Disposable Prefilled Syringes Market
Disposable Prefilled Syringes Market Size and Share Forecast Outlook 2025 to 2035
Disposable prefilled syringes market is projected to grow from USD 8.1 billion in 2025 to USD 29.8 billion by 2035, at a CAGR of 13.9%. Glass Syringes will dominate with a 57.4% market share, while vaccines & immunizations will lead the application segment with a 26.2% share.
Disposable Prefilled Syringes Market Forecast and Outlook 2025 to 2035
The global disposable prefilled syringes market is projected to reach USD 29.8 billion by 2035, recording an absolute increase of USD 21.7 billion over the forecast period. The market is valued at USD 8.1 billion in 2025 and is set to rise at a CAGR of 13.9% during the assessment period.
Quick Stats for Disposable Prefilled Syringes Market
- Disposable Prefilled Syringes Market Value (2025): USD 8.1 billion
- Disposable Prefilled Syringes Market Forecast Value (2035): USD 29.8 billion
- Disposable Prefilled Syringes Market Forecast CAGR: 13.9%
- Leading Material Type in Disposable Prefilled Syringes Market: Glass Syringes (57.4%)
- Key Growth Regions in Disposable Prefilled Syringes Market: Asia Pacific, North America, and Europe
- Top Players in Disposable Prefilled Syringes Market: Becton, Dickinson and Company (BD), Gerresheimer AG, SCHOTT AG, West Pharmaceutical Services, Inc., Medtronic, Roche Holding AG, Fresenius Kabi AG, SteriPack, Ypsomed AG, Meda Pharmaceuticals
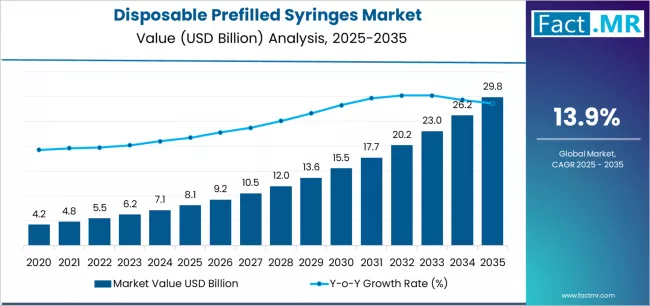
The market is expected to grow by approximately 3.7 times during the same period, supported by increasing prevalence of chronic diseases requiring injectable biologics and biosimilars worldwide, driving demand for ready-to-use drug delivery systems and increasing investments in patient-centric self-administration devices with enhanced safety features across vaccination campaigns and specialty pharmaceutical applications globally.
Healthcare providers and patients face mounting pressure to ensure medication accuracy and reduce administration errors while addressing safety concerns and contamination risks, with modern disposable prefilled syringes providing documented therapeutic benefits including precise dosing capabilities, reduced preparation time, and minimized needlestick injuries compared to conventional vial-and-syringe systems alone.
Rising adoption of home healthcare models and expanding biologics portfolio enabling convenient self-injection protocols create substantial opportunities for manufacturers and healthcare partners. However, higher unit costs compared to traditional syringes and material compatibility challenges with certain drug formulations may pose obstacles to widespread adoption across price-sensitive markets.
The glass syringes segment dominates market activity, driven by extensive pharmaceutical compatibility supporting safe storage of sensitive biologics and vaccines across diverse therapeutic applications worldwide. Healthcare facilities increasingly recognize the superior barrier properties of glass prefilled syringes, with typical product offerings providing excellent chemical inertness and dimensional stability at accessible price points through established pharmaceutical distribution networks.
The plastic syringes segment demonstrates robust growth potential, supported by rising demand for lightweight alternatives and innovation in cyclic olefin polymer technologies enabling break-resistant designs in modern drug delivery approaches. Vaccines & immunizations emerge as the dominant application segment, reflecting global immunization program expansion and pandemic preparedness initiatives in public health management routines. Hospitals represent the leading distribution channel, driven by bulk procurement contracts and centralized medication management systems enabling standardized administration protocols for injectable therapies.
Regional dynamics show North America maintaining market leadership, supported by high biologics consumption rates and established prefilled syringe adoption patterns across specialty pharmaceutical categories. Asia Pacific demonstrates the fastest growth trajectory driven by expanding pharmaceutical manufacturing capabilities and increasing chronic disease burden adopting advanced drug delivery approaches, while Europe emphasizes regulatory compliance and pharmaceutical-grade quality standards for injectable device systems. India leads country-level growth through extensive manufacturing expansion and cost-competitive production capabilities, followed by China supported by domestic biologics development and regional capacity scale-up initiatives.
The competitive landscape features moderate concentration with Becton, Dickinson and Company maintaining market leadership position at 19.0% share, while specialized players including Gerresheimer AG, SCHOTT AG, West Pharmaceutical Services, and Medtronic compete through advanced material technologies and comprehensive pharmaceutical partnerships across diverse therapeutic applications.
Disposable Prefilled Syringes Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2029, the disposable prefilled syringes market is projected to expand from USD 8.1 billion to USD 13.6 billion, resulting in a value increase of USD 5.5 billion, which represents 25.3% of the total forecast growth for the period. This phase of development will be shaped by rising demand for biologics and biosimilar injectables addressing chronic diseases, product innovation in safety-engineered devices with passive needle protection mechanisms and enhanced usability features, as well as expanding integration with digital health platforms and patient adherence monitoring systems. Companies are establishing competitive positions through investment in advanced glass formulation technologies, automated filling line capabilities, and strategic market expansion across hospital procurement channels, retail pharmacy networks, and direct-to-patient distribution models.
From 2029 to 2035, the market is forecast to grow from USD 13.6 billion to USD 29.8 billion, adding another USD 16.2 billion, which constitutes 74.7% of the overall expansion. This period is expected to be characterized by the expansion of specialized product applications, including high-concentration biologics requiring advanced container closure systems and wearable injector-compatible formats tailored for specific therapeutic areas, strategic collaborations between pharmaceutical companies and device manufacturers, and an enhanced focus on sustainability initiatives and eco-friendly material alternatives. The growing emphasis on personalized medicine protocols and rising patient preference for self-administration devices addressing multiple chronic conditions will drive demand for comprehensive drug delivery solutions across diverse therapeutic populations.
Disposable Prefilled Syringes Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 8.1 billion |
| Market Forecast Value (2035) | USD 29.8 billion |
| Forecast CAGR (2025-2035) | 13.9% |
Why is the Disposable Prefilled Syringes Market Growing?
The disposable prefilled syringes market grows by enabling healthcare providers and patients to administer injectable medications with enhanced safety, accuracy, and convenience while addressing medication errors and contamination risks without exclusive reliance on traditional vial-and-syringe preparation methods.
Healthcare facilities and patients face mounting pressure to ensure precise dosing and reduce needlestick injuries while managing complex biologic therapies, chronic disease treatments, and emergency medications, with modern prefilled syringe systems typically providing ready-to-use functionality including pre-measured doses for optimal therapeutic outcomes, integrated safety mechanisms preventing accidental needle exposure, and tamper-evident packaging ensuring medication integrity compared to conventional preparation workflows alone, making disposable prefilled syringes essential for comprehensive pharmaceutical care delivery.
The pharmaceutical industry's need for reliable and patient-friendly drug delivery systems creates demand for specialized prefilled syringe solutions that can provide consistent administration experiences, enhance medication adherence, and support home-based therapy without compromising sterility standards or causing preparation-related complications.
Healthcare provider endorsements and clinical evidence supporting prefilled syringe benefits drive adoption in hospital environments, ambulatory care settings, and home healthcare applications, where administration efficiency and safety outcomes have direct impact on patient quality of care and treatment success rates. The increasing development of biologics and biosimilars requiring injectable delivery, representing approximately 40% of pharmaceutical pipeline products, creates expanding market opportunities for advanced drug delivery systems.
Rising patient empowerment trends and preference for self-administration options enable informed treatment choices and adherence to evidence-based medication protocols. Compatibility challenges with certain drug formulations and higher unit costs compared to traditional vial systems may limit adoption rates and optimal implementation across resource-constrained healthcare environments serving diverse patient populations with different economic capabilities.
Segmental Analysis
The market is segmented by material, application, distribution channel, and region. By material, the market is divided into glass syringes and plastic syringes. Based on application, the market is categorized into vaccines & immunizations, anaphylaxis, rheumatoid arthritis, diabetes, autoimmune diseases, oncology, and others. By distribution channel, the market includes hospitals, retail pharmacies, and online pharmacies. Regionally, the market is divided into Asia Pacific, Europe, North America, Latin America, and Middle East & Africa.
By Material, Which Segment Accounts for the Dominant Market Share?
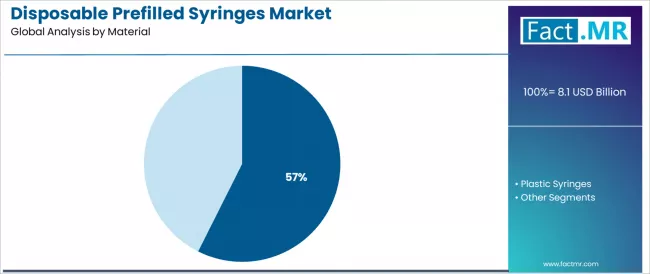
The glass syringes segment represents the dominant force in the disposable prefilled syringes market, capturing approximately 57.4% of the total market share in 2025. This established material category encompasses solutions featuring superior chemical resistance properties and dimensional stability characteristics, including advanced borosilicate glass formulations and surface treatment technologies that enable optimal drug compatibility and extended shelf-life performance across biologics delivery and vaccine storage applications worldwide.
The glass syringes segment's market leadership stems from its superior pharmaceutical compatibility profile, with solutions capable of maintaining drug stability for sensitive biologics including monoclonal antibodies, peptides, and vaccines while providing excellent barrier properties against oxygen and moisture permeation across diverse storage conditions and temperature ranges.
The plastic syringes segment maintains a substantial market share in the 42-43% range, serving cost-conscious healthcare facilities and emergency care applications that require break-resistant containers addressing safety concerns during transport and handling scenarios including pre-hospital emergency services and disaster response situations.
These solutions offer lightweight construction benefits for large-scale immunization campaigns and mass vaccination programs while providing sufficient compatibility to support conventional injectable medications and select biologics formulations. The plastic segment demonstrates strong growth potential, driven by expanding adoption of cyclic olefin copolymer and cyclic olefin polymer technologies offering glass-like transparency and drug compatibility without fragility concerns.
Within the material category, hybrid systems combining glass barrels with plastic components demonstrate growing interest, driven by pharmaceutical industry demands for optimized performance characteristics and manufacturing flexibility. This sub-segment benefits from component innovation and material science advances enabling customized drug delivery solutions.
Key therapeutic advantages driving the glass syringes segment include:
- Advanced chemical inertness mechanisms with clinically demonstrated compatibility across sensitive biologics and vaccines without leachables compromising drug stability across pharmaceutical applications
- Established manufacturing infrastructure allowing pharmaceutical-grade quality standards and regulatory compliance without extensive validation complexity for new material platforms
- Enhanced barrier properties features enabling extended shelf-life and temperature stability while maintaining formulation integrity and therapeutic consistency across global distribution networks
- Superior transparency profile providing optimal visual inspection capabilities for particulate detection and quality assurance protocols across various healthcare administration settings
By Application, Which Segment Accounts for the Largest Market Share?
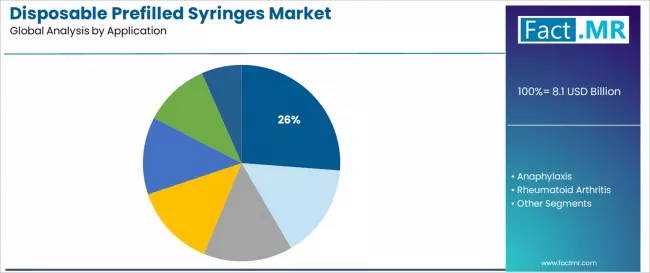
Vaccines & immunizations dominate the disposable prefilled syringes application landscape with a 26.2% market share in 2025, reflecting the critical role of standardized vaccination delivery systems in supporting global immunization programs, pandemic preparedness initiatives, and routine childhood vaccination schedules across healthcare facilities worldwide.
The vaccines & immunizations segment's market leadership is reinforced by public health priorities for mass immunization campaigns, cold-chain distribution requirements, and standardized dosing protocols that characterize comprehensive vaccination program implementations.
Within this segment, influenza vaccines represent a substantial application area, driven by annual vaccination campaigns and seasonal immunization requirements across global populations. This sub-segment benefits from established procurement patterns and healthcare provider familiarity with prefilled vaccine delivery systems.
The anaphylaxis segment represents a rapidly growing application category at 14.0% share, demonstrating strong expansion through specialized requirements for emergency epinephrine administration, auto-injector device integration, and life-saving medication accessibility in allergic emergency scenarios. This segment benefits from increasing food allergy prevalence patterns and regulatory mandates for epinephrine availability in schools and public facilities.
The rheumatoid arthritis segment maintains meaningful presence at 11.5% through chronic disease management requirements and biologic therapy administration protocols, while diabetes applications at 10.0% serve insulin delivery needs and emerging GLP-1 agonist markets supporting metabolic disease treatment. Autoimmune diseases command 9.0% share, oncology applications represent 8.5%, and other therapeutic areas including cardiovascular conditions and hormonal therapies collectively account for 20.8% of market activity.
Key market dynamics supporting application growth include:
- Vaccine delivery expansion driven by global immunization initiatives and pandemic preparedness programs, requiring rapid deployment capabilities and standardized administration protocols
- Emergency medication modernization trends require user-friendly designs for non-medical personnel administration and critical care accessibility
- Integration of chronic disease management protocols enabling patient self-administration and treatment adherence optimization across diverse therapeutic areas
- Growing emphasis on specialty pharmaceutical delivery supporting complex biologic therapies and targeted treatment approaches
By Distribution Channel, Which Segment Accounts for the Dominant Market Share?
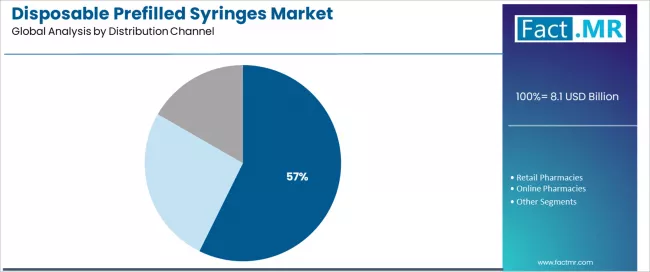
Hospitals dominate the disposable prefilled syringes distribution landscape with a 57.3% market share in 2025, reflecting the critical role of institutional healthcare facilities in supporting bulk medication procurement, centralized pharmacy operations, and standardized drug administration protocols across inpatient care, emergency departments, and surgical services worldwide. The hospitals segment's market leadership is reinforced by healthcare system preferences for group purchasing organizations, value-based contracting models, and integrated supply chain management systems that reduce medication preparation time and enhance patient safety outcomes.
Within this segment, large academic medical centers and tertiary care hospitals represent major procurement volumes, driven by complex patient populations requiring specialty medications and advanced biologic therapies. This sub-segment benefits from established formulary processes and pharmacy oversight supporting evidence-based device selection.
The retail pharmacies segment represents an important distribution category at 28.0% share demonstrating steady growth through community healthcare access, prescription fulfillment services, and patient counseling capabilities. Retail pharmacists provide valuable medication management support including proper administration technique education, storage requirement guidance, and adverse event monitoring that enhances patient safety and therapeutic outcomes in ambulatory care settings.
The online pharmacies segment maintains growing presence at 14.7% through digital health platform integration, home delivery convenience, and direct-to-patient distribution models, while specialty pharmacies serve dedicated patient populations requiring complex biologic therapies and comprehensive care coordination services.
Key market dynamics supporting distribution channel growth include:
- Hospital channel expansion driven by value-based care initiatives and medication safety programs, requiring standardized drug delivery systems and error reduction technologies
- Retail pharmacy modernization trends require patient education programs and refrigerated storage infrastructure supporting temperature-sensitive biologic medications
- Integration of e-commerce platforms enabling convenient home delivery and automatic refill services optimization
- Growing emphasis on specialty pharmacy networks supporting high-cost biologic therapies and comprehensive patient support programs
What are the Drivers, Restraints, and Key Trends of the Disposable Prefilled Syringes Market?
The market is driven by three concrete demand factors tied to pharmaceutical delivery outcomes. First, rising biologics and biosimilars development creates expanding therapeutic portfolios requiring advanced drug delivery systems, with prefilled syringes representing a critical enabling technology for temperature-sensitive medications in comprehensive pharmaceutical care protocols, requiring widespread product availability. Second, growing emphasis on medication safety and error reduction drives healthcare facility adoption and regulatory support, with numerous studies demonstrating significant benefits in dosing accuracy, contamination prevention, and needlestick injury reduction through standardized prefilled syringe systems by 2030. Third, increasing patient preference for self-administration and home-based therapy enables more convenient chronic disease management approaches that improve treatment adherence while reducing healthcare facility visits and associated costs.
Market restraints include higher unit costs compared to traditional vial-and-syringe systems that can challenge adoption rates and budget allocations across cost-sensitive healthcare markets, particularly in regions where reimbursement structures remain limited and procurement decisions prioritize lowest-cost alternatives over safety and efficiency benefits. Drug-container compatibility challenges for certain pharmaceutical formulations pose another significant obstacle, as disposable prefilled syringes require extensive compatibility testing and stability studies, potentially affecting product development timelines and formulation flexibility. Limited recycling infrastructure and environmental concerns regarding single-use medical devices create additional sustainability pressures, demanding innovative material solutions and waste management initiatives.
Key trends indicate accelerated connected device integration in developed markets, particularly North America and Europe, where healthcare systems demonstrate willingness to invest in smart prefilled syringes with dose tracking capabilities, temperature monitoring sensors, and digital health platform connectivity. Self-administration technology trends toward patient-centric designs with ergonomic features, visual feedback systems, and simplified operation protocols enable convenient home-based therapy that optimizes treatment adherence and clinical outcomes. However, the market thesis could face disruption if significant advances in alternative drug delivery technologies including oral biologics formulations or transdermal delivery systems reduce reliance on traditional injectable administration approaches.
Analysis of the Disposable Prefilled Syringes Market by Key Countries
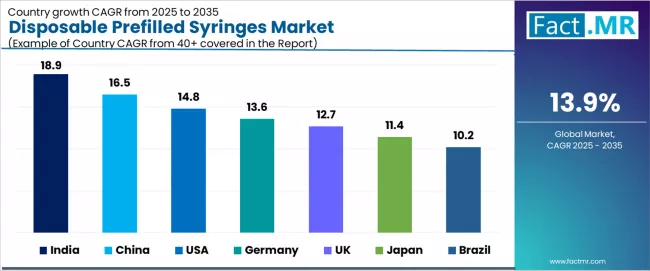
| Country | CAGR (2025-2035) |
|---|---|
| India | 18.9% |
| China | 16.5% |
| USA | 14.8% |
| Germany | 13.6% |
| UK | 12.7% |
| Japan | 11.4% |
| Brazil | 10.2% |
The global disposable prefilled syringes market is expanding rapidly, with India leading at an 18.9% CAGR through 2035, driven by cost-competitive manufacturing expansion, rising chronic disease treatment access, and local capacity builds supporting domestic and export markets. China follows at 16.5%, supported by rapid capacity expansion, rising domestic biologics development, and regional production scale-up initiatives. USA records 14.8%, reflecting strong biologics adoption, high hospital procurement volumes, and e-grocery/pharmacy integration.
Germany advances at 13.6%, leveraging mature pharmaceutical manufacturing base, regulatory-driven single-use adoption, and specialty glass demand. UK posts 12.7%, focusing on aging population pressure, NHS procurement shifts, and growth in self-administration applications, while Japan grows steadily at 11.4%, emphasizing high per-capita biologic use, automation, and regulatory support for advanced delivery systems. Brazil demonstrates 10.2% growth, anchored by growing vaccination programs, improving cold-chain distribution, and regional manufacturing investments.
How is India Leading Global Market Expansion?
India demonstrates the strongest growth potential in the disposable prefilled syringes market with a CAGR of 18.9% through 2035. The country's leadership position stems from rapidly expanding pharmaceutical manufacturing capabilities, cost-competitive production infrastructure, and growing domestic demand for injectable biologics and vaccines driving adoption of advanced drug delivery systems.
Growth is concentrated in major pharmaceutical manufacturing clusters and metropolitan healthcare centers, including Mumbai, Hyderabad, Bangalore, and Ahmedabad, where manufacturers are increasingly establishing prefilled syringe production facilities for domestic consumption and international export markets.
Distribution channels through hospital procurement systems, retail pharmacy networks, and government immunization programs expand product accessibility across urban populations and rural healthcare initiatives. The country's growing integration of Good Manufacturing Practice standards with international quality requirements provides strong momentum for pharmaceutical-grade device production, including comprehensive adoption across income segments seeking affordable yet high-quality injectable medications.
Key market factors:
- Manufacturing hub development concentrated in pharmaceutical zones and special economic areas with advanced production capabilities
- Export growth through international pharmaceutical partnerships, regulatory approvals, and cost-competitive manufacturing advantages
- Comprehensive healthcare infrastructure expansion ecosystem, including hospital modernization programs with proven medication safety capabilities
- Local production development featuring companies establishing state-of-the-art filling lines offering competitively priced formulations
Why is China Emerging as a High-Growth Market?
In major urban centers including Beijing, Shanghai, Guangzhou, and Shenzhen, the adoption of disposable prefilled syringe solutions is accelerating across healthcare facilities and pharmaceutical manufacturers, driven by increasing domestic biologics development and growing emphasis on medication safety standards. The market demonstrates strong growth momentum with a CAGR of 16.5% through 2035, linked to comprehensive pharmaceutical industry modernization trends and increasing focus on high-value drug delivery systems.
Chinese pharmaceutical companies are implementing advanced manufacturing technologies and quality control systems to meet international standards while serving growing domestic demand for specialty medications. The country's expanding middle class creates ongoing demand for premium healthcare products, while increasing regulatory emphasis on pharmaceutical packaging safety drives adoption of standardized prefilled syringe systems.
Key development areas:
- Pharmaceutical manufacturing modernization leading prefilled syringe adoption with emphasis on biologics production and vaccine manufacturing
- Distribution expansion through both hospital group purchasing organizations and rapidly growing retail pharmacy chains
- Technology integration enabling automated filling lines and quality control systems meeting international pharmaceutical standards
- Growing investment in domestic glass production capabilities alongside contract manufacturing services offering integrated solutions
What drives USA’s Market Resilience?
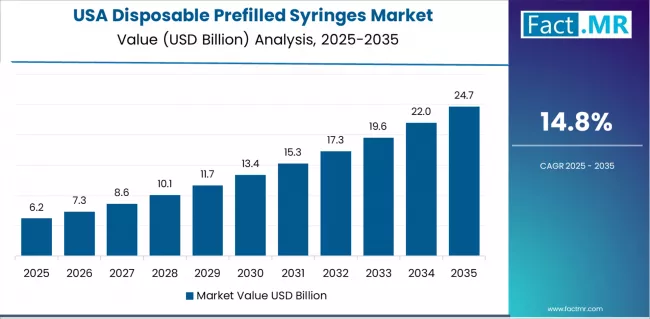
USA’s market expansion is driven by diverse healthcare delivery preferences, including specialty pharmaceutical distribution in integrated health systems and convenient self-administration solutions across home healthcare segments. The country demonstrates steady growth potential with a CAGR of 14.8% through 2035, supported by continuous innovation from established device manufacturers and pharmaceutical companies prioritizing patient-centric delivery systems.
American healthcare providers face implementation challenges related to reimbursement complexity and formulary management requirements, requiring manufacturers to demonstrate clear clinical value propositions and total cost-of-care benefits. However, established biologics consumption patterns and high medication safety standards create stable baseline demand for disposable prefilled syringes, particularly among specialty pharmacy networks and integrated delivery systems where evidence-based drug delivery technologies drive primary procurement decisions.
Market characteristics:
- Specialty pharmaceutical distribution and hospital group purchasing showing robust demand with substantial annual procurement across therapeutic applications
- Regional preferences varying between safety-engineered device emphasis in major healthcare systems and cost-effective solutions in ambulatory care settings
- Future projections indicate continued innovation with emphasis on connected devices with digital health integration and patient adherence monitoring
- Growing emphasis on sustainability initiatives and pharmaceutical waste reduction supporting eco-friendly packaging innovations
How does Germany Demonstrate Manufacturing Excellence Leadership?
The market in Germany leads in pharmaceutical-grade device manufacturing based on integration with rigorous quality standards and advanced glass formulation technologies for enhanced drug compatibility. The country shows strong potential with a CAGR of 13.6% through 2035, driven by strict regulatory oversight and pharmaceutical industry preferences for premium-quality devices in major markets, including Bavaria, North Rhine-Westphalia, Baden-Württemberg, and Hesse.
German pharmaceutical companies are adopting disposable prefilled syringes through established relationships with device manufacturers and emphasis on medication safety protocols for comprehensive drug delivery, particularly in healthcare-conscious populations and quality-focused treatment protocols demanding rigorous validation credentials. Distribution channels through hospital pharmacy departments and specialized pharmaceutical distributors expand coverage across healthcare facilities and research institutions.
Leading market segments:
- Pharmaceutical-grade device adoption in major manufacturing centers implementing comprehensive quality management systems
- Strategic partnerships between pharmaceutical companies and device manufacturers achieving high integration rates
- Technology collaborations between German manufacturers and international partners expanding innovation capabilities
- Focus on sustainability initiatives and recyclable materials addressing environmental requirements
What positions UK for Healthcare Integration Leadership?
In London, Manchester, Birmingham, and other major cities, healthcare providers are implementing disposable prefilled syringe solutions through NHS procurement frameworks and clinical guideline recommendations, with documented case studies showing substantial improvements in medication administration efficiency through standardized delivery protocols.
The market shows steady growth potential with a CAGR of 12.7% through 2035, linked to ongoing healthcare system modernization, aging population medication needs, and emerging self-administration models in community care regions.
Healthcare facilities are adopting evidence-based prefilled syringes with NHS guidance to enhance medication safety while maintaining standards demanded by national healthcare quality frameworks. The country's established pharmaceutical distribution infrastructure creates ongoing opportunities for innovative device launches that differentiate through clinical validation and healthcare professional endorsement.
Market development factors:
- Aging population demographics leading adoption of chronic disease medications across UK
- NHS procurement modernization providing growth opportunities in standardized medication delivery systems
- Strategic partnerships between pharmaceutical companies and healthcare trusts expanding formulary inclusion
- Emphasis on patient self-administration training and home healthcare expansion supporting community-based therapy
How does Japan Show Quality Innovation Leadership?
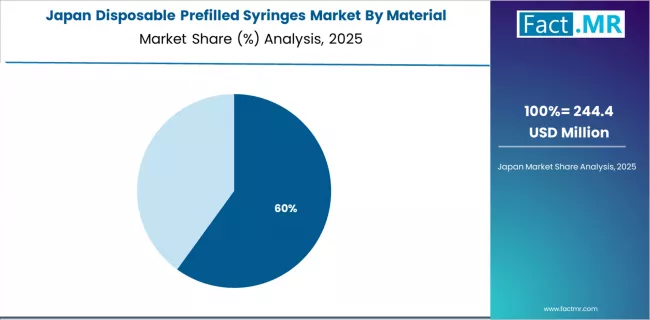
Japan's disposable prefilled syringes market demonstrates sophisticated preferences focused on device precision and pharmaceutical compatibility optimization, with documented integration of advanced glass manufacturing technologies achieving substantial improvement in drug stability and administration accuracy across specialty pharmaceutical applications.
The country maintains steady growth momentum with a CAGR of 11.4% through 2035, driven by mature market dynamics emphasizing device excellence and continuous quality improvement methodologies that align with Japanese pharmaceutical standards applied to injectable medication delivery. Major metropolitan areas, including Tokyo, Osaka, Nagoya, and Fukuoka, showcase advanced adoption of prefilled syringe protocols where pharmaceutical-grade materials integrate seamlessly with automated administration systems and comprehensive patient safety programs.
Key market characteristics:
- Quality-conscious healthcare providers driving demand for premium devices with emphasis on precision and reliability
- Manufacturing excellence enabling consistent device performance with comprehensive validation programs
- Technology collaboration between Japanese companies and international device manufacturers expanding product portfolios
- Emphasis on aging population care and chronic disease management addressing demographic healthcare needs
What Characterizes Brazil's Market Development?
In major metropolitan centers including São Paulo, Rio de Janeiro, Brasília, and Belo Horizonte, the adoption of disposable prefilled syringe solutions is expanding across public and private healthcare facilities, driven by increasing vaccination program requirements and growing chronic disease prevalence. The market demonstrates solid growth potential with a CAGR of 10.2% through 2035, linked to comprehensive healthcare infrastructure improvements and increasing focus on medication safety standardization in hospital and primary care channels.
Brazilian healthcare providers are implementing modern drug delivery practices and purchasing standardized prefilled products to enhance vaccination efficiency while meeting growing expectations in urban healthcare environments. The country's expanding pharmaceutical manufacturing sector creates ongoing demand for locally produced devices, while increasing cold-chain infrastructure investments drive adoption of temperature-sensitive biologic formulations.
Key development areas:
- Public health vaccination programs leading prefilled syringe adoption with emphasis on immunization campaign efficiency
- Distribution expansion through public hospital networks and private healthcare facilities providing accessible product availability
- Local manufacturing development supporting competitive pricing and market-appropriate device specifications
- Integration of pharmaceutical partnerships and technology transfer initiatives supporting domestic production capabilities
Europe Market Split by Country
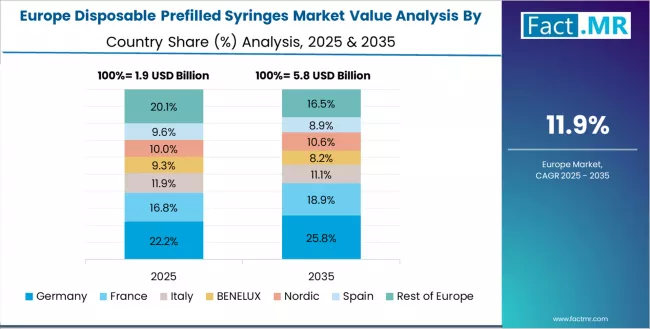
The disposable prefilled syringes market in Europe is projected to grow from USD 2.4 billion in 2025 to USD 9.1 billion by 2035, registering a CAGR of 14.2% over the forecast period. Germany is expected to maintain its leadership position with a 29.5% market share in 2025, adjusting to 28.8% by 2035, supported by its advanced pharmaceutical manufacturing infrastructure, specialty glass production capabilities, and comprehensive regulatory compliance frameworks serving major European markets.
France follows with a 20.5% share in 2025, projected to reach 21.0% by 2035, driven by comprehensive biologics development programs and hospital procurement modernization in major healthcare regions implementing standardized medication delivery protocols. UK holds an 18.0% share in 2025, expected to maintain 18.2% by 2035 through ongoing NHS formulary integration and self-administration program expansion.
Italy commands a 16.0% share, while Spain accounts for 12.5% in 2025. The rest of Europe region is anticipated to gain momentum, expanding its collective share from 3.5% to 4.5% by 2035, attributed to increasing prefilled syringe adoption in Nordic countries and emerging Eastern European markets implementing modern pharmaceutical distribution practices.
Competitive Landscape of the Disposable Prefilled Syringes Market
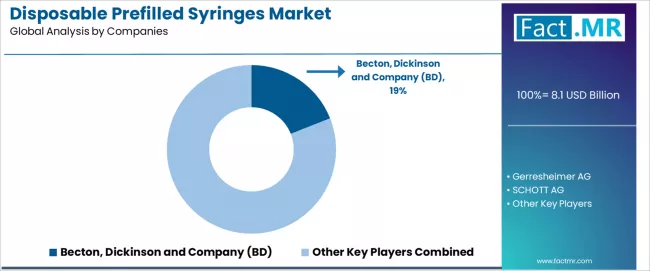
The disposable prefilled syringes market features approximately 10-15 meaningful players with moderate concentration, where the top three companies control roughly 40-50% of global market share through established manufacturing capabilities, extensive pharmaceutical partnerships, and multi-region production networks. Competition centers on material innovation, pharmaceutical compatibility validation, and integrated drug-device development rather than price competition alone.
Market leaders include Becton, Dickinson and Company (BD) with a 19.0% market share, along with Gerresheimer AG and SCHOTT AG, which maintain competitive advantages through comprehensive glass and polymer manufacturing expertise, advanced filling technology capabilities, and deep relationships with pharmaceutical companies, creating high credibility among drug developers seeking reliable container closure systems. These companies leverage proprietary glass formulations and coating technologies to defend market positions while expanding into adjacent categories including autoinjector platforms and combination product applications.
Challengers encompass specialized device manufacturers including West Pharmaceutical Services and contract development organizations like SteriPack, which compete through targeted pharmaceutical service offerings and strong engagement with biologics developers through integrated drug-device development partnerships. Medical device specialists, including Medtronic, Ypsomed AG, and Meda Pharmaceuticals, focus on specific therapeutic applications or delivery technologies, offering differentiated capabilities in safety-engineered devices, patient-centric designs, and specialty pharmaceutical formulations.
Emerging pharmaceutical packaging companies and regional manufacturers create competitive pressure through innovative material solutions and cost-effective production approaches, particularly in high-growth markets including India and China, where local manufacturing capabilities provide advantages in domestic market access and competitive pricing strategies.
Market dynamics favor companies that combine pharmaceutical-grade manufacturing standards with customer-centric development partnerships that address the complete drug lifecycle from formulation compatibility through commercial-scale production and post-market surveillance. Strategic emphasis on sustainability initiatives, break-resistant materials, and connected device technologies enables differentiation in increasingly competitive pharmaceutical packaging segments across developed and emerging markets.
Global Disposable Prefilled Syringes Market — Stakeholder Contribution Framework
Disposable prefilled syringes represent a critical pharmaceutical delivery system that enables healthcare providers and patients to administer injectable medications with enhanced safety, accuracy, and convenience while addressing medication errors and contamination risks without exclusive pharmaceutical preparation dependency, typically providing ready-to-use functionality including pre-measured doses for optimal therapeutic outcomes, integrated safety mechanisms preventing needlestick injuries, and tamper-evident packaging ensuring medication integrity compared to conventional vial-and-syringe systems alone while ensuring improved administration efficiency and comprehensive patient safety outcomes.
With the market projected to grow from USD 8.1 billion in 2025 to USD 29.8 billion by 2035 at a 13.9% CAGR, these solutions offer compelling advantages for biologics delivery applications, hospital distribution channels, and diverse therapeutic populations seeking standardized medication administration. Scaling market penetration and clinical acceptance requires coordinated action across pharmaceutical policy, device manufacturing standards, pharmaceutical companies, healthcare providers, and patient safety initiatives.
How Could Governments Spur Local Development and Adoption?
- Healthcare Infrastructure Programs: Include medication safety initiatives in national quality improvement programs, providing targeted support for prefilled syringe adoption campaigns in hospital settings and supporting research institutions through development grants and compatibility testing funding.
- Tax Policy & Investment Support: Implement reduced tax rates for pharmaceutical device manufacturers, provide tax incentives for companies investing in advanced filling line technologies and quality assurance systems, and establish favorable import duty structures that encourage pharmaceutical-grade glass procurement over conventional alternatives.
- Regulatory Framework Development: Create streamlined approval processes for new prefilled syringe configurations across pharmaceutical applications, establish clear compatibility testing standards and stability documentation requirements for drug-device combinations, and develop international harmonization protocols that facilitate cross-border regulatory acceptance.
- Healthcare Integration & Coverage: Fund integration of standardized drug delivery systems in public healthcare facilities, invest in healthcare provider training initiatives that bridge conventional preparation methods with prefilled device adoption, and explore reimbursement enhancement models for safety-engineered medication delivery systems.
- Research & Innovation Support: Establish public-private partnerships for device-drug compatibility research, support academic-industry collaborations investigating material science innovations and optimal formulation protocols, and create regulatory environments that encourage innovation in sustainable packaging approaches.
How Could Industry Bodies Support Market Development?
- Clinical Standards & Evidence: Define standardized compatibility testing endpoints for prefilled syringe materials across pharmaceutical formulation types, establish universal quality specifications and extractables testing protocols, and create evidence databases that pharmaceutical developers can rely on for device selection decisions.
- Market Education & Best Practices: Lead messaging that demonstrates prefilled syringe benefits, emphasizing medication safety improvements, administration efficiency gains, and realistic cost-benefit analyses compared to traditional preparation-only approaches.
- Quality Assurance Standards: Develop guidelines for material selection, manufacturing processes, sterilization validation requirements, and stability testing protocols, ensuring pharmaceutical compatibility across production and distribution operations.
- Professional Development: Run certification programs for healthcare providers, pharmacy technicians, and patient educators on optimizing prefilled syringe protocols, proper handling procedures, and patient administration training in diverse healthcare populations.
How Could Manufacturers and Technology Players Strengthen the Ecosystem?
- Advanced Product Development: Develop next-generation prefilled syringe platforms with enhanced drug compatibility, optimized needle configurations, and application-specific characteristics that enhance pharmaceutical stability while improving patient experience and administration success rates.
- Clinical Validation Programs: Provide comprehensive compatibility testing data, stability study results, and usability research that supports pharmaceutical developer confidence and informed device selection aligned with formulation requirements.
- Healthcare Professional Education: Offer comprehensive training programs about prefilled syringe handling, administration techniques, and patient counseling approaches that help providers deliver safe medication administration aligned with institutional protocols.
- Research & Development Networks: Build comprehensive R&D capabilities, collaborative pharmaceutical partnerships, and compatibility testing systems that ensure prefilled syringes maintain high performance standards and consistent quality across diverse formulation requirements.
How Could Healthcare Providers and Educators Navigate the Market?
- Evidence-Based Protocol Integration: Incorporate validated prefilled syringe systems into medication administration protocols, with particular focus on high-risk medications, emergency drugs, and patient self-administration programs for comprehensive pharmaceutical care.
- Staff Training Excellence: Establish comprehensive education programs addressing device selection, proper handling techniques, and patient counseling frameworks through optimized training protocols and competency assessments.
- Multidisciplinary Care Models: Implement integrated medication management teams combining pharmacists, nurses, physicians, and patient educators that provide comprehensive drug delivery oversight addressing clinical, operational, and patient experience aspects.
- Outcomes Monitoring Systems: Develop standardized medication error tracking, needlestick injury surveillance, and patient satisfaction assessments that enable continuous protocol optimization and safety improvement.
How Could Investors and Financial Enablers Unlock Value?
- Manufacturing Capacity Financing: Provide growth capital for established companies like BD, Gerresheimer, and SCHOTT to fund production line expansions and advanced filling technology investments that support increasing pharmaceutical demand.
- Innovation Investment: Back startups developing advanced materials, connected device technologies, and sustainable packaging solutions that enhance pharmaceutical compatibility and environmental performance.
- Market Expansion Funding: Finance distribution infrastructure development for device manufacturers establishing operations in high-growth regions including India and China, supporting localization initiatives that address regional pharmaceutical manufacturing needs while maintaining quality standards.
- Digital Health Integration: Support companies developing smart syringe platforms, dose tracking systems, and patient engagement applications that enhance medication adherence and clinical outcomes through technology-enabled pharmaceutical delivery.
Key Players in the Disposable Prefilled Syringes Market
- Becton, Dickinson and Company (BD)
- Gerresheimer AG
- SCHOTT AG
- West Pharmaceutical Services, Inc.
- Medtronic
- Roche Holding AG
- Fresenius Kabi AG
- SteriPack
- Ypsomed AG
- Meda Pharmaceuticals
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 8.1 Billion |
| Material | Glass Syringes, Plastic Syringes |
| Application | Vaccines & Immunizations, Anaphylaxis, Rheumatoid Arthritis, Diabetes, Autoimmune Diseases, Oncology, Others |
| Distribution Channel | Hospitals, Retail Pharmacies, Online Pharmacies |
| Regions Covered | Asia Pacific, Europe, North America, Latin America, Middle East & Africa |
| Country Covered | India, China, USA, Germany, UK, Japan, Brazil, and 40+ countries |
| Key Companies Profiled | Becton, Dickinson and Company (BD), Gerresheimer AG, SCHOTT AG, West Pharmaceutical Services, Inc., Medtronic, Roche Holding AG, Fresenius Kabi AG, SteriPack, Ypsomed AG, Meda Pharmaceuticals |
| Additional Attributes | Dollar sales by material and application categories, regional adoption trends across Asia Pacific, Europe, and North America, competitive landscape with device manufacturers and pharmaceutical packaging companies, product specifications and compatibility requirements, integration with pharmaceutical development programs and biologics manufacturing, innovations in material science and safety-engineered designs, and development of specialized applications with drug-device combination products and patient-centric delivery capabilities. |
Disposable Prefilled Syringes Market by Segments
-
Material :
- Glass Syringes
- Plastic Syringes
-
Application :
- Vaccines & Immunizations
- Anaphylaxis
- Rheumatoid Arthritis
- Diabetes
- Autoimmune Diseases
- Oncology
- Others
-
Distribution Channel :
- Hospitals
- Retail Pharmacies
- Online Pharmacies
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia
- Thailand
- Rest of Asia Pacific
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Denmark
- Sweden
- Norway
- Rest of Europe
- North America
- USA
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Rest of Latin America
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
- Kuwait
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Material
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Material, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Material, 2025 to 2035
- Glass Syringes
- Plastic Syringes
- Y to o to Y Growth Trend Analysis By Material, 2020 to 2024
- Absolute $ Opportunity Analysis By Material, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Application
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Application, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Application, 2025 to 2035
- Vaccines & Immunizations
- Anaphylaxis
- Rheumatoid Arthritis
- Diabetes
- Autoimmune Diseases
- Oncology
- Others
- Y to o to Y Growth Trend Analysis By Application, 2020 to 2024
- Absolute $ Opportunity Analysis By Application, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Distribution Channel
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Distribution Channel, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Distribution Channel, 2025 to 2035
- Hospitals
- Retail Pharmacies
- Online Pharmacies
- Y to o to Y Growth Trend Analysis By Distribution Channel, 2020 to 2024
- Absolute $ Opportunity Analysis By Distribution Channel, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Material
- By Application
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Material
- By Application
- By Distribution Channel
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Material
- By Application
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Material
- By Application
- By Distribution Channel
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Material
- By Application
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Material
- By Application
- By Distribution Channel
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Material
- By Application
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Material
- By Application
- By Distribution Channel
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Material
- By Application
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Material
- By Application
- By Distribution Channel
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Material
- By Application
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Material
- By Application
- By Distribution Channel
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Material
- By Application
- By Distribution Channel
- By Country
- Market Attractiveness Analysis
- By Country
- By Material
- By Application
- By Distribution Channel
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Material
- By Application
- By Distribution Channel
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Material
- By Application
- By Distribution Channel
- Competition Analysis
- Competition Deep Dive
- Becton, Dickinson and Company (BD)
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Gerresheimer AG
- SCHOTT AG
- West Pharmaceutical Services, Inc.
- Medtronic
- Roche Holding AG
- Fresenius Kabi AG
- SteriPack
- Ypsomed AG
- Meda Pharmaceuticals
- Becton, Dickinson and Company (BD)
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Material, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Material, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Material, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Material, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Material, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Material, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Material, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Material, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Application, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by Distribution Channel, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Material, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Material, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Material
- Figure 6: Global Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Application
- Figure 9: Global Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by Distribution Channel
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Material, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Material, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Material
- Figure 26: North America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Application
- Figure 29: North America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by Distribution Channel
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Material, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Material, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Material
- Figure 36: Latin America Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Application
- Figure 39: Latin America Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by Distribution Channel
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Material, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Material, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Material
- Figure 46: Western Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Application
- Figure 49: Western Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by Distribution Channel
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Material, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Material, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Material
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Application
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by Distribution Channel
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Material, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Material, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Material
- Figure 66: East Asia Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Application
- Figure 69: East Asia Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by Distribution Channel
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Material, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Material, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Material
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Application
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by Distribution Channel
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Material, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Material, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Material
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Application, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Application, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Application
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by Distribution Channel, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by Distribution Channel, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by Distribution Channel
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the disposable prefilled syringes market in 2025?
The global disposable prefilled syringes market is estimated to be valued at USD 8.1 billion in 2025.
What will be the size of disposable prefilled syringes market in 2035?
The market size for the disposable prefilled syringes market is projected to reach USD 29.8 billion by 2035.
How much will be the disposable prefilled syringes market growth between 2025 and 2035?
The disposable prefilled syringes market is expected to grow at a 13.9% CAGR between 2025 and 2035.
What are the key product types in the disposable prefilled syringes market?
The key product types in disposable prefilled syringes market are glass syringes and plastic syringes.
Which application segment to contribute significant share in the disposable prefilled syringes market in 2025?
In terms of application, vaccines & immunizations segment to command 26.2% share in the disposable prefilled syringes market in 2025.