Blood Cancer Diagnostics Market
Blood Cancer Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
The blood cancer diagnostics market is projected to grow from USD 14.2 billion in 2025 to USD 29.5 billion by 2035, at a CAGR of 7.6%. Molecular/NGS will dominate with a 39.0% market share, while hospital labs will lead the setting type segment with a 0.0% share.
Blood Cancer Diagnostics Market Analysis - Size, Share, and Forecast Outlook 2025 to 2035
The blood cancer diagnostics industry stands at the threshold of a decade-long expansion trajectory that promises to reshape oncological testing technology and hematology diagnostic enhancement systems. The market's journey from USD 14.2 billion in 2025 to USD 29.5 billion by 2035 represents substantial growth, the market will rise at a CAGR of 7.6% which demonstrating the accelerating adoption of molecular diagnostic systems and next-generation sequencing technologies across healthcare providers, diagnostic laboratories, and oncology service providers worldwide.
The first half of the decade (2025-2030) will witness the market climbing from USD 14.2 billion to approximately USD 19.8 billion, adding USD 5.6 billion in value, which constitutes 36.6% of the total forecast growth period. This phase will be characterized by the rapid adoption of NGS diagnostic systems, driven by increasing cancer detection regulations and molecular diagnostic modernization programs worldwide. Advanced sequencing capabilities and integrated biomarker analysis features will become standard expectations rather than premium options.
The latter half (2030-2035) will witness sustained growth from USD 19.8 billion to USD 29.5 billion, representing an addition of USD 9.7 billion or 63.4% of the decade's expansion. This period will be defined by mass market penetration of comprehensive molecular diagnostic platforms, integration with personalized medicine systems, and seamless compatibility with existing laboratory management architecture. The market trajectory signals fundamental shifts in how healthcare providers approach cancer diagnostics design, with participants positioned to benefit from sustained demand across multiple diagnostic segments.
Quick Stats for Blood Cancer Diagnostics Market
- Blood Cancer Diagnostics Market Value (2025): USD 14.2 billion
- Blood Cancer Diagnostics Market Forecast Value (2035): USD 29.5 billion
- Blood Cancer Diagnostics Market Forecast CAGR: 7.6%
- Leading Modality Type in Blood Cancer Diagnostics Market: Molecular/NGS
- Key Growth Regions in Blood Cancer Diagnostics Market: Asia Pacific, North America, and Europe
- Top Key Players in Blood Cancer Diagnostics Market: Roche, Illumina, Thermo Fisher, Qiagen, BD

The blood cancer diagnostics market demonstrates distinct growth phases with varying market characteristics and competitive dynamics. Between 2025 and 2030, the market progresses through its molecular diagnostics adoption phase, expanding from USD 14.2 billion to USD 19.8 billion with steady annual increments averaging 6.9% growth. This period showcases the transition from traditional diagnostic methods to advanced NGS platforms with enhanced precision capabilities and integrated biomarker analysis systems becoming mainstream features.
The 2025-2030 phase adds USD 5.6 billion to market value, representing 36.6% of total decade expansion. Market maturation factors include standardization of molecular diagnostic protocols, declining sequencing costs for NGS systems, and increasing healthcare provider awareness of molecular diagnostic benefits, reaching 92-95% accuracy in cancer detection applications. Competitive landscape evolution during this period features established diagnostic companies like Roche and Illumina expanding their NGS portfolios while new entrants focus on specialized sequencing algorithms and enhanced integration capabilities.
From 2030 to 2035, market dynamics shift toward advanced integration and multi-platform deployment, with growth accelerating from USD 19.8 billion to USD 29.5 billion, adding USD 9.7 billion or 63.4% of total expansion. This phase transition logic centers on comprehensive diagnostic platforms, integration with precision medicine ecosystems, and deployment across diverse oncology specialties, becoming standard rather than specialized applications. The competitive environment matures with focus shifting from basic diagnostic capability to comprehensive cancer detection ecosystems and integration with automated pathology and treatment selection platforms.
Blood Cancer Diagnostics Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| $ Market Value (2025) → | USD 14.2 billion |
| $ Market Forecast (2035) ↑ | USD 29.5 billion |
| # Growth Rate ★ | 7.6% CAGR |
| Leading Modality Type → | Molecular/NGS |
| Primary Setting → | Hospital Labs |
The market demonstrates strong fundamentals with molecular/NGS capturing a dominant share through advanced diagnostic features and precision testing implementation capabilities. Hospital labs are the most preferred settings for blood cancer diagnostics, supported by increasing healthcare provider spending on diagnostic enhancement tools and cancer detection systems.
Geographic expansion remains concentrated in developed markets with established healthcare infrastructure, while emerging economies show accelerating adoption rates driven by oncology modernization and rising diagnostic testing budgets.
Why is the Blood Cancer Diagnostics Market Growing?
Cancer detection demand creates compelling operational advantages through molecular diagnostic systems that provide precise identification without traditional diagnostic dependency risks, enabling healthcare providers to maintain testing performance while achieving accuracy superiority and reducing misdiagnosis costs.
Healthcare diagnostic modernization programs accelerate as medical providers worldwide seek advanced NGS systems that complement traditional testing methods, enabling precise biomarker analysis and personalized applications that align with treatment standards and accuracy requirements.
Medical technology enhancement drives adoption from hospital laboratories and diagnostic centers requiring effective testing tools that minimize diagnostic errors while maintaining detection efficacy during complex oncology procedures and patient management.
However, growth faces headwinds from cost pressure challenges that vary across healthcare providers regarding the deployment of NGS diagnostic systems and pricing protocols, which may limit operational flexibility in certain medical environments. Technical limitations also persist regarding system adaptability and integration complexity that may reduce diagnostic performance with diverse cancer types or non-standardized testing formats that limit detection capabilities.
Opportunity Pathways - Blood Cancer Diagnostics Market
The blood cancer diagnostics market represents a transformative growth opportunity, expanding from USD 14.2 billion in 2025 to USD 29.5 billion by 2035 at a 7.6% CAGR. As healthcare systems worldwide prioritize precision medicine, early detection, and personalized treatment, blood cancer diagnostics have evolved from optional testing to essential medical infrastructure, enabling accurate diagnosis, reducing treatment delays, and supporting operational excellence across hospital laboratories, reference labs, and point-of-care testing applications.
The convergence of precision medicine mandates, increasing cancer detection quality requirements, molecular technology maturation, and regulatory acceptance of advanced diagnostic systems creates unprecedented adoption momentum. Advanced NGS algorithms offering superior effectiveness, seamless healthcare integration, and regulatory compliance will capture premium market positioning, while geographic expansion into emerging healthcare markets and scalable diagnostic deployment will drive volume leadership. Government healthcare programs and diagnostic standardization provide structural support.
- Pathway A - Molecular/NGS Dominance: Leading with 39.0% market share through superior diagnostic precision, genomic analysis, and biomarker identification capabilities, molecular solutions enable comprehensive cancer detection across diverse healthcare settings without significant laboratory modifications. Advanced features, including mutation analysis, copy number variation detection, and seamless integration with laboratory information systems, command premium pricing while reducing total diagnostic costs. Expected revenue pool: USD 11.5-13.4 billion.
- Pathway B - Hospital Labs Leadership: Dominating with 47.0% market share, hospital applications drive primary demand through integrated diagnostic systems for inpatient care, emergency diagnostics, and clinical laboratory operations. Specialized systems for hospital integration, workflow optimization, and quality standards that exceed diagnostic requirements while maintaining operational efficiency capture significant premiums from healthcare providers and laboratory suppliers. Opportunity: USD 13.9-16.2 billion.
- Pathway C - North American Market Acceleration: USA (8.3% CAGR) and China (8.1% CAGR) lead global growth through aggressive healthcare modernization programs, government precision medicine initiatives, and diagnostic infrastructure development. Local partnerships enabling compliance with domestic healthcare regulations, diagnostic standards, and cost-effective solutions tailored for emerging market price points capture expanding demand. Geographic expansion upside: USD 11.8-15.4 billion.
- Pathway D - Leukemia Applications: Beyond traditional diagnostic integration, blood cancer applications in leukemia detection, minimal residual disease monitoring, and treatment response assessment represent high-growth segments. Advanced diagnostic systems for acute leukemia, chronic conditions, and specialized hematology that improve outcomes while ensuring diagnostic accuracy create differentiated value propositions with premium pricing potential. Revenue opportunity: USD 9.7-12.7 billion.
- Pathway E - Technology Integration & Automation: Healthcare automation acceleration drives demand for smart diagnostic systems, enabling automated analysis, real-time reporting, and integrated workflow management. Advanced solutions supporting laboratory automation, AI integration, and predictive analytics expand addressable markets beyond traditional diagnostic applications. Technology advancement pool: USD 8.3-10.9 billion.
- Pathway F - Reference Lab Solutions: Growing demand for specialized testing services enabling comprehensive diagnostics across healthcare provider operations, outsourced testing, and specialized diagnostic scenarios. Reference lab solutions supporting operational efficiency, testing expertise, and multi-site deployment create new market opportunities with moderate premium potential. Reference lab opportunity: USD 6.8-9.0 billion.
- Pathway G - Point-of-Care Testing Enhancement: Increasing accessibility requirements drive demand for near-patient diagnostic systems with rapid results, portable testing, and decentralized capabilities. POCT solutions supporting immediate care, rural healthcare, and operational convenience expand addressable markets with accessibility premium positioning. POCT solutions pool: USD 5.4-7.1 billion.
Segmental Analysis
The market segments by modality type into molecular/NGS, flow cytometry, and immunoassays/others categories, representing the evolution from traditional immunological solutions to advanced molecular diagnostic systems for comprehensive cancer detection coverage.
Setting segmentation divides the market into hospital labs, reference labs, and POCT/near-patient sectors, reflecting distinct requirements for integrated hospital diagnostics, specialized testing services, decentralized care, and point-of-care applications.
Indication distribution covers leukemias, lymphomas, and myeloma/MDS categories, with leukemia diagnostics leading adoption while lymphoma and myeloma segments show steady growth patterns driven by specialized oncology programs.
Geographic distribution covers Asia Pacific, North America, Europe, Latin America, and the Middle East & Africa, with developed markets leading adoption while emerging economies show accelerating growth patterns driven by healthcare modernization programs.
The segmentation structure reveals technology progression from traditional immunoassay diagnostics toward integrated molecular platforms with enhanced precision and genomic capabilities, while application diversity spans from hospital diagnostics to comprehensive reference laboratory solutions requiring precise cancer detection assistance.
By Modality Type, the Molecular/NGS Segment Accounts for Dominant Market Share

Molecular/NGS commands the leading position in the blood cancer diagnostics market with approximately 39.0% market share through advanced diagnostic features, including superior genomic analysis, mutation detection, and biomarker identification that enable healthcare providers to deploy molecular diagnostics across diverse laboratory platforms without significant infrastructure modifications.
The segment benefits from healthcare provider preference for precision diagnostic systems that provide superior accuracy without requiring extensive laboratory infrastructure or specialized maintenance protocols.
NGS design features enable deployment in hospital labs, reference laboratories, and specialized applications where diagnostic precision and genomic analysis represent critical operational requirements.
Molecular systems differentiate through established diagnostic effectiveness, proven genomic capabilities, and integration with existing laboratory information systems that enhance testing effectiveness while maintaining cost-effective operational profiles suitable for healthcare providers of all sizes.
Key market characteristics:
- Advanced sequencing algorithms with established accuracy capabilities and validated genomic parameters
- High-precision resources enabling comprehensive cancer detection and consistent diagnostic outcomes
- Integration capabilities with laboratory information systems, diagnostic platforms, and healthcare management systems for comprehensive testing workflows
By Setting, the Hospital Labs Segment Accounts for the Largest Market Share
Hospital labs applications dominate the blood cancer diagnostics market with approximately 47.0% market share due to widespread adoption of integrated diagnostic systems and increasing focus on precision medicine, rapid diagnosis, and treatment optimization applications that optimize testing delivery while maintaining diagnostic effectiveness.
Hospital customers prioritize diagnostic reliability, testing speed, and integration with existing clinical workflows that enables coordinated diagnostic systems across multiple testing types and medical functions. The segment benefits from substantial healthcare provider spending and medical technology programs that emphasize advanced diagnostic acquisition for improved patient outcomes and treatment optimization.
Healthcare modernization programs incorporate molecular diagnostics as standard equipment for hospital laboratory testing and precision medicine applications. At the same time, increasing clinical standards are driving demand for diagnostic capabilities that maintain testing requirements and minimize diagnostic delays.
Application dynamics include:
- Strong growth in hospital laboratories requiring comprehensive diagnostic assistance capabilities
- Increasing adoption in specialized oncology departments for enhanced precision medicine support applications
- Rising integration with clinical decision support platforms for automated diagnostic interpretation and adaptive treatment selection
What are the Drivers, Restraints, and Key Trends of the Blood Cancer Diagnostics Market?
Healthcare precision medicine drives primary adoption as diagnostic systems provide cancer detection capabilities that enable accurate identification without traditional testing dependency risks, supporting clinical decision-making and treatment missions that require precise diagnostic management.
The demand for medical modernization accelerates market expansion as healthcare providers seek effective diagnostic enhancement tools that minimize detection errors while maintaining operational effectiveness during complex oncology procedures and patient care scenarios. Healthcare spending increases worldwide, creating sustained demand for molecular diagnostic systems that complement traditional testing methods and provide operational flexibility in diverse medical environments.
Cost pressure challenges vary across healthcare providers regarding the deployment of NGS diagnostic systems and pricing allocation protocols, which may limit market penetration and operational flexibility in regions with constrained healthcare budgets.
Technical performance limitations persist regarding system adaptability and integration complexity that may reduce effectiveness with diverse cancer types, non-standardized testing formats, or complex laboratory workflows that limit detection capabilities. Market fragmentation across multiple healthcare standards and operational requirements creates compatibility concerns between different diagnostic providers and existing laboratory infrastructure.
Adoption accelerates in hospital laboratory and reference lab sectors where precision medicine justifies system costs, with geographic concentration in developed markets transitioning toward mainstream adoption in emerging economies driven by healthcare modernization and diagnostic infrastructure development.
Technology development focuses on enhanced diagnostic effectiveness, improved NGS capabilities, and compatibility with diverse healthcare systems that optimize testing workflow and operational effectiveness. The market could face disruption if alternative diagnostic technologies or cost restrictions significantly limit molecular diagnostic deployment in healthcare or oncology applications.
Analysis of the Blood Cancer Diagnostics Market by Key Country
The blood cancer diagnostics market demonstrates varied regional dynamics with growth leaders including USA (8.3% CAGR) and China (8.1% CAGR) driving expansion through healthcare modernization and diagnostic infrastructure development.
Steady performers encompass Germany (7.8% CAGR), UK (7.4% CAGR), and Japan (7.2% CAGR), benefiting from established healthcare industries and advanced diagnostic technology adoption. Emerging markets feature India (7.0% CAGR) and France (6.7% CAGR), where specialized healthcare applications and diagnostic technology integration support consistent growth patterns.
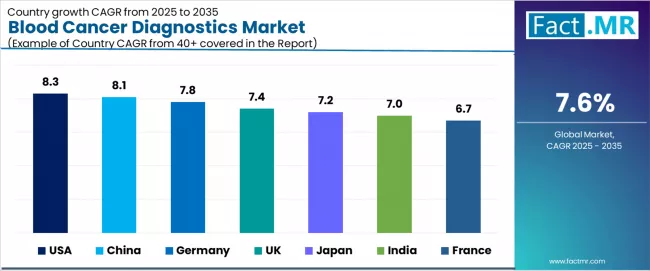
| Country | CAGR (2025-2035) |
|---|---|
| USA | 8.3% |
| China | 8.1% |
| Germany | 7.8% |
| UK | 7.4% |
| Japan | 7.2% |
| India | 7.0% |
| France | 6.7% |
Regional synthesis reveals North American markets leading growth through healthcare modernization and precision medicine infrastructure development, while European and Asian countries maintain steady expansion supported by diagnostic technology advancement and healthcare standardization requirements. Emerging markets show moderate growth driven by diagnostic applications and medical technology integration trends.
USA Drives Global Market Leadership
USA establishes market leadership through aggressive healthcare modernization programs and comprehensive precision medicine infrastructure development, integrating advanced molecular diagnostic systems as standard components in hospital laboratory operations and oncology care.
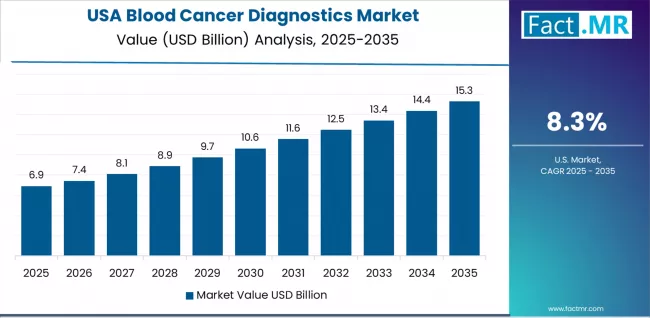
The country's 8.3% CAGR through 2035 reflects government initiatives promoting precision medicine and domestic healthcare capabilities that mandate advanced diagnostic systems in medical facility installations.
Growth concentrates in major medical centers, including New York, California, and Texas, where healthcare technology development showcases integrated diagnostic systems that appeal to domestic healthcare providers seeking advanced precision medicine capabilities and operational enhancement applications.
American manufacturers are developing advanced molecular diagnostic solutions that combine domestic innovation advantages with cutting-edge features, including precision sequencing algorithms and comprehensive genomic analysis capabilities.
Distribution channels through healthcare procurement and laboratory suppliers expand market access, while government funding for precision medicine development supports adoption across diverse healthcare and oncology segments.
Strategic Market Indicators:
- Healthcare systems leading adoption with 82% deployment rate in hospital laboratory and oncology departments
- Government healthcare programs providing substantial funding for domestic precision medicine development
- Domestic manufacturers capturing 71% market share through innovation leadership and localized healthcare support
- Clinical segment growth driven by oncology provider requirements for enhanced diagnostic systems
- Technology export development for advanced molecular diagnostic solutions targeting global healthcare markets
China Emerges as High-Growth Market
In Beijing, Shanghai, and Guangzhou, healthcare providers and diagnostic laboratories are implementing advanced molecular diagnostic systems as standard equipment for cancer detection and precision medicine applications, driven by increasing healthcare spending and modernization programs that emphasize the use of advanced diagnostic capabilities.
The market is projected to demonstrate an 8.1% CAGR through 2035, supported by government healthcare initiatives and medical infrastructure development programs that promote the use of advanced diagnostic tools for healthcare providers and laboratory operators. Chinese healthcare companies are adopting molecular diagnostic systems that provide superior detection capabilities and precision medicine enhancement features, particularly appealing in urban regions where healthcare quality represents critical operational requirements.
Market expansion benefits from growing healthcare technology capabilities and international technology transfer agreements that enable domestic development of advanced diagnostic systems for healthcare and medical applications. Technology adoption follows patterns established in healthcare infrastructure, where precision medicine enhancement and diagnostic quality drive procurement decisions and system deployment.
Market Intelligence Brief:
- Healthcare and diagnostic laboratory segments are driving initial adoption with 67% annual growth in molecular diagnostic system procurement
- Medical modernization programs emphasizing advanced diagnostic tools for precision medicine enhancement and patient management
- Local healthcare technology companies partnering with international providers for system development
- Hospital and laboratory services implementing molecular diagnostics for detection assistance and patient management
Germany Maintains Technology Leadership
Germany's advanced healthcare technology market demonstrates sophisticated molecular diagnostic deployment with documented detection effectiveness in hospital laboratory departments and diagnostic centers through integration with existing healthcare systems and medical infrastructure. The country leverages engineering expertise in healthcare technology and diagnostic systems integration to maintain a 7.8% CAGR through 2035.
Medical centers, including Munich, Berlin, and Hamburg, showcase premium installations where molecular diagnostics integrate with comprehensive hospital information systems and clinical platforms to optimize detection accuracy and operational workflow effectiveness.
German healthcare technology providers prioritize system reliability and regulatory compliance in molecular diagnostic development, creating demand for premium systems with advanced features, including clinical validation and integration with European healthcare standards. The market benefits from established healthcare industry infrastructure and a willingness to invest in advanced diagnostic technologies that provide long-term clinical benefits and compliance with medical regulations.
Market Intelligence Brief:
- Engineering focuses on healthcare standards and system integration, driving premium clinical segment growth
- Healthcare technology partnerships providing 47% faster clinical validation cycles
- Technology collaboration between German healthcare providers and international diagnostic companies
- Medical training programs are expanding molecular diagnostic integration in clinical management and patient care scenarios
UK Shows Strong Technology Innovation
UK's market expansion benefits from diverse healthcare demand, including medical modernization in London and Manchester, healthcare system upgrades, and government medical programs that increasingly incorporate advanced diagnostic solutions for cancer detection applications. The country maintains a 7.4% CAGR through 2035, driven by rising healthcare awareness and increasing adoption of molecular diagnostic benefits, including superior detection capabilities and improved patient outcomes.
Market dynamics focus on high-quality molecular diagnostic solutions that balance advanced detection features with clinical effectiveness considerations important to British healthcare providers. Growing healthcare infrastructure creates sustained demand for modern diagnostic systems in new medical facilities and healthcare equipment modernization projects.
Strategic Market Considerations:
- Healthcare and medical segments leading growth with focus on diagnostic automation and precision medicine enhancement applications
- Regional healthcare requirements are driving a diverse product portfolio from basic diagnostics to advanced precision medicine platforms
- NHS integration challenges offset by potential innovation partnerships with international healthcare technology manufacturers
- Government healthcare initiatives beginning to influence procurement standards and diagnostic requirements
Japan Demonstrates Precision and Integration Excellence
Japan demonstrates steady market development with a 7.2% CAGR through 2035, distinguished by healthcare providers' preference for high-quality molecular diagnostic systems that integrate seamlessly with existing medical equipment and provide reliable long-term operation in specialized healthcare applications. The market prioritizes advanced features, including precision diagnostic algorithms, clinical validation, and integration with comprehensive healthcare platforms that reflect Japanese medical expectations for technological sophistication and operational excellence.
- Premium focus on molecular systems with advanced diagnostic algorithms and high-precision capabilities
- Integration requirements with existing laboratory information systems and clinical management platforms
- Emphasis on diagnostic reliability and long-term performance in healthcare applications
India Emerges as Growing Market
In Delhi, Mumbai, and Bangalore, healthcare providers and diagnostic laboratories are implementing advanced molecular diagnostic systems to enhance detection capabilities and support clinical decision-making that aligns with Indian healthcare standards and medical regulations. The India market is expected to demonstrate sustained growth with a 7.0% CAGR through 2035, driven by healthcare modernization programs and medical equipment upgrades that emphasize advanced diagnostic tools for healthcare and clinical applications. Indian healthcare facilities are prioritizing molecular diagnostic systems that provide superior detection capabilities while maintaining compliance with healthcare regulations and minimizing operational costs, particularly important in oncology and specialized medical applications.
Market expansion benefits from healthcare procurement programs that support automated diagnostic capabilities in medical equipment specifications, creating sustained demand across India's healthcare sectors where operational effectiveness and regulatory compliance represent critical requirements. The regulatory framework supports molecular diagnostic adoption through healthcare device standards and medical technology requirements that promote advanced diagnostic systems aligned with national healthcare capabilities.
Strategic Market Indicators:
- Healthcare and medical systems are leading the adoption with healthcare equipment modernization programs requiring advanced diagnostic systems
- Government healthcare procurement providing regulatory support for advanced molecular diagnostic system acquisition
- Indian compatibility requirements are driving demand for standardized systems with national healthcare interoperability
- Specialized medical departments adopting comprehensive diagnostic solutions for precision medicine automation and patient management
France Drives European Innovation
France's market expansion benefits from diverse healthcare demand, including medical modernization in Paris and Lyon, healthcare system upgrades, and government medical programs that increasingly incorporate advanced diagnostic solutions for cancer detection applications. The country maintains a 6.7% CAGR through 2035, driven by rising healthcare awareness and increasing adoption of molecular diagnostic benefits, including superior detection capabilities and enhanced clinical outcomes.
Market dynamics focus on innovative molecular diagnostic solutions that balance advanced detection features with clinical effectiveness considerations important to French healthcare providers. Growing healthcare infrastructure creates sustained demand for modern diagnostic systems in new medical facilities and healthcare equipment modernization projects.
Strategic Market Considerations:
- Healthcare and clinical segments leading growth with focus on diagnostic automation and precision medicine enhancement applications
- Regional healthcare requirements are driving a diverse product portfolio from basic diagnostics to advanced clinical platforms
- EU regulatory compliance benefits offset by potential innovation partnerships with international healthcare technology manufacturers
- Government healthcare initiatives beginning to influence procurement standards and diagnostic requirements
Europe Market Split by Country
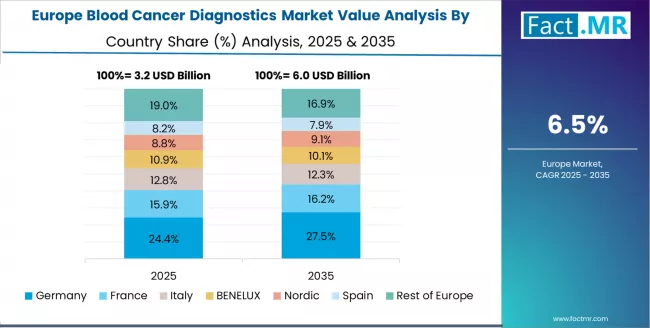
The Blood Cancer Diagnostics market in Europe is projected to grow from USD 3.1 billion in 2025 to USD 6.4 billion by 2035, registering a CAGR of 7.5% over the forecast period. Germany is expected to maintain its leadership position with a 28.3% market share in 2025, rising slightly to 29.1% by 2035, supported by its advanced healthcare technology sector and major medical research centers, including Munich and Berlin.
The United Kingdom follows with a 22.4% share in 2025, projected to reach 23.2% by 2035, driven by comprehensive healthcare modernization programs and precision medicine development initiatives. France holds a 17.8% share in 2025, expected to increase to 18.4% by 2035 through specialized healthcare applications and regulatory standardization requirements. Italy commands a 13.9% share in 2025, rising to 14.5% by 2035, while Spain accounts for 10.2% in 2025, increasing to 10.6% by 2035. The Rest of Europe region, including Nordic countries, Eastern Europe, BENELUX, and other markets, is anticipated to hold 7.4% in 2025, declining slightly to 4.2% by 2035, attributed to mixed growth patterns with moderate expansion in some advanced healthcare markets balanced by steady growth in smaller countries implementing medical technology development programs.
Competitive Landscape of the Blood Cancer Diagnostics Market
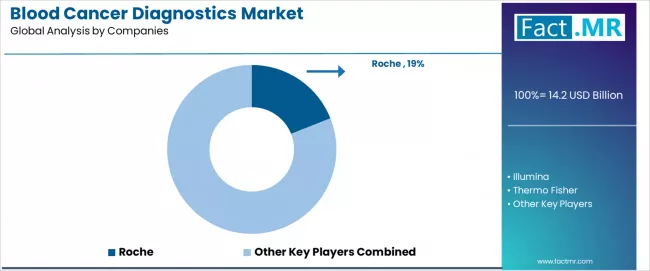
The Blood Cancer Diagnostics market operates with moderate concentration, featuring approximately 15-20 meaningful participants, where leading companies control roughly 58-63% of the global market share through established healthcare technology relationships and comprehensive diagnostic system portfolios. Competition emphasizes advanced diagnostic capabilities, system accuracy, and integration with healthcare platforms rather than price-based rivalry.
Market Leaders encompass Roche, Illumina, Thermo Fisher, Qiagen, and BD, which maintain competitive advantages through extensive healthcare technology expertise, global medical provider networks, and comprehensive system integration capabilities that create customer switching costs and support premium pricing. These companies leverage decades of diagnostic experience and ongoing research investments to develop advanced molecular diagnostic systems with precision detection algorithms and clinical validation features.
Technology Challengers include Sysmex, Agilent, Bio-Rad, Guardant, and Natera, which compete through specialized healthcare diagnostic focus and innovative detection interfaces that appeal to healthcare customers seeking advanced diagnostic capabilities and operational flexibility. These companies differentiate through rapid technology development cycles and specialized medical application focus.
Regional Specialists feature companies with focus on specific geographic markets and specialized applications, including hospital laboratory systems and integrated precision medicine platforms. Market dynamics favor participants that combine reliable diagnostic algorithms with advanced healthcare software, including precision cancer detection control and automatic clinical decision support capabilities. Competitive pressure intensifies as traditional healthcare equipment contractors expand into molecular diagnostic systems. At the same time, specialized diagnostic companies challenge established players through innovative cancer detection solutions and cost-effective platforms targeting specialized healthcare segments.
Key Players in the Blood Cancer Diagnostics Market
- Roche
- Illumina
- Thermo Fisher
- Qiagen
- BD
- Sysmex
- Agilent
- Bio-Rad
- Guardant
- Natera
Scope of the Report
| Item | Value |
|---|---|
| Quantitative Units | USD 14.2 Billion |
| Modality Type | Molecular/NGS, Flow Cytometry, Immunoassays/Others |
| Setting Type | Hospital Labs, Reference Labs, POCT/Near-patient |
| Indication Type | Leukemias, Lymphomas, Myeloma/MDS |
| Regions Covered | Asia Pacific, North America, Europe, Latin America, Middle East & Africa |
| Countries Covered | USA, China, Germany, UK, Japan, India, France, and 25+ additional countries |
| Key Companies Profiled | Roche, Illumina, Thermo Fisher, Qiagen, BD, Sysmex, Agilent, Bio-Rad, Guardant, Natera |
| Additional Attributes | Dollar sales by modality type and setting categories, regional adoption trends across Asia Pacific, North America, and Europe, competitive landscape with healthcare technology providers and diagnostic specialists, healthcare provider preferences for diagnostic effectiveness and system reliability, integration with laboratory information systems and clinical workflows, innovations in molecular algorithms and automated detection, and development of advanced solutions with enhanced precision and genomic capabilities. |
Frequently Asked Questions
How big is the Blood Cancer Diagnostics market in 2025?
The global Blood Cancer Diagnostics market is valued at USD 14.2 billion in 2025.
What will be the size of the Blood Cancer Diagnostics market in 2035?
The size of the Blood Cancer Diagnostics market is projected to reach USD 29.5 billion by 2035.
How much will the Blood Cancer Diagnostics market grow between 2025 and 2035?
The Blood Cancer Diagnostics market is expected to grow at a 7.6% CAGR between 2025 and 2035.
What are the key modality type segments in the Blood Cancer Diagnostics market?
The key modality type segments in the Blood Cancer Diagnostics market are Molecular/NGS, Flow Cytometry, and Immunoassays/Others.
Which setting segment is expected to contribute a significant share to the Blood Cancer Diagnostics market in 2025?
In terms of setting type, the Hospital Labs segment is set to command the dominant share in the Blood Cancer Diagnostics market in 2025.
Blood Cancer Diagnostics Market by Segments
-
Modality Type:
- Molecular/NGS
- Flow Cytometry
- Immunoassays/Others
-
Setting Type:
- Hospital Labs
- Reference Labs
- POCT/Near-patient
-
Indication Type:
- Leukemias
- Lymphomas
- Myeloma/MDS
-
Region:
- Asia Pacific
- China
- Japan
- South Korea
- India
- ASEAN
- Australia & New Zealand
- Rest of Asia Pacific
- North America
- United States
- Canada
- Mexico
- Europe
- Germany
- United Kingdom
- France
- Italy
- Spain
- Nordic
- BENELUX
- Rest of Europe
- Latin America
- Brazil
- Chile
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Other African Union
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Modality Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Modality Type , 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Modality Type , 2025 to 2035
- Molecular/NGS
- Flow Cytometry
- Immunoassays/Others
- Y to o to Y Growth Trend Analysis By Modality Type , 2020 to 2024
- Absolute $ Opportunity Analysis By Modality Type , 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Setting Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Setting Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Setting Type, 2025 to 2035
- Hospital Labs
- Reference Labs
- POCT/Near-patient
- Y to o to Y Growth Trend Analysis By Setting Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Setting Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Modality Type
- By Setting Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Modality Type
- By Setting Type
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Modality Type
- By Setting Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Modality Type
- By Setting Type
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Modality Type
- By Setting Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Modality Type
- By Setting Type
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Modality Type
- By Setting Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Modality Type
- By Setting Type
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Modality Type
- By Setting Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Modality Type
- By Setting Type
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Modality Type
- By Setting Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Modality Type
- By Setting Type
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Modality Type
- By Setting Type
- By Country
- Market Attractiveness Analysis
- By Country
- By Modality Type
- By Setting Type
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Modality Type
- By Setting Type
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Modality Type
- By Setting Type
- Competition Analysis
- Competition Deep Dive
- Roche
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Illumina
- Thermo Fisher
- Qiagen
- BD
- Sysmex
- Agilent
- Bio-Rad
- Guardant
- Natera
- Roche
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Modality Type , 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Setting Type, 2020 to 2035
- Table 4: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Modality Type , 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Setting Type, 2020 to 2035
- Table 7: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 8: Latin America Market Value (USD Million) Forecast by Modality Type , 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Setting Type, 2020 to 2035
- Table 10: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 11: Western Europe Market Value (USD Million) Forecast by Modality Type , 2020 to 2035
- Table 12: Western Europe Market Value (USD Million) Forecast by Setting Type, 2020 to 2035
- Table 13: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Eastern Europe Market Value (USD Million) Forecast by Modality Type , 2020 to 2035
- Table 15: Eastern Europe Market Value (USD Million) Forecast by Setting Type, 2020 to 2035
- Table 16: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 17: East Asia Market Value (USD Million) Forecast by Modality Type , 2020 to 2035
- Table 18: East Asia Market Value (USD Million) Forecast by Setting Type, 2020 to 2035
- Table 19: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 20: South Asia and Pacific Market Value (USD Million) Forecast by Modality Type , 2020 to 2035
- Table 21: South Asia and Pacific Market Value (USD Million) Forecast by Setting Type, 2020 to 2035
- Table 22: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 23: Middle East & Africa Market Value (USD Million) Forecast by Modality Type , 2020 to 2035
- Table 24: Middle East & Africa Market Value (USD Million) Forecast by Setting Type, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020-2035
- Figure 3: Global Market Value Share and BPS Analysis by Modality Type , 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Modality Type , 2025-2035
- Figure 5: Global Market Attractiveness Analysis by Modality Type
- Figure 6: Global Market Value Share and BPS Analysis by Setting Type, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Setting Type, 2025-2035
- Figure 8: Global Market Attractiveness Analysis by Setting Type
- Figure 9: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 11: Global Market Attractiveness Analysis by Region
- Figure 12: North America Market Incremental Dollar Opportunity, 2025-2035
- Figure 13: Latin America Market Incremental Dollar Opportunity, 2025-2035
- Figure 14: Western Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 15: Eastern Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 16: East Asia Market Incremental Dollar Opportunity, 2025-2035
- Figure 17: South Asia and Pacific Market Incremental Dollar Opportunity, 2025-2035
- Figure 18: Middle East & Africa Market Incremental Dollar Opportunity, 2025-2035
- Figure 19: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 20: North America Market Value Share and BPS Analysis by Modality Type , 2025 and 2035
- Figure 21: North America Market Y to o to Y Growth Comparison by Modality Type , 2025-2035
- Figure 22: North America Market Attractiveness Analysis by Modality Type
- Figure 23: North America Market Value Share and BPS Analysis by Setting Type, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Setting Type, 2025-2035
- Figure 25: North America Market Attractiveness Analysis by Setting Type
- Figure 26: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 27: Latin America Market Value Share and BPS Analysis by Modality Type , 2025 and 2035
- Figure 28: Latin America Market Y to o to Y Growth Comparison by Modality Type , 2025-2035
- Figure 29: Latin America Market Attractiveness Analysis by Modality Type
- Figure 30: Latin America Market Value Share and BPS Analysis by Setting Type, 2025 and 2035
- Figure 31: Latin America Market Y to o to Y Growth Comparison by Setting Type, 2025-2035
- Figure 32: Latin America Market Attractiveness Analysis by Setting Type
- Figure 33: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 34: Western Europe Market Value Share and BPS Analysis by Modality Type , 2025 and 2035
- Figure 35: Western Europe Market Y to o to Y Growth Comparison by Modality Type , 2025-2035
- Figure 36: Western Europe Market Attractiveness Analysis by Modality Type
- Figure 37: Western Europe Market Value Share and BPS Analysis by Setting Type, 2025 and 2035
- Figure 38: Western Europe Market Y to o to Y Growth Comparison by Setting Type, 2025-2035
- Figure 39: Western Europe Market Attractiveness Analysis by Setting Type
- Figure 40: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 41: Eastern Europe Market Value Share and BPS Analysis by Modality Type , 2025 and 2035
- Figure 42: Eastern Europe Market Y to o to Y Growth Comparison by Modality Type , 2025-2035
- Figure 43: Eastern Europe Market Attractiveness Analysis by Modality Type
- Figure 44: Eastern Europe Market Value Share and BPS Analysis by Setting Type, 2025 and 2035
- Figure 45: Eastern Europe Market Y to o to Y Growth Comparison by Setting Type, 2025-2035
- Figure 46: Eastern Europe Market Attractiveness Analysis by Setting Type
- Figure 47: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 48: East Asia Market Value Share and BPS Analysis by Modality Type , 2025 and 2035
- Figure 49: East Asia Market Y to o to Y Growth Comparison by Modality Type , 2025-2035
- Figure 50: East Asia Market Attractiveness Analysis by Modality Type
- Figure 51: East Asia Market Value Share and BPS Analysis by Setting Type, 2025 and 2035
- Figure 52: East Asia Market Y to o to Y Growth Comparison by Setting Type, 2025-2035
- Figure 53: East Asia Market Attractiveness Analysis by Setting Type
- Figure 54: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 55: South Asia and Pacific Market Value Share and BPS Analysis by Modality Type , 2025 and 2035
- Figure 56: South Asia and Pacific Market Y to o to Y Growth Comparison by Modality Type , 2025-2035
- Figure 57: South Asia and Pacific Market Attractiveness Analysis by Modality Type
- Figure 58: South Asia and Pacific Market Value Share and BPS Analysis by Setting Type, 2025 and 2035
- Figure 59: South Asia and Pacific Market Y to o to Y Growth Comparison by Setting Type, 2025-2035
- Figure 60: South Asia and Pacific Market Attractiveness Analysis by Setting Type
- Figure 61: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 62: Middle East & Africa Market Value Share and BPS Analysis by Modality Type , 2025 and 2035
- Figure 63: Middle East & Africa Market Y to o to Y Growth Comparison by Modality Type , 2025-2035
- Figure 64: Middle East & Africa Market Attractiveness Analysis by Modality Type
- Figure 65: Middle East & Africa Market Value Share and BPS Analysis by Setting Type, 2025 and 2035
- Figure 66: Middle East & Africa Market Y to o to Y Growth Comparison by Setting Type, 2025-2035
- Figure 67: Middle East & Africa Market Attractiveness Analysis by Setting Type
- Figure 68: Global Market - Tier Structure Analysis
- Figure 69: Global Market - Company Share Analysis
- FAQs -
How big is the blood cancer diagnostics market in 2025?
The global blood cancer diagnostics market is estimated to be valued at USD 14.2 billion in 2025.
What will be the size of blood cancer diagnostics market in 2035?
The market size for the blood cancer diagnostics market is projected to reach USD 29.5 billion by 2035.
How much will be the blood cancer diagnostics market growth between 2025 and 2035?
The blood cancer diagnostics market is expected to grow at a 7.6% CAGR between 2025 and 2035.
What are the key product types in the blood cancer diagnostics market?
The key product types in blood cancer diagnostics market are molecular/ngs, flow cytometry and immunoassays/others.
Which setting type segment to contribute significant share in the blood cancer diagnostics market in 2025?
In terms of setting type, hospital labs segment to command 0.0% share in the blood cancer diagnostics market in 2025.