Pediatric Cardiac Tumor Diagnostics Market
Pediatric Cardiac Tumor Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
Pediatric cardiac tumor diagnostics market is projected to grow from USD 0.7 billion in 2025 to USD 1.6 billion by 2035, at a CAGR of 8.2%. Primary Cardiac Tumors will dominate with a 84.5% market share, while echocardiography will lead the diagnostics type segment with a 61.0% share.
Pediatric Cardiac Tumor Diagnostics Market Forecast and Outlook 2025 to 2035
The pediatric cardiac tumor diagnostics market stands at the threshold of transformative expansion, with projections indicating growth from USD 0.72 billion in 2025 to USD 1.58 billion by 2035. This trajectory, characterized by a compound annual growth rate of 8.2%, reflects the critical evolution in early detection capabilities, advanced imaging technologies, and specialized diagnostics protocols designed to address the unique challenges of identifying cardiac tumors in pediatric populations where clinical presentation often differs significantly from adult manifestations.
Quick Stats for Pediatric Cardiac Tumor Diagnostics Market
- Pediatric Cardiac Tumor Diagnostics Market Value (2025): USD 0.72 billion
- Pediatric Cardiac Tumor Diagnostics Market Forecast Value (2035): USD 1.58 billion
- Pediatric Cardiac Tumor Diagnostics Market Forecast CAGR: 8.2%
- Leading Diagnostics Type in Pediatric Cardiac Tumor Diagnostics Market: Echocardiography
- Key Growth Regions in Pediatric Cardiac Tumor Diagnostics Market: North America, Europe, and Asia Pacific
- Top Players in Pediatric Cardiac Tumor Diagnostics Market: GE HealthCare, Siemens Healthineers AG, Koninklijke Philips N.V., Canon Medical Systems Corp., Boston Scientific Corp.
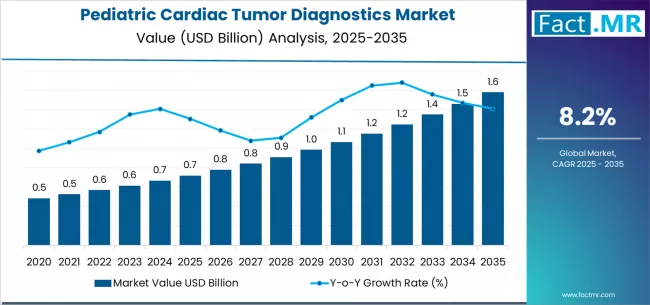
The first half of the forecast period (2025-2030) will witness the market ascending from USD 0.72 billion to approximately USD 1.08 billion, adding USD 0.36 billion in incremental value. This phase represents 42% of the total decade-long expansion and will be characterized by accelerating adoption of AI-enhanced echocardiography systems, non-invasive real-time MRI protocols reducing sedation requirements, and high-resolution imaging platforms capable of detecting tumors in early developmental stages.
Enhanced diagnostics accuracy through machine learning algorithms and automated image analysis will transition from experimental applications to standard clinical practice across specialized pediatric cardiac centers and tertiary care facilities.
The latter half of the period (2030-2035) will demonstrate sustained momentum, with the market expanding from USD 1.08 billion to USD 1.58 billion, representing an addition of USD 0.50 billion or 58% of the decade's total growth. This accelerated expansion phase will be defined by widespread integration of photon-counting CT technology, real-time MRI systems eliminating anesthesia requirements for younger patients, and comprehensive diagnostics platforms combining multiple imaging modalities with biomarker analysis for definitive tumor characterization.
The market trajectory signals fundamental transformations in how pediatric cardiologists and oncologists approach cardiac tumor detection, moving from reactive diagnosis following symptom presentation to proactive screening protocols in high-risk populations.
Geographic diversity will characterize market expansion, with North America maintaining technological leadership through advanced AI-driven diagnostics systems and comprehensive pediatric cardiac programs. Europe will demonstrate innovation strength particularly in MRI development and research collaborations advancing non-invasive imaging protocols.
Asia Pacific will emerge as the fastest-growing region driven by government healthcare infrastructure investments, expanding pediatric oncology awareness, and increasing accessibility to advanced diagnostics equipment across major metropolitan hospitals and specialized cardiac centers.
The clinical imperative driving market growth extends beyond technological advancement to fundamental improvements in patient outcomes. Early and accurate diagnosis of pediatric cardiac tumors, particularly rhabdomyomas representing 42% of primary cardiac tumors, enables timely intervention decisions, surgical planning optimization, and appropriate clinical management strategies that significantly impact long-term survival rates and quality of life for affected children.
The integration of artificial intelligence, enhanced imaging resolution, and multi-modal diagnostics approaches represents not merely technological progress but essential evolution in pediatric cardiac care delivery.
Where Revenue Comes From — Now vs Next (Industry-Level View)
| Period | Primary Revenue Buckets | Share | Notes |
|---|---|---|---|
| Today | Primary Cardiac Tumors | 84.5% | Rhabdomyoma prevalence, infant detection |
| Secondary Cardiac Tumors | 15.5% | Metastatic imaging advancement | |
| Echocardiography | 61.0% | Non-invasive standard, real-time diagnosis | |
| Magnetic Resonance Imaging | 25.2% | Superior tissue characterization | |
| Computed Tomography | 8.5% | Rapid assessment capability | |
| Hospitals and Clinics | 74.0% | Specialized infrastructure dominance | |
| Government Health Programs | 16.1% | Funding expansion initiatives | |
| Future (3-5 yrs) | Primary Tumor Detection | 82-84% | Sustained rhabdomyoma screening |
| Secondary Tumor Imaging | 16-18% | AI metastatic detection growth | |
| AI-Enhanced Echo Systems | 63-66% | Machine learning integration | |
| Real-Time MRI Platforms | 27-30% | Sedation-free pediatric imaging | |
| Photon-Counting CT | 9-11% | Radiation reduction technology | |
| Specialized Cardiac Centers | 75-78% | Equipment concentration expansion | |
| Telemedicine Diagnostics | 3-5% | Remote consultation emergence |
Pediatric Cardiac Tumor Diagnostics Market Key Takeaways
At-a-Glance Metrics
| Metric | Value |
|---|---|
| Market Value (2025) → | USD 0.72 billion |
| Market Forecast (2035) ↑ | USD 1.58 billion |
| Growth Rate ★ | 8.2% CAGR |
| Leading Diagnostics Type → | Echocardiography |
| Primary End Use → | Hospitals and Clinics |
The market demonstrates specialized fundamentals with echocardiography capturing the dominant share through non-invasive real-time imaging capabilities and proven effectiveness in detecting primary cardiac tumors during routine pediatric examinations. Hospital and clinic infrastructure maintains primary diagnostics leadership supported by specialized pediatric cardiac expertise, advanced imaging equipment availability, and comprehensive multidisciplinary care coordination essential for accurate tumor characterization.
Geographic expansion remains concentrated in North America with established pediatric cardiac programs and advanced AI diagnostics integration, while Asia Pacific shows accelerating adoption rates driven by government healthcare investments and expanding pediatric oncology awareness. MRI technology exhibits rapid growth trajectory, reflecting clinical preference for superior soft tissue visualization and elimination of ionizing radiation exposure critical for pediatric patient safety.
Imperatives for Stakeholders in Pediatric Cardiac Tumor Diagnostics Market
Design for Pediatric-Specific Requirements, Not Adult Protocol Adaptation
- Offer complete diagnostics ecosystems: age-appropriate imaging systems + sedation-free protocols + pediatric-trained technologists + child-friendly examination environments + family education resources.
- Preconfigured clinical workflows: rapid scanning protocols, motion artifact reduction algorithms, age-specific reference databases, and automated measurement tools optimized for pediatric cardiac anatomy.
Technology Readiness for AI Integration
- Real-time image analysis with automated tumor detection, predictive diagnostics algorithms identifying high-risk presentations, and clinical decision support systems (machine learning pattern recognition, multi-modal data fusion, longitudinal monitoring capabilities).
Safety-by-Design Approach
- Minimal radiation exposure protocols, sedation elimination through rapid acquisition, child-appropriate equipment sizing, and comprehensive safety documentation meeting pediatric imaging standards.
Value-Based Care Models
- Clear equipment pricing + transparent service packages (installation support, pediatric protocol development, ongoing technical training); outcome-based reimbursement alignment and telemedicine consultation integration for remote expert interpretation.
Segmental Analysis
The market is segmented by tumor type into primary cardiac tumors and secondary cardiac tumors, representing the fundamental diagnostics differentiation between tumors originating within cardiac tissue versus metastatic lesions from systemic malignancies requiring distinct imaging protocols and clinical management approaches.
The diagnostics type segmentation divides the market into echocardiography, magnetic resonance imaging (MRI), computed tomography (CT), and others, reflecting varied clinical applications based on tumor characteristics, patient age, hemodynamic stability, and required diagnostics precision for treatment planning.
End-use segmentation encompasses hospitals and clinics, government health departments, and others, demonstrating diverse healthcare delivery settings with varying equipment accessibility, specialized expertise availability, and patient population characteristics.
The segmentation structure reveals diagnostics evolution from conventional echocardiography-based screening toward comprehensive multi-modal imaging strategies incorporating AI-enhanced analysis and sedation-free protocols, while clinical delivery spans from specialized pediatric cardiac centers with cutting-edge technology to government health initiatives expanding diagnostics accessibility across underserved populations.
Why do Primary Cardiac Tumors Account for Dominant Market Share?
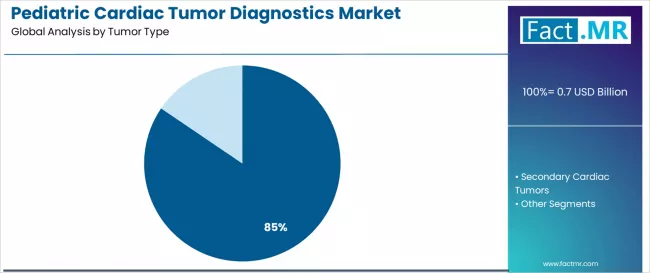
Primary cardiac tumors command the leading position in the pediatric cardiac tumor diagnostics market with 84.5% market share. This is attributed to clinical prevalence characteristics, including rhabdomyoma, congenital presentation patterns enabling early detection, and benign tumor characteristics requiring definitive diagnostics characterization to differentiate from malignant pathologies and guide clinical management decisions.
The segment benefits from established screening protocols during routine prenatal and postnatal cardiac examinations that enable incidental tumor detection before symptomatic presentation, creating opportunities for early diagnostics intervention and longitudinal monitoring. Rhabdomyomas' strong association with tuberous sclerosis complex necessitates comprehensive cardiac imaging as part of syndromic evaluation protocols, driving consistent diagnostics volume.
Advanced echocardiography capabilities enable detailed tumor characterization including size measurement, chamber involvement assessment, and hemodynamic impact evaluation that inform surgical planning and conservative management strategies without exposing pediatric patients to ionizing radiation or invasive procedures.
Primary cardiac tumors treatment differentiates through well-established natural history understanding, predictable imaging characteristics enabling confident diagnosis, and clinical management protocols integrating diagnostics findings with multidisciplinary care coordination involving pediatric cardiology, cardiac surgery, and genetics specialties.
The segment's diagnostics approaches leverage non-invasive imaging modalities appropriate for repeated monitoring during tumor evolution, particularly relevant for rhabdomyomas demonstrating spontaneous regression patterns requiring serial assessment without cumulative radiation exposure.
Key Market Characteristics:
- Advanced echocardiographic protocols with harmonic imaging, strain analysis, and three-dimensional reconstruction capabilities optimizing tumor visualization
- Enhanced MRI sequences enabling tissue characterization distinguishing benign rhabdomyomas from rare malignant cardiac tumors requiring aggressive intervention
- Prenatal diagnostics capabilities identifying cardiac masses during fetal echocardiography, enabling delivery planning and immediate postnatal management coordination
What drives Secondary Cardiac Tumors Growth Trajectory?
Secondary cardiac tumors maintain a 15.5% market share in 2025. The market is expected to advance at a CAGR of 9.6% until 2035. This is due to advancing metastatic detection capabilities through AI-powered imaging analysis and improved systemic oncology surveillance protocols. The segment appeals to comprehensive cancer staging requirements where cardiac involvement significantly impacts treatment selection and prognosis assessment.
The segment’s expansion is driven by sophisticated cross-sectional imaging technologies including whole-body MRI and PET-CT integration that identify cardiac metastases during systematic staging evaluations, combined with heightened clinical awareness of cardiac involvement patterns in pediatric malignancies requiring thorough cardiac assessment.
By Diagnostics Type, which Category will Maintain Market Leadership?
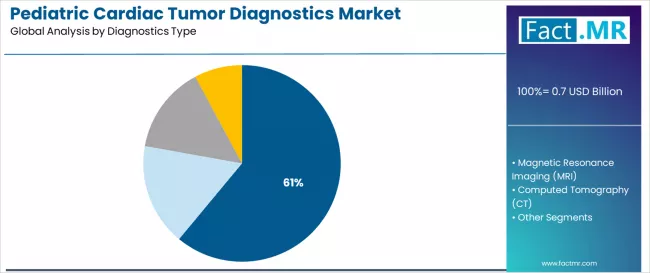
Echocardiography demonstrates market leadership in the pediatric cardiac tumor diagnostics market with 61.0% share due to its non-invasive examination characteristics, real-time imaging capabilities, portability enabling bedside assessment, and absence of ionizing radiation. This makes its modality ideal for repeated examinations in pediatric populations requiring serial monitoring throughout tumor evolution and treatment response assessment.
Clinicians prioritize echocardiography's immediate availability in most pediatric cardiac centers, comprehensive hemodynamic assessment capabilities evaluating tumor impact on cardiac function, and patient tolerance advantages eliminating sedation requirements for cooperative children.
Echocardiography-based diagnostics preference is supported by established technical expertise among pediatric sonographers, standardized examination protocols optimizing tumor detection, and integration with Doppler capabilities assessing blood flow patterns around cardiac masses. Cost-effectiveness compared to advanced cross-sectional imaging modalities supports utilization as first-line diagnostics approach and primary monitoring tool throughout clinical management.
AI-enhanced echocardiography systems are experiencing increasing adoption, driven by automated border detection, tumor measurement algorithms, and decision support tools that improve diagnostics accuracy and examination efficiency. Technology innovations including three-dimensional echocardiography, contrast-enhanced techniques, and strain imaging expand diagnostics capabilities beyond simple tumor identification to comprehensive functional impact assessment informing clinical decision-making.
Why does MRI Demonstrate the Fastest Growth among all Diagnostics Modalities?
Magnetic resonance imaging (MRI) captures 25.2% market share and is expected to progress at a 9.4% CAGR until 2035. This is attributed to its superior soft tissue characterization capabilities, multiparametric imaging sequences distinguishing tumor composition, and elimination of ionizing radiation exposure critical for pediatric safety considerations.
The segment benefits from real-time MRI technological advances reducing examination duration and eliminating sedation requirements that historically limited pediatric applicability, while cardiac-gated sequences enable motion-artifact-free tumor visualization throughout cardiac cycle phases.
By End Use, What Drives Hospitals and Clinics' Dominant Position?
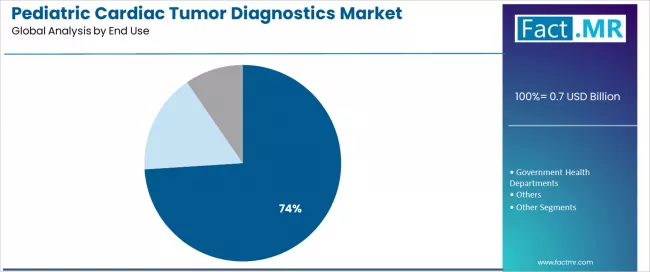
Hospitals and clinics demonstrate market leadership in the pediatric cardiac tumor diagnostics market with 74.0% share, reflecting specialized infrastructure requirements for advanced cardiac imaging equipment, multidisciplinary expertise concentration enabling comprehensive diagnostics evaluation, and integrated care delivery models coordinating diagnostics findings with immediate therapeutic intervention capabilities essential for managing critically ill pediatric cardiac tumor patients.
Healthcare facilities prioritize equipment investment concentration in high-volume centers achieving operational efficiency and technical expertise development, comprehensive support services including pediatric anesthesiology for sedation protocols and pediatric radiology specialists interpreting complex cardiac imaging studies.
The segment benefits from tertiary referral patterns directing suspected cardiac tumor cases to specialized centers possessing advanced imaging capabilities and experienced multidisciplinary teams, while quality assurance programs and accreditation requirements drive continuous technology upgrades and protocol optimization.
Specialized pediatric cardiac centers expanding globally increase diagnostics accessibility, while hospital networks investing in AI-enhanced imaging platforms and telemedicine consultation capabilities extend diagnostics expertise to broader geographic regions. Integration with pediatric oncology programs creates comprehensive care pathways streamlining diagnosis-to-treatment timelines and improving patient outcomes.
Application Dynamics Include:
- Strong growth in dedicated pediatric cardiac imaging centers establishing specialized protocols and child-friendly examination environments
- Increasing adoption of hybrid imaging suites enabling seamless transition from diagnostics evaluation to interventional procedures or surgical planning
- Rising integration with electronic health records and picture archiving systems facilitating multidisciplinary tumor board discussions and remote expert consultations
How do Government Health Departments Accelerate Market Expansion?
Government health departments capture 16.1% market share in 2025. The segment is expected to advance at a 9.0% CAGR from 2025 to 2035. This is attributed to public health initiatives funding pediatric cardiac screening programs, equipment procurement subsidies expanding diagnostics accessibility in underserved regions, and awareness campaigns improving early detection rates through systematic referral protocols for suspected cardiac abnormalities in pediatric populations.
What are the Drivers, Restraints, and Key Trends of the Pediatric Cardiac Tumor Diagnostics Market?
| Category | Factor | Impact | Why It Matters |
|---|---|---|---|
| Driver | AI integration & automated diagnostics algorithms (machine learning tumor detection, image analysis automation) | ★★★★★ | Artificial intelligence enhances diagnostics accuracy, reduces interpretation time, and enables standardized tumor characterization across varying operator expertise levels; creates competitive differentiation for technology providers. |
| Driver | Prenatal diagnostics advancement & fetal imaging protocols (improved fetal echocardiography, early detection capabilities) | ★★★★★ | Earlier tumor identification enables delivery planning, immediate postnatal intervention readiness, and improved outcomes through proactive management; expands diagnostics market to prenatal screening applications. |
| Driver | Sedation-free imaging technology development (rapid acquisition MRI, cooperative imaging protocols) | ★★★★☆ | Elimination of anesthesia requirements reduces procedural risks, healthcare costs, and examination complexity; improves patient safety and expands diagnostics accessibility for younger children. |
| Restraint | Limited pediatric cardiac imaging expertise & specialized training requirements (technologist shortages, interpretation complexity) | ★★★★☆ | Specialized knowledge requirements for pediatric cardiac anatomy and tumor characteristics limit diagnostics program expansion; creates workforce development challenges and geographic access disparities. |
| Restraint | High equipment costs & infrastructure investment barriers (advanced imaging system pricing, facility requirements) | ★★★☆☆ | Capital-intensive technology limits diagnostics availability in resource-constrained settings; concentrates diagnostics capabilities in major academic centers creating referral bottlenecks and access inequities. |
| Trend | Multi-modal imaging integration & comprehensive diagnostics platforms (echo-MRI-CT correlation, biomarker integration) | ★★★★★ | Growing demand for definitive tumor characterization combining multiple imaging modalities with molecular diagnostics; creates opportunities for integrated diagnostics solutions and comprehensive care pathways. |
| Trend | Telemedicine consultation & remote expert interpretation (image sharing platforms, virtual tumor boards) | ★★★★☆ | Technology-enabled expertise distribution improves diagnostics quality in community hospitals; expands market reach for specialized interpretation services and educational partnerships. |
Analysis of the Pediatric Cardiac Tumor Diagnostics Market by Key Countries
The pediatric cardiac tumor diagnostics market demonstrates diverse regional dynamics with growth leaders including India (9.7% growth rate) and China (9.4% growth rate) driving expansion through government-backed healthcare infrastructure investments, domestic medical equipment manufacturing initiatives, and systematic pediatric oncology program development.
Strong performers encompass Brazil (8.8% growth rate), Germany (8.3% growth rate), and the USA (8.0% growth rate), benefiting from established pediatric cardiac programs, advanced diagnostics technology adoption, and comprehensive research initiatives. Developed markets feature UK (7.7% growth rate) and Japan (7.4% growth rate), where specialized pediatric cardiac centers and cutting-edge imaging research support consistent growth patterns.
Regional synthesis reveals Asian markets leading adoption through government healthcare modernization initiatives, expanding pediatric specialty hospital networks, and increasing accessibility to advanced diagnostics equipment previously concentrated in developed economies, while Western countries maintain strong expansion supported by AI technology integration, clinical research advancement, and comprehensive care model refinement. Emerging markets show robust growth driven by awareness campaigns improving early detection and systematic screening protocol implementation.
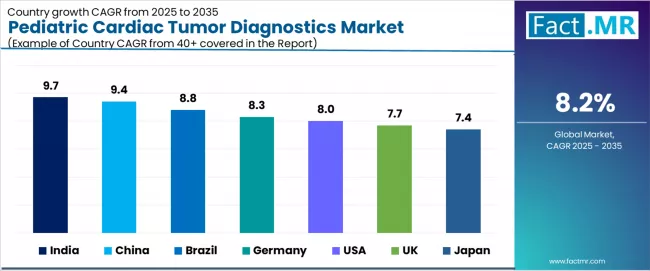
| Region/Country | 2025-2035 Growth | How to Win | What to Watch Out |
|---|---|---|---|
| India | 9.7% | Focus on government partnerships and affordable AI systems | Infrastructure gaps; trained workforce shortages |
| China | 9.4% | Lead with domestic manufacturing and AI integration | Quality standardization; regional access disparities |
| Brazil | 8.8% | Expand public health diagnostics programs | Economic volatility; equipment import costs |
| USA | 8.0% | Emphasize AI-enabled advanced imaging | Market maturity; reimbursement complexity |
| Germany | 8.3% | Invest in pediatric oncology R&D partnerships | Regulatory requirements; specialized expertise concentration |
| UK | 7.7% | Leverage NHS pediatric cardiac networks | Budget constraints; Brexit supply chain impacts |
| Japan | 7.4% | Position photon-counting CT innovations | Aging infrastructure; demographic challenges |
Why is India Driving the Fastest Market Growth?
India establishes fastest market growth through comprehensive government healthcare initiatives targeting pediatric cardiac care accessibility and systematic investments in domestic medical equipment manufacturing capabilities reducing import dependency for advanced diagnostics systems.
The country's 9.7% growth rate reflects National Health Mission programs expanding pediatric specialty services, increasing institutional capacity for complex cardiac diagnostics, and growing awareness among healthcare providers regarding systematic cardiac screening protocols for suspected pediatric tumors.
Growth concentrates in major metropolitan medical centers including AIIMS Delhi, Apollo Hospitals network facilities, and specialized pediatric cardiac institutions where advanced imaging equipment deployment showcases integration with comprehensive multidisciplinary care teams.
Indian medical equipment manufacturers are developing cost-effective diagnostics solutions that balance affordability requirements with clinical performance standards, incorporating AI-enhanced analysis capabilities and telemedicine connectivity enabling remote expert consultation from tertiary centers to peripheral facilities. Government partnerships with international imaging companies facilitate technology transfer, local production initiatives, and training program development creating sustainable diagnostics capacity expansion across diverse geographic regions.
How does China Emerge as a High-Volume Diagnostics Hub?
In Beijing, Shanghai, Guangzhou, and Chengdu, specialized pediatric cardiac centers are implementing advanced tumor diagnostics protocols integrating AI-powered imaging analysis with comprehensive clinical evaluation, driven by government healthcare modernization initiatives and systematic pediatric oncology program development.
The market holds a 9.4% growth rate, supported by domestic medical equipment manufacturing leadership enabling cost-effective deployment of advanced echocardiography and MRI systems across expanding hospital networks. Chinese healthcare institutions are adopting cutting-edge diagnostics technologies that provide rapid tumor characterization and treatment planning support, particularly appealing in urban regions where specialized pediatric cardiac surgery capabilities enable immediate therapeutic intervention following diagnostics confirmation.
Market expansion benefits from massive healthcare infrastructure investment programs targeting maternal and child health services, creating dedicated pediatric cardiac diagnostics facilities within major hospital systems. Technology adoption follows patterns established in adult cardiac imaging, where domestic manufacturers including Mindray and United Imaging Healthcare develop competitive alternatives to international premium brands, enabling broader diagnostics accessibility across tier-2 and tier-3 cities beyond coastal metropolitan concentrations.
What drives Brazil's Government Investment-Led Growth?
Brazil establishes government investment-led growth through systematic pediatric oncology program expansion and public health initiatives improving early cardiac tumor detection through standardized referral protocols from primary care settings to specialized diagnostics centers.
The country's 8.8% growth rate reflects Ministry of Health programs funding advanced imaging equipment procurement for public hospital networks, increasing diagnostics accessibility for underserved populations, and growing clinical awareness regarding cardiac tumor presentations in pediatric patients.
Growth concentrates in major metropolitan regions including São Paulo, Rio de Janeiro, and Brasília, where university-affiliated pediatric hospitals showcase comprehensive diagnostics capabilities appealing to both public insurance beneficiaries and private healthcare consumers.
Brazilian healthcare institutions focus on optimizing diagnostics efficiency through protocol standardization and technologist training programs, creating demand for imaging systems with simplified operation, automated analysis capabilities, and robust telemedicine connectivity enabling expert consultation from specialized academic centers.
The market benefits from established pediatric cardiology networks and commitment to reducing childhood mortality through systematic screening programs that identify cardiac abnormalities requiring further diagnostics evaluation.
How does USA Demonstrate AI Technology Leadership?
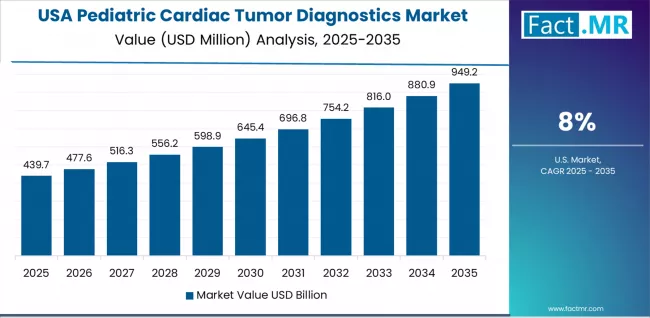
The USA establishes AI technology leadership through extensive research funding supporting machine learning algorithm development, comprehensive clinical validation studies establishing diagnostics accuracy benchmarks, and rapid commercial deployment of AI-enhanced imaging systems across major pediatric cardiac centers.
The country's 8.0% growth rate reflects mature diagnostics infrastructure with high baseline adoption rates, ongoing technology refinement improving examination efficiency, and increasing integration of artificial intelligence throughout diagnostics workflows from image acquisition to automated reporting.
Growth concentrates across major academic medical centers including Boston Children's Hospital, Children's Hospital of Philadelphia, and Texas Children's Hospital, where cutting-edge research programs showcase AI application development that subsequently diffuses to broader clinical practice.
American healthcare institutions leverage established pediatric cardiac imaging expertise and comprehensive clinical research infrastructure, including FDA-approved AI diagnostics algorithms and commercial partnerships between academic centers and medical device manufacturers accelerating innovation translation to clinical deployment.
The market benefits from sophisticated reimbursement frameworks supporting advanced diagnostics technology utilization and outcome-based care models incentivizing accurate early diagnosis improving long-term patient outcomes while reducing overall healthcare expenditure through timely intervention.
Why does Germany Show Pediatric Oncology R&D Leadership?
Germany's advanced pediatric healthcare system demonstrates sophisticated cardiac tumor diagnostics integration with documented clinical effectiveness through comprehensive research programs and systematic quality improvement initiatives.
The country leverages engineering excellence in medical imaging technology development and collaborative research networks connecting university hospitals, equipment manufacturers, and clinical research organizations to maintain an 8.3% growth rate.
Major pediatric cardiac centers including German Heart Center Berlin, University Hospital Heidelberg, and Hannover Medical School showcase premium diagnostics capabilities where imaging systems integrate with comprehensive tumor registry programs and longitudinal outcome tracking supporting continuous protocol optimization.
German healthcare institutions prioritize evidence-based diagnostics approaches and systematic quality assurance programs, creating demand for imaging systems with comprehensive documentation capabilities, standardized examination protocols, and integration with clinical research databases.
The market benefits from established pediatric subspecialty training programs producing highly skilled cardiac imaging experts and commitment to technology innovation through public-private research partnerships advancing diagnostics capabilities.
How does the UK Demonstrate NHS Integration Leadership?
The UK's comprehensive healthcare system demonstrates strategic pediatric cardiac tumor diagnostics integration through National Health Service coordination networks connecting specialized cardiac centers with community pediatric services via systematic referral pathways and telemedicine consultation programs.
The country maintains a 7.7% growth rate, leveraging established pediatric cardiac surgery centers including Great Ormond Street Hospital and Royal Brompton Hospital serving as diagnostics hubs with advanced imaging capabilities. Major population centers demonstrate integrated diagnostics approaches where imaging findings coordinate seamlessly with multidisciplinary tumor boards and treatment planning discussions optimizing clinical management decisions.
British healthcare institutions prioritize diagnostics efficiency and systematic care pathway optimization, creating demand for imaging systems with rapid examination protocols, automated analysis reducing interpretation time, and comprehensive connectivity supporting remote expert consultation across NHS trust networks. The market benefits from Siemens Healthineers Oxford MRI research hub driving innovation in pediatric cardiac imaging and commitment to evidence-based protocol development through collaborative clinical research programs.
What explains Japan's Photon-Counting CT Innovation Focus?
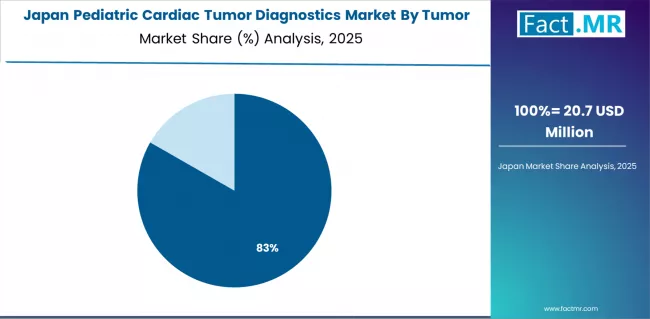
Japan's technology-advanced healthcare system demonstrates sophisticated pediatric cardiac tumor diagnostics capabilities through cutting-edge imaging technology development and systematic radiation dose reduction initiatives critical for pediatric patient safety. The country maintains a 7.4% growth rate, leveraging traditional medical technology manufacturing excellence and collaborative research programs advancing diagnostics innovation.
Major urban medical centers including National Center for Child Health and Development in Tokyo and Osaka Medical Center showcase premium diagnostics systems where photon-counting CT technology enables superior spatial resolution with minimal radiation exposure, while advanced MRI protocols eliminate sedation requirements through rapid acquisition techniques.
Japanese medical equipment manufacturers prioritize pediatric-specific technology refinement and comprehensive clinical validation programs, creating demand for imaging systems with advanced safety features, child-appropriate protocols, and integration with comprehensive electronic health record systems. The market benefits from established pediatric subspecialty care networks and commitment to continuous quality improvement through systematic outcome monitoring and protocol optimization based on accumulated clinical experience.
Europe Market Split by Country
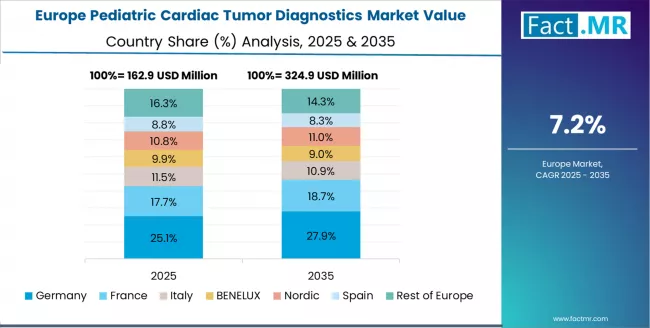
The pediatric cardiac tumor diagnostics market in Europe is projected to grow from USD 0.21 billion in 2025 to USD 0.39 billion by 2035, registering a CAGR of 8.1% over the forecast period. Germany is expected to maintain its leadership position with a 29.3% market share in 2025, stabilizing at 29.0% by 2035, supported by its advanced pediatric cardiac research infrastructure and major specialized centers including German Heart Center Berlin and University Hospital Heidelberg driving diagnostics innovation.
The UK follows with a 24.2% share in 2025, projected to reach 24.5% by 2035, driven by comprehensive NHS pediatric cardiac networks and Siemens Healthineers Oxford MRI research hub advancing imaging technology. France holds a 18.7% share in 2025, expected to reach 19.1% by 2035 due to expanding pediatric cardiology programs at major university hospitals.
Italy commands a 13.5% share, while Spain accounts for 10.8% in 2025. The rest of Europe region is anticipated to expand its collective share from 3.5% to 3.7% by 2035, attributed to increasing pediatric cardiac diagnostics capacity in Nordic countries and emerging Eastern European specialized cardiac centers implementing comprehensive tumor screening protocols as part of broader pediatric oncology program development.
How are South Korea and Japan Advancing Radiation-Free Imaging Solutions?
South Korea demonstrates rapid pediatric cardiac diagnostics advancement through government healthcare technology initiatives funding advanced MRI system deployment and comprehensive pediatric hospital network expansion, where cardiac tumor protocols integrate with systematic congenital heart disease screening programs. The market benefits from domestic medical equipment manufacturing capabilities and established telemedicine infrastructure enabling remote expert consultation from specialized cardiac centers to regional pediatric hospitals.
Japan maintains established market maturity through medical technology innovation leadership and systematic pediatric patient safety initiatives, where photon-counting CT technology development and rapid-acquisition MRI protocols eliminate traditional diagnostics barriers including radiation exposure concerns and sedation requirements. The market leverages collaborative research programs connecting university hospitals with medical device manufacturers advancing pediatric-specific imaging solutions and comprehensive clinical validation ensuring diagnostics accuracy comparable to adult imaging standards.
Competitive Landscape of the Pediatric Cardiac Tumor Diagnostics Market
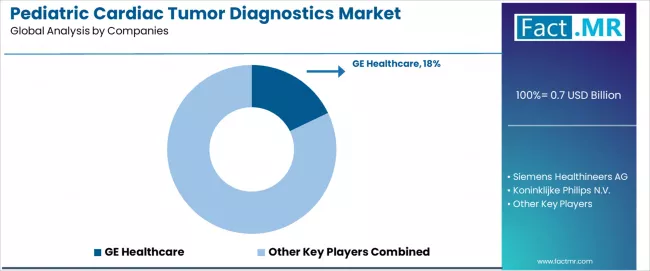
The pediatric cardiac tumor diagnostics market exhibits a moderately consolidated competitive structure with approximately 15-25 specialized players operating across imaging equipment manufacturing, diagnostics software development, and clinical service provision segments.
The top 3-4 medical imaging companies collectively command roughly 45-50% of total market revenue, with GE Healthcare maintaining market leadership at 17.9% share through comprehensive product portfolio spanning echocardiography, MRI, and CT systems with pediatric-specific protocols and AI-enhanced analysis capabilities.
This competitive concentration reflects the capital-intensive nature of advanced medical imaging technology development, stringent regulatory approval requirements, and established relationships between major equipment manufacturers and prominent pediatric cardiac centers that create significant barriers to market entry for new competitors.
Market leadership is sustained through several critical competitive advantages extending beyond basic imaging hardware capabilities. Global distribution networks with specialized pediatric cardiac application specialists enable leading players to provide comprehensive implementation support, protocol optimization, and ongoing technical training essential for successful diagnostics program establishment. Research and development investments in artificial intelligence, pediatric-specific imaging protocols, and radiation dose reduction technologies create innovation advantages that support premium pricing and clinical differentiation.
Regulatory expertise navigating FDA approval processes, CE marking requirements, and pediatric-specific safety standards represents crucial capability differentiating established manufacturers from potential market entrants. The combination of clinical evidence generation through academic partnerships, comprehensive service networks providing rapid technical support, and integration capabilities with hospital information systems creates switching costs that reinforce market position and customer retention throughout technology refresh cycles.
The market demonstrates limited commoditization in basic echocardiography systems where multiple manufacturers offer comparable imaging performance for routine cardiac screening applications, creating price competition in entry-level product segments serving community hospitals and outpatient imaging centers. However, significant margin opportunities persist in advanced diagnostics technologies and specialized applications.
AI-enhanced imaging platforms incorporating automated tumor detection algorithms, tissue characterization capabilities, and decision support tools command premium pricing through demonstrated workflow efficiency improvements and diagnostics accuracy advantages supported by clinical validation studies. Real-time MRI systems eliminating sedation requirements through rapid acquisition protocols create substantial value propositions for pediatric applications where anesthesia risks and costs represent significant clinical and economic concerns.
| Stakeholder | What They Actually Control | Typical Strengths | Typical Blind Spots |
|---|---|---|---|
| Global Imaging Equipment Manufacturers | Technology platforms; R&D capabilities; regulatory expertise | Comprehensive product portfolios; established hospital relationships; global service networks | Pediatric-specific protocol refinement; local market customization; specialized workforce training |
| AI Diagnostics Software Developers | Algorithm development; clinical validation; cloud platforms | Cutting-edge machine learning; workflow automation; continuous improvement | Hardware integration complexity; regulatory pathway navigation; clinical adoption barriers |
| Specialized Pediatric Cardiac Centers | Clinical expertise; protocol development; outcome data | Deep pediatric cardiac knowledge; multidisciplinary coordination; research capabilities | Technology commercialization; scalability beyond single institution; equipment procurement resources |
| Medical Device Distributors | Local market access; customer relationships; technical support | Geographic coverage; installation expertise; responsive service | Technology innovation; clinical evidence generation; comprehensive solution integration |
| Telemedicine Platform Providers | Remote consultation infrastructure; image sharing; specialist networks | Expertise distribution; access expansion; cost-effective scaling | Diagnostics equipment control; clinical credibility; regulatory compliance complexity |
Key Players in the Pediatric Cardiac Tumor Diagnostics Market
- GE Healthcare
- Siemens Healthineers AG
- Koninklijke Philips N.V.
- Canon Medical Systems Corp.
- Boston Scientific Corp.
- Abbott
- Bio-Rad Laboratories
- F. Hoffmann-La Roche Ltd
- Medtronic
- Lantheus
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units (2025) | USD 0.72 billion |
| Tumor Type | Primary Cardiac Tumors, Secondary Cardiac Tumors |
| Diagnostics Type | Echocardiography, Magnetic Resonance Imaging (MRI), Computed Tomography (CT), Others |
| End Use | Hospitals and Clinics, Government Health Departments, Others |
| Regions Covered | North America, Europe, Asia Pacific, Latin America, Middle East & Africa |
| Countries Covered | India, China, Brazil, USA, Germany, UK, Japan, and 20+ additional countries |
| Key Companies Profiled | GE HealthCare, Siemens Healthineers AG, Koninklijke Philips N.V., Canon Medical Systems Corp., Boston Scientific Corp., Abbott, Bio-Rad Laboratories, F. Hoffmann-La Roche Ltd, Medtronic, Lantheus |
| Additional Attributes | Dollar sales by tumor type and diagnostics modality categories, regional adoption trends across North America, Europe, and Asia Pacific, competitive landscape with medical imaging equipment manufacturers and diagnostics service providers, clinical preferences for non-invasive imaging and radiation-free protocols, integration with AI-enhanced analysis platforms and telemedicine consultation networks, innovations in pediatric-specific imaging protocols and sedation-free examination techniques, and development of comprehensive diagnostics solutions with enhanced accuracy and patient safety optimization capabilities. |
Pediatric Cardiac Tumor Diagnostics Market by Segments
-
Tumor Type :
- Primary Cardiac Tumors
- Secondary Cardiac Tumors
-
Diagnostics Type :
- Echocardiography
- Magnetic Resonance Imaging (MRI)
- Computed Tomography (CT)
- Others
-
End Use :
- Hospitals and Clinics
- Government Health Departments
- Others
-
Region :
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Rest of Europe
- Asia Pacific
- India
- China
- Japan
- South Korea
- Australia & New Zealand
- Rest of Asia Pacific
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
- North America
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Tumor Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Tumor Type , 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Tumor Type , 2025 to 2035
- Primary Cardiac Tumors
- Secondary Cardiac Tumors
- Y to o to Y Growth Trend Analysis By Tumor Type , 2020 to 2024
- Absolute $ Opportunity Analysis By Tumor Type , 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Diagnostics Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Diagnostics Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Diagnostics Type, 2025 to 2035
- Echocardiography
- Magnetic Resonance Imaging (MRI)
- Computed Tomography (CT)
- Others
- Y to o to Y Growth Trend Analysis By Diagnostics Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Diagnostics Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Hospitals and Clinics
- Government Health Departments
- Others
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Tumor Type
- By Diagnostics Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Tumor Type
- By Diagnostics Type
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Tumor Type
- By Diagnostics Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Tumor Type
- By Diagnostics Type
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Tumor Type
- By Diagnostics Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Tumor Type
- By Diagnostics Type
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Tumor Type
- By Diagnostics Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Tumor Type
- By Diagnostics Type
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Tumor Type
- By Diagnostics Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Tumor Type
- By Diagnostics Type
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Tumor Type
- By Diagnostics Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Tumor Type
- By Diagnostics Type
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Tumor Type
- By Diagnostics Type
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Tumor Type
- By Diagnostics Type
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Tumor Type
- By Diagnostics Type
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Tumor Type
- By Diagnostics Type
- By End Use
- Competition Analysis
- Competition Deep Dive
- GE Healthcare
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Siemens Healthineers AG
- Koninklijke Philips N.V.
- Canon Medical Systems Corp.
- Boston Scientific Corp.
- Abbott
- Bio-Rad Laboratories
- F. Hoffmann-La Roche Ltd
- Medtronic
- Lantheus
- GE Healthcare
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Tumor Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Diagnostics Type, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Tumor Type, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Diagnostics Type, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Tumor Type, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Diagnostics Type, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Tumor Type, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Diagnostics Type, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Tumor Type, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Diagnostics Type, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Tumor Type, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Diagnostics Type, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Tumor Type, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Diagnostics Type, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Tumor Type, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Diagnostics Type, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Tumor Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Tumor Type, 2025 to 2035
- Figure 5: Global Market Attractiveness Analysis by Tumor Type
- Figure 6: Global Market Value Share and BPS Analysis by Diagnostics Type, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Diagnostics Type, 2025 to 2035
- Figure 8: Global Market Attractiveness Analysis by Diagnostics Type
- Figure 9: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 11: Global Market Attractiveness Analysis by End Use
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025 to 2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025 to 2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Tumor Type, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Tumor Type, 2025 to 2035
- Figure 25: North America Market Attractiveness Analysis by Tumor Type
- Figure 26: North America Market Value Share and BPS Analysis by Diagnostics Type, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Diagnostics Type, 2025 to 2035
- Figure 28: North America Market Attractiveness Analysis by Diagnostics Type
- Figure 29: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 31: North America Market Attractiveness Analysis by End Use
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Tumor Type, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Tumor Type, 2025 to 2035
- Figure 35: Latin America Market Attractiveness Analysis by Tumor Type
- Figure 36: Latin America Market Value Share and BPS Analysis by Diagnostics Type, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Diagnostics Type, 2025 to 2035
- Figure 38: Latin America Market Attractiveness Analysis by Diagnostics Type
- Figure 39: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 41: Latin America Market Attractiveness Analysis by End Use
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Tumor Type, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Tumor Type, 2025 to 2035
- Figure 45: Western Europe Market Attractiveness Analysis by Tumor Type
- Figure 46: Western Europe Market Value Share and BPS Analysis by Diagnostics Type, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Diagnostics Type, 2025 to 2035
- Figure 48: Western Europe Market Attractiveness Analysis by Diagnostics Type
- Figure 49: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 51: Western Europe Market Attractiveness Analysis by End Use
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Tumor Type, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Tumor Type, 2025 to 2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Tumor Type
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Diagnostics Type, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Diagnostics Type, 2025 to 2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Diagnostics Type
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Tumor Type, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Tumor Type, 2025 to 2035
- Figure 65: East Asia Market Attractiveness Analysis by Tumor Type
- Figure 66: East Asia Market Value Share and BPS Analysis by Diagnostics Type, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Diagnostics Type, 2025 to 2035
- Figure 68: East Asia Market Attractiveness Analysis by Diagnostics Type
- Figure 69: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 71: East Asia Market Attractiveness Analysis by End Use
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Tumor Type, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Tumor Type, 2025 to 2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Tumor Type
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Diagnostics Type, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Diagnostics Type, 2025 to 2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Diagnostics Type
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Tumor Type, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Tumor Type, 2025 to 2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Tumor Type
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Diagnostics Type, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Diagnostics Type, 2025 to 2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Diagnostics Type
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025 to 2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the pediatric cardiac tumor diagnostics market in 2025?
The global pediatric cardiac tumor diagnostics market is estimated to be valued at USD 0.7 billion in 2025.
What will be the size of pediatric cardiac tumor diagnostics market in 2035?
The market size for the pediatric cardiac tumor diagnostics market is projected to reach USD 1.6 billion by 2035.
How much will be the pediatric cardiac tumor diagnostics market growth between 2025 and 2035?
The pediatric cardiac tumor diagnostics market is expected to grow at a 8.2% CAGR between 2025 and 2035.
What are the key product types in the pediatric cardiac tumor diagnostics market?
The key product types in pediatric cardiac tumor diagnostics market are primary cardiac tumors and secondary cardiac tumors.
Which diagnostics type segment to contribute significant share in the pediatric cardiac tumor diagnostics market in 2025?
In terms of diagnostics type, echocardiography segment to command 61.0% share in the pediatric cardiac tumor diagnostics market in 2025.