompassing serum and plasma analysis platforms, represents the most clinically practical and patient-acceptable sample type requiring minimally invasive TBI biomarker measurement due to accessibility and point-of-care compatibility.
Growth is supported by the expanding nature of blood-based diagnostic technology and point-of-care testing advancement, driven by rapid turnaround needs, minimal invasiveness preferences, and FDA regulatory pathway establishment requiring validated blood specimen protocols. Additionally, healthcare providers are increasingly focusing on blood-based assay platforms that enable emergency department integration and widespread clinical adoption while maintaining analytical performance standards.
What Makes Research Institutes the Leading End-Use Segment?
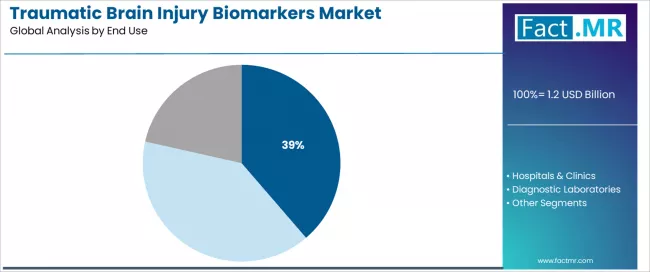
The research institutes end-use segment is projected to account for 38.70% of the traumatic brain injury biomarkers market in 2026, establishing its position as the dominant user category. Research organizations increasingly recognize that academic and government research programs, encompassing biomarker discovery initiatives, clinical validation studies, and therapeutic development trials, represent the most innovation-intensive and scientifically critical end use requiring comprehensive TBI biomarker capabilities due to research advancement needs and clinical translation objectives.
This end use addresses both fundamental neuroscience research requirements and clinical development expectations while providing essential scientific investigation capabilities across neurological research operations. The segment is supported by the expanding nature of TBI research funding and translational neuroscience initiatives, driven by military healthcare investments, sports concussion research priorities, and chronic traumatic encephalopathy investigation programs requiring sophisticated biomarker measurement.
What are the Drivers, Restraints, and Key Trends of the Traumatic Brain Injury Biomarkers Market?
The traumatic brain injury biomarkers market is advancing rapidly due to increasing recognition of objective brain injury assessment importance and growing demand for evidence-based diagnostic solutions across the emergency care sector.
The market faces challenges, including reimbursement uncertainties in some healthcare systems, limited awareness among healthcare providers in certain regions, and concerns about analytical validation complexity in diverse patient populations. Innovation in ultrasensitive detection platforms and multiplex biomarker panels continues to influence test development and market expansion patterns.
Proliferation of Regulatory Approvals and Clinical Validation Evidence
The accelerating progress in FDA clearance pathways is enabling the development of more clinically validated TBI biomarker tests and diagnostic protocols that can meet stringent regulatory and clinical utility requirements.
Healthcare providers demand comprehensive clinical evidence for TBI biomarkers, including large-scale validation studies and prospective clinical trial data that are particularly important for achieving widespread adoption requirements in emergency care applications.
FDA-cleared biomarker tests provide access to reimbursement pathways that can optimize healthcare system integration and enhance diagnostic accessibility while maintaining clinical performance standards for diverse patient populations.
Integration of Point-of-Care Testing Technologies and Rapid Diagnostic Platforms
Modern emergency departments are incorporating advanced technologies such as handheld analyzers, rapid immunoassay platforms, and automated result interpretation systems to enhance TBI biomarker accessibility and clinical workflow efficiency.
These systems improve diagnostic turnaround opportunities, enable seamless emergency department protocol integration, and provide better coordination between triage decisions and comprehensive neurological assessment throughout the patient care experience.
Advanced point-of-care capabilities also enable decentralized testing deployment and early identification of brain injury or neurological deterioration, supporting proactive clinical management and improved patient outcomes.
Analysis of the Traumatic Brain Injury Biomarkers Market by Key Countries
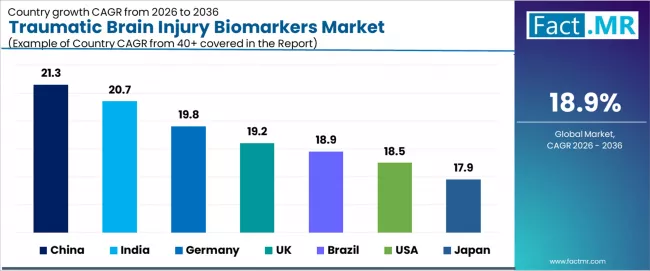
| Country | CAGR (2026-2036) |
|---|---|
| USA | 18.5% |
| Germany | 19.8% |
| UK | 19.2% |
| Japan | 17.9% |
| China | 21.3% |
| India | 20.7% |
| Brazil | 18.9% |
The traumatic brain injury biomarkers market is experiencing exceptional growth globally, with China leading at a 21.3% CAGR through 2036, driven by expanding trauma care infrastructure, growing sports medicine awareness, and increasing neurological diagnostic capability development across major healthcare centers. India follows at 20.7%, supported by rising road traffic accident incidence, expanding emergency care networks, and comprehensive healthcare infrastructure modernization. Germany records 19.8% growth, benefiting from advanced trauma care systems and comprehensive concussion protocol implementation. UK demonstrates 19.2% growth, emphasizing sports-related TBI awareness and emergency department diagnostic advancement.
Brazil shows 18.9% growth, representing expanding trauma care capacity and neurological healthcare development. USA records 18.5% growth, representing established regulatory framework and widespread clinical adoption across emergency care systems, while Japan shows 17.9% growth, representing aging population trauma concerns and advanced healthcare technology integration.
How is Expanding Trauma Care Infrastructure Broadening Growth Prospects for China?
The traumatic brain injury biomarkers market in China is projected to exhibit exceptional growth with a CAGR of 21.3% through 2036, driven by rapidly expanding trauma care infrastructure and increasing recognition of objective neurological assessment as an essential component for emergency medicine modernization and patient safety enhancement.
The country's enormous population and growing availability of advanced diagnostic technologies are creating significant opportunities for TBI biomarker deployment across both urban emergency departments and developing regional trauma centers.
Major international diagnostic companies and domestic biotechnology firms are establishing comprehensive distribution and clinical education capabilities to serve the expanding population of emergency physicians and neurologists requiring TBI biomarker tests across tertiary hospitals, sports medicine facilities, and trauma centers throughout China's diverse healthcare regions.
- Government initiatives supporting healthcare modernization and trauma care enhancement are driving demand for objective diagnostic tools across emergency care segments
- Hospital capacity expansion and emergency medicine education advancement are supporting appropriate utilization of TBI biomarkers among clinicians and trauma centers nationwide
How do Prospects Appear for Traumatic Brain Injury Biomarkers in India?
The traumatic brain injury biomarkers market in India is expanding at a CAGR of 20.7%, supported by rising road traffic accident incidence, expanding emergency care infrastructure, and advancing neurological diagnostic integration across the country's developing trauma care corridors.
The country's significant TBI burden and increasing healthcare system sophistication are driving demand for objective diagnostic tools in both urban trauma centers and emerging regional hospital applications. International diagnostic providers and domestic healthcare companies are establishing market presence to serve the growing need for TBI biomarker testing while supporting the country's healthcare quality improvement initiatives.
- Rising awareness about concussion management and improving emergency care capabilities are creating opportunities for TBI biomarker solutions
- Growing trauma center development and emergency medicine training are supporting increased deployment of neurological diagnostic technologies
How are Advanced Trauma Care Facilities driving TBI Biomarkers Demand in Germany?
Germany's expanding traumatic brain injury biomarkers market demonstrates accelerating diagnostic adoption with a 19.8% CAGR through 2036, driven by advanced trauma care infrastructure, comprehensive concussion protocol implementation, and evidence-based medicine emphasis across major emergency care regions.
The country's leadership in trauma system organization and sports safety standards is creating substantial demand for validated TBI biomarkers across diverse emergency department and athletic medicine platforms. German healthcare providers and sports medicine facilities are prioritizing objective diagnostic tools that incorporate regulatory-cleared biomarker tests for optimal clinical decision-making and athlete safety management.
Market dynamics focus on clinically validated TBI biomarkers that balance advanced diagnostic capabilities with evidence requirements important to German healthcare standards and clinical guideline adherence. Strong medical research infrastructure creates opportunities for integrated clinical validation programs and advanced biomarker technology deployment.
Strategic Market Considerations:
- Emergency care and sports medicine segments leading growth with focus on evidence-based protocols and clinical guideline implementation
- Stringent clinical validation requirements are driving adoption of FDA-cleared and CE-marked biomarker tests with comprehensive clinical evidence
- Trauma care excellence and research leadership supporting competitive positioning in European diagnostic markets
- Physician preferences emphasizing proven clinical utility and comprehensive validation data in TBI biomarker applications
How is Sports-Related TBI Awareness encouraging Uptake in the UK?
The UK's expanding traumatic brain injury biomarkers market demonstrates strong diagnostic adoption with a 19.2% CAGR through 2036, driven by heightened sports-related concussion awareness, comprehensive emergency department modernization, and rugby/football safety protocol implementation across healthcare and athletic organizations.
The country's focus on athlete safety and emergency care quality improvement is creating substantial demand for objective TBI assessment tools across diverse professional sports and emergency medicine platforms. The healthcare providers and sports governing bodies are prioritizing validated biomarker tests that enable evidence-based concussion management and clinical decision support.
Strategic Market Considerations:
- Sports concussion and emergency care segments demonstrating focused growth with emphasis on athlete safety and clinical quality applications
- Healthcare system requirements driving adoption of validated biomarker tests with proven clinical utility and cost-effectiveness
How does USA Maintain Diagnostic Innovation Leadership?
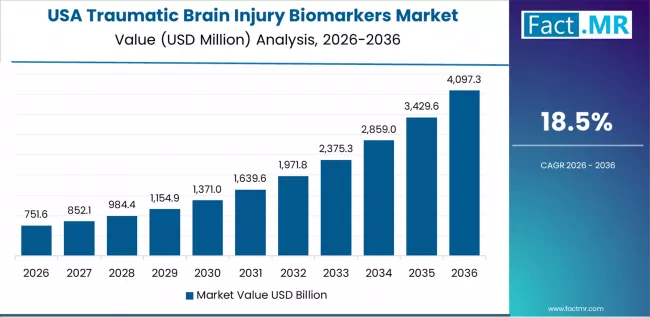
The USA's established TBI biomarkers market demonstrates mature diagnostic adoption with an 18.5% CAGR through 2036, driven by comprehensive FDA regulatory framework, widespread emergency department implementation, and extensive sports concussion protocol integration across healthcare and athletic systems.
The country's leadership in biomarker development and regulatory approval pathways sustains strong demand for validated diagnostic tools across diverse trauma care and sports medicine settings. American healthcare providers maintain focus on FDA-cleared biomarker tests that integrate with established clinical workflows and reimbursement systems.
Strategic Market Considerations:
- Emergency department and sports medicine segments maintaining growth with focus on established diagnostic protocols and guideline-concordant care
- Reimbursement establishment and clinical adoption maturity supporting continued market expansion across diverse healthcare settings
- Regulatory leadership and clinical evidence generation supporting competitive positioning in global diagnostic markets
- Provider preferences emphasizing proven performance and comprehensive reimbursement support in TBI biomarker applications
What drives Brazil’s Market Growth with Expanding Trauma Care Capacity?
Brazil's expanding traumatic brain injury biomarkers market demonstrates accelerating diagnostic adoption with an 18.9% CAGR through 2036, driven by expanding trauma care infrastructure, growing sports medicine development, and comprehensive emergency care modernization across major healthcare regions.
The country's increasing investment in trauma systems and neurological care capabilities is creating substantial demand for objective diagnostic technologies across diverse emergency department and athletic medicine platforms. Brazilian healthcare providers are prioritizing advanced diagnostic tools that enable evidence-based clinical decision-making and improved patient outcomes.
Strategic Market Considerations:
- Trauma care and urban emergency medicine segments leading growth with focus on healthcare quality improvement and diagnostic capability enhancement
- Healthcare system development driving interest in validated diagnostic technologies with proven clinical utility
What drives Japan’s Market Growth with Aging Population Trauma Concerns?
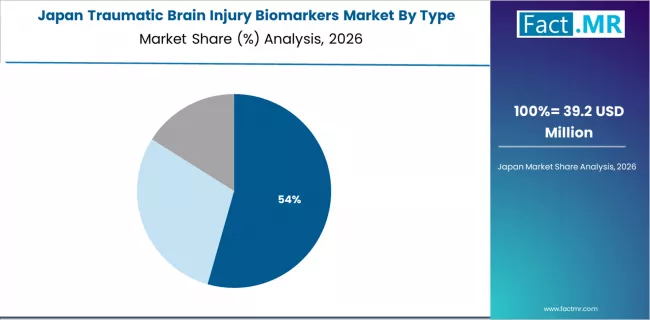
Japan's expanding traumatic brain injury biomarkers market demonstrates consistent diagnostic adoption with a 17.9% CAGR through 2036, driven by aging population fall-related TBI concerns, advanced healthcare technology integration, and comprehensive emergency care quality standards across major healthcare regions.
The country's emphasis on medical technology innovation and elderly care improvement sustains demand for objective neurological assessment tools across diverse geriatric emergency and trauma care platforms. Japanese healthcare providers maintain focus on high-quality diagnostic technologies that incorporate precision biomarker measurement for optimal clinical decision support.
Strategic Market Considerations:
- Geriatric trauma care and emergency medicine segments demonstrating focused growth with emphasis on fall-related injury assessment and elderly patient management
- Healthcare quality requirements maintaining demand for validated biomarker tests with proven analytical performance
Competitive Landscape of the Traumatic Brain Injury Biomarkers Market
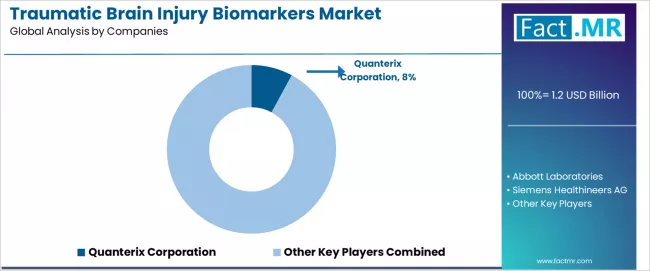
The traumatic brain injury biomarkers market is characterized by intense competition among established diagnostic companies, specialized neurology-focused biotechnology firms, and comprehensive medical device organizations focused on delivering clinically validated, rapid, and accessible TBI diagnostic solutions.
Companies are investing in clinical validation studies, regulatory approval initiatives, strategic healthcare system partnerships, and comprehensive physician education programs to deliver effective, reliable, and evidence-based biomarker tests that meet stringent regulatory standards and clinical utility requirements. FDA clearance achievement, clinical evidence generation, and reimbursement establishment strategies are central to strengthening product portfolios and market presence.
Quanterix Corporation offers ultrasensitive protein detection platforms with a focus on advanced Simoa technology and comprehensive neurological biomarker measurement capabilities for research and clinical applications. Abbott Laboratories provides FDA-cleared TBI biomarker tests with emphasis on point-of-care accessibility and comprehensive emergency department integration across global healthcare markets.
Siemens Healthineers focuses on laboratory automation platforms and diagnostic system integration serving hospital-based TBI testing applications. GE Healthcare delivers comprehensive medical imaging and diagnostic solutions with neurological assessment capabilities.
Koninklijke Philips N.V. operates with a focus on connected care solutions and emergency department workflow optimization for trauma patient management. Thermo Fisher Scientific provides research reagents and analytical platforms emphasizing TBI biomarker discovery and validation. BioMérieux specializes in infectious disease diagnostics with expanding neurological biomarker interests. Banyan Biomarkers focuses exclusively on TBI diagnostic development with FDA-cleared blood-based tests.
Key Players in the Traumatic Brain Injury Biomarkers Market
- Quanterix Corporation
- Abbott Laboratories
- Siemens Healthineers AG
- GE HealthCare Technologies Inc.
- Koninklijke Philips N.V.
- Thermo Fisher Scientific Inc.
- bioMérieux SA
- Banyan Biomarkers, Inc.
- Oculogica Inc.
- Randox Laboratories Ltd.
- QIAGEN N.V.
- Fujirebio Inc.
- Myriad Genetics, Inc.
- F. Hoffmann-La Roche Ltd
- Abcam plc
- BRAINBox Solutions, Inc.
- NeuroTrauma Sciences, LLC
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2026) | USD 1.19 Billion |
| Type | Protein Biomarkers (GFAP, UCH-L1, NF-L, Tau Protein), Genetic Biomarkers, Metabolomic Biomarkers |
| Sample Type | Blood-based (Serum-based Assays, Plasma-based Assays), Cerebrospinal Fluid (CSF)-based, Urine-based |
| Application | Diagnosis (Mild TBI Detection, Moderate-to-Severe TBI, CT-scan Reduction Biomarker Tests), Prognosis, Monitoring Treatment Response |
| End Use | Research Institutes (Academic Centers, Government Research Programs, Private Research Labs), Hospitals & Clinics, Diagnostic Laboratories |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | USA, Germany, UK, Japan, India, China, Brazil and 40+ countries |
| Key Companies Profiled | Quanterix, Abbott, Siemens Healthineers, GE Healthcare, Koninklijke Philips N.V., Thermo Fisher Scientific, BioMérieux, Banyan Biomarkers, Oculogica, Randox, QIAGEN, Fujirebio, Myriad Genetics, F. Hoffmann-La Roche, Abcam Limited, BRAINBox Solutions, NeuroTrauma Sciences |
| Additional Attributes | Dollar sales by type, sample type, application, end use, regional demand trends, competitive landscape, clinician preferences for specific biomarker tests, integration with comprehensive emergency care protocols, innovations in ultrasensitive detection technology development, point-of-care platform advancement, and clinical utility optimization capabilities |
Traumatic Brain Injury Biomarkers Market by Segments
-
Type :
- Protein Biomarkers
- GFAP
- UCH-L1
- NF-L
- Tau Protein
- Genetic Biomarkers
- Metabolomic Biomarkers
- Protein Biomarkers
-
Sample Type :
- Blood-based
- Serum-based Assays
- Plasma-based Assays
- Cerebrospinal Fluid (CSF)-based
- Urine-based
- Blood-based
-
Application :
- Diagnosis
- Mild TBI Detection
- Moderate-to-Severe TBI
- CT-scan Reduction Biomarker Tests
- Prognosis
- Monitoring Treatment Response
- Diagnosis
-
End Use :
- Research Institutes
- Academic Centers
- Government Research Programs
- Private Research Labs
- Hospitals & Clinics
- Diagnostic Laboratories
- Research Institutes
-
Region :
-
North America
- United States
- Canada
- Mexico
-
Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
-
Asia Pacific
- China
- India
- Japan
- South Korea
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
-
Latin America
- Brazil
- Argentina
- Chile
- Rest of Latin America
-
Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Countries
- Rest of Middle East & Africa
-
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2021 to 2025 and Forecast, 2026 to 2036
- Historical Market Size Value (USD Million) Analysis, 2021 to 2025
- Current and Future Market Size Value (USD Million) Projections, 2026 to 2036
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2021 to 2025 and Forecast 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Type, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Type, 2026 to 2036
- Protein Biomarkers
- Genetic Biomarkers
- Metabolomic Biomarkers
- Y to o to Y Growth Trend Analysis By Type, 2021 to 2025
- Absolute $ Opportunity Analysis By Type, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Sample Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Sample Type, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Sample Type, 2026 to 2036
- Blood-based
- Cerebrospinal Fluid (CSF)-based
- Urine-based
- Y to o to Y Growth Trend Analysis By Sample Type, 2021 to 2025
- Absolute $ Opportunity Analysis By Sample Type, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2026 to 2036
- Diagnosis
- Prognosis
- Monitoring Treatment Response
- Y to o to Y Growth Trend Analysis By Application, 2021 to 2025
- Absolute $ Opportunity Analysis By Application, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2026 to 2036
- Research Institutes
- Hospitals & Clinics
- Diagnostic Laboratories
- Y to o to Y Growth Trend Analysis By End Use, 2021 to 2025
- Absolute $ Opportunity Analysis By End Use, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2021 to 2025
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2026 to 2036
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- USA
- Canada
- Mexico
- By Type
- By Sample Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Sample Type
- By Application
- By End Use
- Key Takeaways
- Latin America Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Type
- By Sample Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Sample Type
- By Application
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Type
- By Sample Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Sample Type
- By Application
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Type
- By Sample Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Sample Type
- By Application
- By End Use
- Key Takeaways
- East Asia Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- China
- Japan
- South Korea
- By Type
- By Sample Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Sample Type
- By Application
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Type
- By Sample Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Sample Type
- By Application
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Type
- By Sample Type
- By Application
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Sample Type
- By Application
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2025
- By Type
- By Sample Type
- By Application
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Type
- By Sample Type
- By Application
- By End Use
- Competition Analysis
- Competition Deep Dive
- Quanterix Corporation
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Abbott Laboratories
- Siemens Healthineers AG
- GE HealthCare Technologies Inc.
- Koninklijke Philips N.V.
- Thermo Fisher Scientific Inc.
- bioMérieux SA
- Banyan Biomarkers Inc.
- Oculogica Inc.
- Randox Laboratories Ltd.
- QIAGEN N.V.
- Fujirebio Inc.
- Myriad Genetics Inc.
- F. Hoffmann-La Roche Ltd.
- Abcam plc
- BRAINBox Solutions Inc.
- NeuroTrauma Sciences LLC
- Quanterix Corporation
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2021 to 2036
- Table 2: Global Market Value (USD Million) Forecast by Type, 2021 to 2036
- Table 3: Global Market Value (USD Million) Forecast by Sample Type, 2021 to 2036
- Table 4: Global Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 5: Global Market Value (USD Million) Forecast by End Use, 2021 to 2036
- Table 6: North America Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 7: North America Market Value (USD Million) Forecast by Type, 2021 to 2036
- Table 8: North America Market Value (USD Million) Forecast by Sample Type, 2021 to 2036
- Table 9: North America Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 10: North America Market Value (USD Million) Forecast by End Use, 2021 to 2036
- Table 11: Latin America Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 12: Latin America Market Value (USD Million) Forecast by Type, 2021 to 2036
- Table 13: Latin America Market Value (USD Million) Forecast by Sample Type, 2021 to 2036
- Table 14: Latin America Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 15: Latin America Market Value (USD Million) Forecast by End Use, 2021 to 2036
- Table 16: Western Europe Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 17: Western Europe Market Value (USD Million) Forecast by Type, 2021 to 2036
- Table 18: Western Europe Market Value (USD Million) Forecast by Sample Type, 2021 to 2036
- Table 19: Western Europe Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 20: Western Europe Market Value (USD Million) Forecast by End Use, 2021 to 2036
- Table 21: Eastern Europe Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 22: Eastern Europe Market Value (USD Million) Forecast by Type, 2021 to 2036
- Table 23: Eastern Europe Market Value (USD Million) Forecast by Sample Type, 2021 to 2036
- Table 24: Eastern Europe Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 25: Eastern Europe Market Value (USD Million) Forecast by End Use, 2021 to 2036
- Table 26: East Asia Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 27: East Asia Market Value (USD Million) Forecast by Type, 2021 to 2036
- Table 28: East Asia Market Value (USD Million) Forecast by Sample Type, 2021 to 2036
- Table 29: East Asia Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 30: East Asia Market Value (USD Million) Forecast by End Use, 2021 to 2036
- Table 31: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 32: South Asia and Pacific Market Value (USD Million) Forecast by Type, 2021 to 2036
- Table 33: South Asia and Pacific Market Value (USD Million) Forecast by Sample Type, 2021 to 2036
- Table 34: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 35: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2021 to 2036
- Table 36: Middle East & Africa Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 37: Middle East & Africa Market Value (USD Million) Forecast by Type, 2021 to 2036
- Table 38: Middle East & Africa Market Value (USD Million) Forecast by Sample Type, 2021 to 2036
- Table 39: Middle East & Africa Market Value (USD Million) Forecast by Application, 2021 to 2036
- Table 40: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2021 to 2036
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2021 to 2036
- Figure 3: Global Market Value Share and BPS Analysis by Type, 2026 and 2036
- Figure 4: Global Market Y to o to Y Growth Comparison by Type, 2026 to 2036
- Figure 5: Global Market Attractiveness Analysis by Type
- Figure 6: Global Market Value Share and BPS Analysis by Sample Type, 2026 and 2036
- Figure 7: Global Market Y to o to Y Growth Comparison by Sample Type, 2026 to 2036
- Figure 8: Global Market Attractiveness Analysis by Sample Type
- Figure 9: Global Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 10: Global Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 11: Global Market Attractiveness Analysis by Application
- Figure 12: Global Market Value Share and BPS Analysis by End Use, 2026 and 2036
- Figure 13: Global Market Y to o to Y Growth Comparison by End Use, 2026 to 2036
- Figure 14: Global Market Attractiveness Analysis by End Use
- Figure 15: Global Market Value (USD Million) Share and BPS Analysis by Region, 2026 and 2036
- Figure 16: Global Market Y to o to Y Growth Comparison by Region, 2026 to 2036
- Figure 17: Global Market Attractiveness Analysis by Region
- Figure 18: North America Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 19: Latin America Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 20: Western Europe Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 21: Eastern Europe Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 22: East Asia Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 23: South Asia and Pacific Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 24: Middle East & Africa Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 25: North America Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 26: North America Market Value Share and BPS Analysis by Type, 2026 and 2036
- Figure 27: North America Market Y to o to Y Growth Comparison by Type, 2026 to 2036
- Figure 28: North America Market Attractiveness Analysis by Type
- Figure 29: North America Market Value Share and BPS Analysis by Sample Type, 2026 and 2036
- Figure 30: North America Market Y to o to Y Growth Comparison by Sample Type, 2026 to 2036
- Figure 31: North America Market Attractiveness Analysis by Sample Type
- Figure 32: North America Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 33: North America Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 34: North America Market Attractiveness Analysis by Application
- Figure 35: North America Market Value Share and BPS Analysis by End Use, 2026 and 2036
- Figure 36: North America Market Y to o to Y Growth Comparison by End Use, 2026 to 2036
- Figure 37: North America Market Attractiveness Analysis by End Use
- Figure 38: Latin America Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 39: Latin America Market Value Share and BPS Analysis by Type, 2026 and 2036
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Type, 2026 to 2036
- Figure 41: Latin America Market Attractiveness Analysis by Type
- Figure 42: Latin America Market Value Share and BPS Analysis by Sample Type, 2026 and 2036
- Figure 43: Latin America Market Y to o to Y Growth Comparison by Sample Type, 2026 to 2036
- Figure 44: Latin America Market Attractiveness Analysis by Sample Type
- Figure 45: Latin America Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 46: Latin America Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 47: Latin America Market Attractiveness Analysis by Application
- Figure 48: Latin America Market Value Share and BPS Analysis by End Use, 2026 and 2036
- Figure 49: Latin America Market Y to o to Y Growth Comparison by End Use, 2026 to 2036
- Figure 50: Latin America Market Attractiveness Analysis by End Use
- Figure 51: Western Europe Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 52: Western Europe Market Value Share and BPS Analysis by Type, 2026 and 2036
- Figure 53: Western Europe Market Y to o to Y Growth Comparison by Type, 2026 to 2036
- Figure 54: Western Europe Market Attractiveness Analysis by Type
- Figure 55: Western Europe Market Value Share and BPS Analysis by Sample Type, 2026 and 2036
- Figure 56: Western Europe Market Y to o to Y Growth Comparison by Sample Type, 2026 to 2036
- Figure 57: Western Europe Market Attractiveness Analysis by Sample Type
- Figure 58: Western Europe Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 59: Western Europe Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 60: Western Europe Market Attractiveness Analysis by Application
- Figure 61: Western Europe Market Value Share and BPS Analysis by End Use, 2026 and 2036
- Figure 62: Western Europe Market Y to o to Y Growth Comparison by End Use, 2026 to 2036
- Figure 63: Western Europe Market Attractiveness Analysis by End Use
- Figure 64: Eastern Europe Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 65: Eastern Europe Market Value Share and BPS Analysis by Type, 2026 and 2036
- Figure 66: Eastern Europe Market Y to o to Y Growth Comparison by Type, 2026 to 2036
- Figure 67: Eastern Europe Market Attractiveness Analysis by Type
- Figure 68: Eastern Europe Market Value Share and BPS Analysis by Sample Type, 2026 and 2036
- Figure 69: Eastern Europe Market Y to o to Y Growth Comparison by Sample Type, 2026 to 2036
- Figure 70: Eastern Europe Market Attractiveness Analysis by Sample Type
- Figure 71: Eastern Europe Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 72: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 73: Eastern Europe Market Attractiveness Analysis by Application
- Figure 74: Eastern Europe Market Value Share and BPS Analysis by End Use, 2026 and 2036
- Figure 75: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2026 to 2036
- Figure 76: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 77: East Asia Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 78: East Asia Market Value Share and BPS Analysis by Type, 2026 and 2036
- Figure 79: East Asia Market Y to o to Y Growth Comparison by Type, 2026 to 2036
- Figure 80: East Asia Market Attractiveness Analysis by Type
- Figure 81: East Asia Market Value Share and BPS Analysis by Sample Type, 2026 and 2036
- Figure 82: East Asia Market Y to o to Y Growth Comparison by Sample Type, 2026 to 2036
- Figure 83: East Asia Market Attractiveness Analysis by Sample Type
- Figure 84: East Asia Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 85: East Asia Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 86: East Asia Market Attractiveness Analysis by Application
- Figure 87: East Asia Market Value Share and BPS Analysis by End Use, 2026 and 2036
- Figure 88: East Asia Market Y to o to Y Growth Comparison by End Use, 2026 to 2036
- Figure 89: East Asia Market Attractiveness Analysis by End Use
- Figure 90: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 91: South Asia and Pacific Market Value Share and BPS Analysis by Type, 2026 and 2036
- Figure 92: South Asia and Pacific Market Y to o to Y Growth Comparison by Type, 2026 to 2036
- Figure 93: South Asia and Pacific Market Attractiveness Analysis by Type
- Figure 94: South Asia and Pacific Market Value Share and BPS Analysis by Sample Type, 2026 and 2036
- Figure 95: South Asia and Pacific Market Y to o to Y Growth Comparison by Sample Type, 2026 to 2036
- Figure 96: South Asia and Pacific Market Attractiveness Analysis by Sample Type
- Figure 97: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 98: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 99: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 100: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2026 and 2036
- Figure 101: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2026 to 2036
- Figure 102: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 103: Middle East & Africa Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 104: Middle East & Africa Market Value Share and BPS Analysis by Type, 2026 and 2036
- Figure 105: Middle East & Africa Market Y to o to Y Growth Comparison by Type, 2026 to 2036
- Figure 106: Middle East & Africa Market Attractiveness Analysis by Type
- Figure 107: Middle East & Africa Market Value Share and BPS Analysis by Sample Type, 2026 and 2036
- Figure 108: Middle East & Africa Market Y to o to Y Growth Comparison by Sample Type, 2026 to 2036
- Figure 109: Middle East & Africa Market Attractiveness Analysis by Sample Type
- Figure 110: Middle East & Africa Market Value Share and BPS Analysis by Application, 2026 and 2036
- Figure 111: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2026 to 2036
- Figure 112: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 113: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2026 and 2036
- Figure 114: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2026 to 2036
- Figure 115: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 116: Global Market - Tier Structure Analysis
- Figure 117: Global Market - Company Share Analysis
- FAQs -
How big is the traumatic brain injury biomarkers market in 2026?
The global traumatic brain injury biomarkers market is estimated to be valued at USD 1.2 billion in 2026.
What will be the size of traumatic brain injury biomarkers market in 2036?
The market size for the traumatic brain injury biomarkers market is projected to reach USD 6.7 billion by 2036.
How much will be the traumatic brain injury biomarkers market growth between 2026 and 2036?
The traumatic brain injury biomarkers market is expected to grow at a 18.9% CAGR between 2026 and 2036.
What are the key product types in the traumatic brain injury biomarkers market?
The key product types in traumatic brain injury biomarkers market are protein biomarkers , genetic biomarkers and metabolomic biomarkers.
Which sample type segment to contribute significant share in the traumatic brain injury biomarkers market in 2026?
In terms of sample type, blood-based segment to command 63.7% share in the traumatic brain injury biomarkers market in 2026.