Bladder Cancer Immunotherapy Market
Bladder Cancer Immunotherapy Market Size and Share Forecast Outlook 2026 to 2036
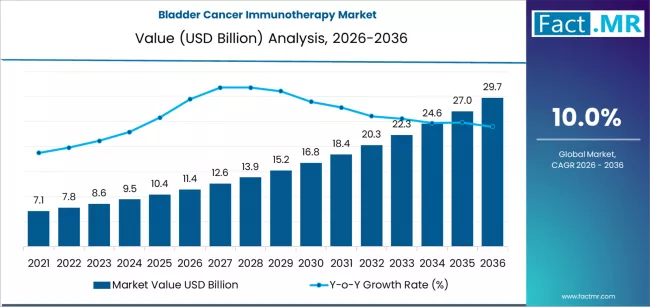
Bladder cancer immunotherapy market is projected to grow from USD 11.4 billion in 2026 to USD 29.7 billion by 2036, at a CAGR of 10.0%. Checkpoint inhibitor therapies (PD-1/PD-L1/CTLA-4) will dominate with a 60.9% market share, while advanced/metastatic urothelial carcinoma will lead the indication segment with a 48.3% share.
Bladder Cancer Immunotherapy Market Forecast and Outlook 2026 to 2036
The global bladder cancer immunotherapy market is projected to total USD 11.44 billion in 2026, advancing to USD 29.75 billion by 2036 at a 10.0% CAGR. Growth is anchored in the paradigm shift toward immune checkpoint inhibitors as first- and second-line treatments for advanced urothelial carcinoma, altering a therapeutic landscape long dominated by chemotherapy.
Key Takeaways from the Bladder Cancer Immunotherapy Market
- Market Value for 2026: USD 11.44 Billion
- Market Value for 2036: USD 29.75 Billion
- Forecast CAGR 2026 to 2036: 10.0%
- Leading Therapy Segment (2026): Checkpoint Inhibitor Therapies (PD-1/PD-L1/CTLA-4) (60.9%)
- Leading Indication Segment (2026): Advanced/Metastatic Urothelial Carcinoma (48.3%)
- Leading End User Segment (2026): Hospital & Comprehensive Cancer Centers (56.7%)
- Key Growth Countries: India (13.2% CAGR), China (13.0% CAGR), Brazil (12.6% CAGR), USA (11.3% CAGR), France (11.2% CAGR), UK (11.1% CAGR), Germany (11.0% CAGR)
- Key Players: Merck & Co. Inc., Bristol Myers Squibb Company, F. Hoffmann-La Roche Ltd, AstraZeneca plc., Pfizer Inc.
Checkpoint inhibitor therapies targeting PD-1, PD-L1, and CTLA-4 pathways command the dominant product share, reflecting their established efficacy and broadening label expansions. The market is stratified across distinct disease stages, from BCG-unresponsive non-muscle invasive disease to peri-operative settings for muscle-invasive cancer, each creating specific demand for different immunotherapeutic modalities.
The integration of biomarker testing, particularly PD-L1 expression and tumor mutational burden, is becoming standard for treatment selection, reinforcing the precision oncology approach. The robust pipeline of next-generation agents, including antibody-drug conjugates combined with immunotherapy, further propels growth and novel intravesical formulations, which promise to address unmet needs in resistant disease states.
Bladder Cancer Immunotherapy Market
| Metric | Value |
|---|---|
| Market Value (2026) | USD 11.44 Billion |
| Market Forecast Value (2036) | USD 29.75 Billion |
| Forecast CAGR 2026 to 2036 | 10.0% |
Category
| Category | Segments |
|---|---|
| Therapy | Checkpoint Inhibitor Therapies (PD-1/PD-L1/CTLA-4), Intravesical Immunotherapies (BCG & Next-Gen), Advanced Immunotherapies (ADC + IO, Vaccines, Cell Therapies) |
| Indication | Advanced/Metastatic Urothelial Carcinoma, Non–Muscle Invasive Bladder Cancer (BCG-Unresponsive), Muscle-Invasive Bladder Cancer (Peri-Operative Use) |
| End User | Hospital & Comprehensive Cancer Centers, Specialty Urology–Oncology Clinics, Specialty Pharmacies & Infusion Networks |
| Region | North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, Middle East & Africa |
What Clinical and Commercial Dynamics are Propelling Immuno-Oncology in Urology?
Adoption is heavily guided by evolving National Comprehensive Cancer Network and European Association of Urology guidelines, which increasingly position checkpoint inhibitors as standard-of-care in multiple lines of therapy. The critical shortage and supply instability of traditional intravesical BCG have accelerated development of novel next-generation immunotherapeutic agents for the non-muscle invasive space.
The expansion of biomarker testing infrastructure is essential for patient stratification, directly influencing therapy selection and supporting the premium pricing of targeted immunotherapies. Furthermore, the trend toward combination regimens, such as antibody-drug conjugates paired with checkpoint blockade, is extending treatment durations and improving outcomes, thereby increasing the total value of therapy per patient.
Segmental Analysis
By Therapy, Which Mechanism of Action Defines the Current Treatment Paradigm?
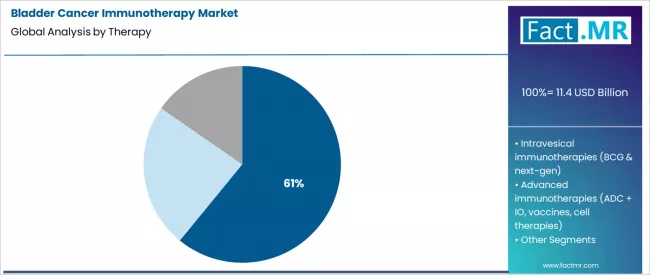
Checkpoint inhibitor therapies command a dominant 60.9% share. This segment’s leadership is built upon the transformative clinical data that established anti-PD-1/L1 agents as superior to chemotherapy for both cisplatin-ineligible and previously treated metastatic bladder cancer.
Their application has since expanded into adjuvant and neoadjuvant settings for muscle-invasive disease. The high annual cost per patient and broad label approvals across multiple disease stages create a substantial and sustained revenue base, making this class the commercial and clinical engine of the market.
By Indication, Which Setting Captures the Largest Share of High-Cost Systemic Therapy?
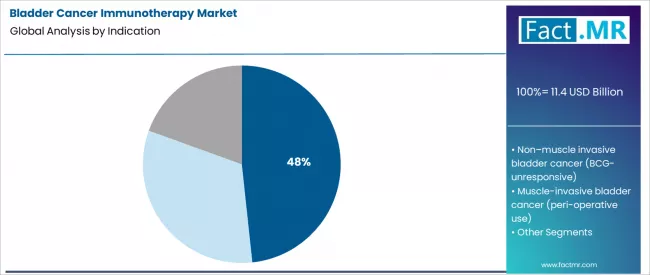
Advanced or metastatic urothelial carcinoma constitutes the primary indication segment at 48.3%. This patient population requires lifelong systemic treatment, often involving sequential lines of immunotherapy.
The high unmet need and poor historical outcomes with chemotherapy established this stage as the initial focus for immunotherapy development and commercialization. The volume of patients progressing to this stage, combined with the premium pricing of life-extending checkpoint inhibitors, ensures this segment’s significant market value.
By End User, Where are Complex Treatment Protocols Initiated and Managed?
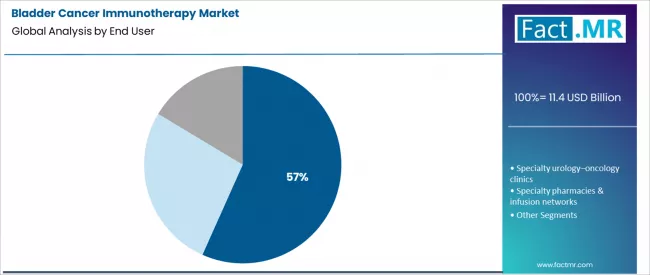
Hospital and comprehensive cancer centers hold a leading 56.7% share. These institutions are equipped to manage the complexities of advanced cancer care, including the administration of intravenous biologics, management of immune-related adverse events, and coordination of multidisciplinary care involving urology, medical oncology, and radiology.
They serve as the central hubs for initial diagnosis, biomarker testing, and the initiation of first-line systemic immunotherapy regimens, capturing the highest-value component of the treatment journey.
What Drivers, Restraints, Opportunities, and Trends are prevalent in the Bladder Cancer Immunotherapy Market?
The rapid integration of PD-L1 and comprehensive genomic profiling as standard diagnostic tests is expanding the eligible patient pool for first-line checkpoint inhibitors, directly increasing treatment volumes. Guideline recommendations now firmly endorse these biomarkers for therapeutic decision-making in metastatic disease.
Extremely high annual treatment costs for checkpoint inhibitors and novel antibody-drug conjugates create severe access limitations, particularly in single-payer or budget-constrained health systems. This financial toxicity often leads to stringent reimbursement criteria and can delay or preclude patient access to optimal therapy.
The BCG-unresponsive non-muscle invasive bladder cancer space represents a high-value niche with significant unmet need. Successful development and approval of next-generation intravesical immunotherapies or systemic checkpoint inhibitors for this indication command premium pricing due to the lack of effective alternatives and the desire to avoid radical cystectomy.
Clinical development is intensely focused on novel antibody-drug conjugate and checkpoint inhibitor combinations. These regimens aim to improve response rates and durability in metastatic disease, potentially moving into earlier treatment lines. Their success would further elevate treatment costs but also set new efficacy benchmarks, reshaping the competitive landscape.
Analysis of the Bladder Cancer Immunotherapy Market by Key Countries
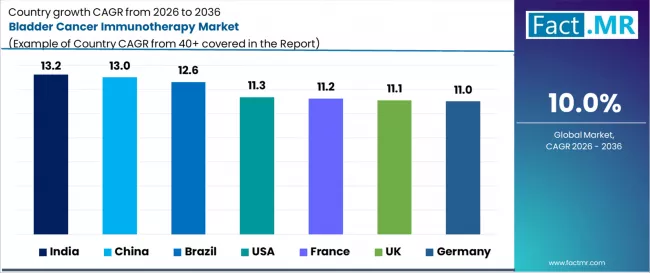
| Country | CAGR 2026 to 2036 |
|---|---|
| USA | 11.3% |
| Germany | 11.0% |
| China | 13.0% |
| India | 13.2% |
| Brazil | 12.6% |
| France | 11.2% |
| UK | 11.1% |
How does the USA's Reimbursement and Specialist Network Drive Market Leadership?
Comprehensive medicare and private insurance coverage for approved immunotherapies, a dense network of specialist cancer centers, and early adoption of novel agents following FDA accelerated approvals fuel an 11.3% CAGR. The commercial model relies on specialist prescribing within major hospital networks, supported by extensive manufacturer patient access programs. High biomarker testing rates further optimize patient selection.
What Role does Germany's Early Diagnostic and Regulatory Framework Play?
Germany's 11.0% CAGR benefits from a structured urological care pathway with early cystoscopic diagnosis and swift adoption of EMA-approved therapies. The country’s strong academic research centers participate in pivotal clinical trials, facilitating early experience with new agents. Reimbursement through the G-BA system, while rigorous, provides clarity for approved indications.
Which Factors are Accelerating China's Rapid Integration of Novel Therapies?
The National Reimbursement Drug List increasingly incorporating key checkpoint inhibitors, dramatically improving patient access, propels China’s 13.0% CAGR. Domestic pharmaceutical companies are also advancing biosimilars and novel agents in the pipeline. The vast patient population and growing investment in modern cancer hospitals create a substantial addressable market for both current and emerging immunotherapies.
Why is India's Growth Potential Tied to Improving Diagnostic Infrastructure?
India's leading 13.2% CAGR is linked to the expansion of private tertiary care hospitals offering advanced oncology services. Improving access to cystoscopy and pathology is raising diagnosis rates of advanced disease. While cost remains a barrier, localized manufacturing, biosimilar development, and innovative access schemes for premium therapies are enabling gradual market penetration.
How does Brazil's Public Health System Influence Immunotherapy Adoption?
Brazil's 12.6% CAGR operates within a dual system where the private sector rapidly adopts new therapies, while the public SUS system faces budgetary constraints for high-cost oncology drugs. Growth is concentrated in major urban private hospital networks, with market expansion dependent on government initiatives to include select immunotherapies in public formularies for specific indications.
What Characterizes France's Protocol-Driven Approach to Treatment Access?
France's 11.2% CAGR is supported by a national healthcare system that provides broad coverage for approved cancer drugs. The market is governed by strict hospital prescribing protocols and recommendations from the Haute Autorité de Santé. Access to novel therapies is relatively rapid post-EMA approval, with treatment centralized in authorized cancer centers, ensuring controlled but consistent uptake.
How is the UK's NICE and NHS Structure Shaping Market Dynamics?
The UK’s 11.1% CAGR is guided by health technology assessments from NICE, which determine NHS reimbursement based on cost-effectiveness. This results in managed access agreements and sometimes delayed or restricted availability for very high-cost drugs. Adoption is consequently high for therapies with positive NICE guidance and structured within commissioned cancer treatment pathways.
Competitive Landscape of the Bladder Cancer Immunotherapy Market
Global pharmaceutical giants with deep expertise in oncology dominate the competitive environment. Merck, Bristol Myers Squibb, and Roche lead the checkpoint inhibitor segment, competing on label breadth, overall survival data, and combination therapy pipelines. Success is determined by securing first-line treatment recommendations in major guidelines, expanding indications into earlier disease stages, and developing differentiated combination regimens.
Companies also invest heavily in companion diagnostic development to link their therapy to specific biomarkers, creating a competitive moat. New entrants aiming to address the BCG shortage with next-generation immuno-stimulatory agents are revitalizing the intravesical therapy segment.
Key Players in the Bladder Cancer Immunotherapy Market
- Merck & Co. Inc.
- Bristol Myers Squibb Company
- F. Hoffmann-La Roche Ltd
- AstraZeneca plc.
- Pfizer Inc.
- Others
Scope of Report
| Items | Metrics |
|---|---|
| Quantitative Units | USD Billion |
| Therapy | Checkpoint Inhibitor Therapies, Intravesical Immunotherapies, Advanced Immunotherapies |
| Indication | Advanced/Metastatic Urothelial Carcinoma, Non–Muscle Invasive Bladder Cancer, Muscle-Invasive Bladder Cancer |
| End User | Hospital & Comprehensive Cancer Centers, Specialty Urology–Oncology Clinics, Specialty Pharmacies & Infusion Networks |
| Key Countries | India, China, Brazil, USA, France, UK, Germany |
| Key Companies | Merck & Co. Inc., Bristol Myers Squibb Company, F. Hoffmann-La Roche Ltd, AstraZeneca plc., Pfizer Inc. |
| Additional Analysis | Comparative efficacy analysis of checkpoint inhibitors in PD-L1 high vs. low subgroups; market impact of BCG shortage and alternatives; health economic modeling of adjuvant immunotherapy; analysis of clinical trial design for BCG-unresponsive NMIBC; review of biomarker testing rates and reimbursement across regions. |
Market by Segments
-
Therapy :
- Checkpoint Inhibitor Therapies (PD-1/PD-L1/CTLA-4)
- Intravesical Immunotherapies (BCG & Next-Gen)
- Advanced Immunotherapies (ADC + IO, Vaccines, Cell Therapies)
-
Indication :
- Advanced/Metastatic Urothelial Carcinoma
- Non–Muscle Invasive Bladder Cancer (BCG-Unresponsive)
- Muscle-Invasive Bladder Cancer (Peri-Operative Use)
-
End User :
- Hospital & Comprehensive Cancer Centers
- Specialty Urology–Oncology Clinics
- Specialty Pharmacies & Infusion Networks
-
Region :
-
North America
- USA
- Canada
-
Latin America
- Brazil
- Mexico
- Argentina
- Rest of Latin America
-
Western Europe
- Germany
- France
- UK
- Italy
- Spain
- BENELUX
- Rest of Western Europe
-
Eastern Europe
- Poland
- Russia
- Czech Republic
- Rest of Eastern Europe
-
East Asia
- China
- Japan
- South Korea
- Rest of East Asia
-
South Asia & Pacific
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia & Pacific
-
Middle East & Africa
- GCC Countries
- South Africa
- Turkiye
- Rest of MEA
-
Bibliography
- Bellmunt, J., & Powles, T. (2024). Immunotherapy in urothelial carcinoma: latest evidence and clinical implications. Nature Reviews Urology.
- European Association of Urology. (2025). EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer.
- National Comprehensive Cancer Network. (2025). NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer.
- Powles, T., et al. (2023). Pembrolizumab versus chemotherapy for previously treated advanced urothelial cancer: Long-term survival results. Journal of Clinical Oncology.
- U.S. Food and Drug Administration. (2024). FDA approves first immunotherapy for BCG-unresponsive bladder cancer.
- Vuky, J., & Balar, A. V. (2025). The evolving role of antibody-drug conjugates in urothelial carcinoma. The Lancet Oncology.
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2021 to 2025 and Forecast, 2026 to 2036
- Historical Market Size Value (USD Million) Analysis, 2021 to 2025
- Current and Future Market Size Value (USD Million) Projections, 2026 to 2036
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2021 to 2025 and Forecast 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Therapy
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Therapy, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Therapy, 2026 to 2036
- Checkpoint inhibitor therapies (PD-1/PD-L1/CTLA-4)
- Intravesical immunotherapies (BCG & next-gen)
- Advanced immunotherapies (ADC + IO, vaccines, cell therapies)
- Checkpoint inhibitor therapies (PD-1/PD-L1/CTLA-4)
- Y to o to Y Growth Trend Analysis By Therapy, 2021 to 2025
- Absolute $ Opportunity Analysis By Therapy, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Indication
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Indication, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Indication, 2026 to 2036
- Advanced/metastatic urothelial carcinoma
- Non–muscle invasive bladder cancer (BCG-unresponsive)
- Muscle-invasive bladder cancer (peri-operative use)
- Advanced/metastatic urothelial carcinoma
- Y to o to Y Growth Trend Analysis By Indication, 2021 to 2025
- Absolute $ Opportunity Analysis By Indication, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By End User
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End User, 2021 to 2025
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End User, 2026 to 2036
- Hospital & comprehensive cancer centers
- Specialty urology–oncology clinics
- Specialty pharmacies & infusion networks
- Hospital & comprehensive cancer centers
- Y to o to Y Growth Trend Analysis By End User, 2021 to 2025
- Absolute $ Opportunity Analysis By End User, 2026 to 2036
- Global Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2021 to 2025
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2026 to 2036
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- USA
- Canada
- Mexico
- By Therapy
- By Indication
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By Indication
- By End User
- Key Takeaways
- Latin America Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Therapy
- By Indication
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By Indication
- By End User
- Key Takeaways
- Western Europe Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Therapy
- By Indication
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By Indication
- By End User
- Key Takeaways
- Eastern Europe Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Therapy
- By Indication
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By Indication
- By End User
- Key Takeaways
- East Asia Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- China
- Japan
- South Korea
- By Therapy
- By Indication
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By Indication
- By End User
- Key Takeaways
- South Asia and Pacific Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Therapy
- By Indication
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By Indication
- By End User
- Key Takeaways
- Middle East & Africa Market Analysis 2021 to 2025 and Forecast 2026 to 2036, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2021 to 2025
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2026 to 2036
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Therapy
- By Indication
- By End User
- By Country
- Market Attractiveness Analysis
- By Country
- By Therapy
- By Indication
- By End User
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Canada
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Mexico
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Brazil
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Chile
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Germany
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- UK
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Italy
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Spain
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- France
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- India
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- China
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Japan
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- South Korea
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Russia
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Poland
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Hungary
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- South Africa
- Pricing Analysis
- Market Share Analysis, 2025
- By Therapy
- By Indication
- By End User
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Therapy
- By Indication
- By End User
- Competition Analysis
- Competition Deep Dive
- Merck & Co. Inc.
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Bristol Myers Squibb Company
- F. Hoffmann-La Roche Ltd
- AstraZeneca plc.
- Pfizer Inc.
- Others
- Merck & Co. Inc.
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2021 to 2036
- Table 2: Global Market Value (USD Million) Forecast by Therapy, 2021 to 2036
- Table 3: Global Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 4: Global Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 5: North America Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 6: North America Market Value (USD Million) Forecast by Therapy, 2021 to 2036
- Table 7: North America Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 8: North America Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 10: Latin America Market Value (USD Million) Forecast by Therapy, 2021 to 2036
- Table 11: Latin America Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 12: Latin America Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 14: Western Europe Market Value (USD Million) Forecast by Therapy, 2021 to 2036
- Table 15: Western Europe Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 16: Western Europe Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Therapy, 2021 to 2036
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 20: Eastern Europe Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 22: East Asia Market Value (USD Million) Forecast by Therapy, 2021 to 2036
- Table 23: East Asia Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 24: East Asia Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Therapy, 2021 to 2036
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by End User, 2021 to 2036
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2021 to 2036
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Therapy, 2021 to 2036
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Indication, 2021 to 2036
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by End User, 2021 to 2036
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2021 to 2036
- Figure 3: Global Market Value Share and BPS Analysis by Therapy, 2026 and 2036
- Figure 4: Global Market Y to o to Y Growth Comparison by Therapy, 2026 to 2036
- Figure 5: Global Market Attractiveness Analysis by Therapy
- Figure 6: Global Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 7: Global Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 8: Global Market Attractiveness Analysis by Indication
- Figure 9: Global Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 10: Global Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 11: Global Market Attractiveness Analysis by End User
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2026 and 2036
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2026 to 2036
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2026 to 2036
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 23: North America Market Value Share and BPS Analysis by Therapy, 2026 and 2036
- Figure 24: North America Market Y to o to Y Growth Comparison by Therapy, 2026 to 2036
- Figure 25: North America Market Attractiveness Analysis by Therapy
- Figure 26: North America Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 27: North America Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 28: North America Market Attractiveness Analysis by Indication
- Figure 29: North America Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 30: North America Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 31: North America Market Attractiveness Analysis by End User
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 33: Latin America Market Value Share and BPS Analysis by Therapy, 2026 and 2036
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Therapy, 2026 to 2036
- Figure 35: Latin America Market Attractiveness Analysis by Therapy
- Figure 36: Latin America Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 38: Latin America Market Attractiveness Analysis by Indication
- Figure 39: Latin America Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 40: Latin America Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 41: Latin America Market Attractiveness Analysis by End User
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 43: Western Europe Market Value Share and BPS Analysis by Therapy, 2026 and 2036
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Therapy, 2026 to 2036
- Figure 45: Western Europe Market Attractiveness Analysis by Therapy
- Figure 46: Western Europe Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 48: Western Europe Market Attractiveness Analysis by Indication
- Figure 49: Western Europe Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 51: Western Europe Market Attractiveness Analysis by End User
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Therapy, 2026 and 2036
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Therapy, 2026 to 2036
- Figure 55: Eastern Europe Market Attractiveness Analysis by Therapy
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 58: Eastern Europe Market Attractiveness Analysis by Indication
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 61: Eastern Europe Market Attractiveness Analysis by End User
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 63: East Asia Market Value Share and BPS Analysis by Therapy, 2026 and 2036
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Therapy, 2026 to 2036
- Figure 65: East Asia Market Attractiveness Analysis by Therapy
- Figure 66: East Asia Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 68: East Asia Market Attractiveness Analysis by Indication
- Figure 69: East Asia Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 70: East Asia Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 71: East Asia Market Attractiveness Analysis by End User
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Therapy, 2026 and 2036
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Therapy, 2026 to 2036
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Therapy
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Indication
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by End User
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2026 and 2036
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Therapy, 2026 and 2036
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Therapy, 2026 to 2036
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Therapy
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Indication, 2026 and 2036
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Indication, 2026 to 2036
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Indication
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by End User, 2026 and 2036
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by End User, 2026 to 2036
- Figure 91: Middle East & Africa Market Attractiveness Analysis by End User
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the bladder cancer immunotherapy market in 2026?
The global bladder cancer immunotherapy market is estimated to be valued at USD 11.4 billion in 2026.
What will be the size of bladder cancer immunotherapy market in 2036?
The market size for the bladder cancer immunotherapy market is projected to reach USD 29.7 billion by 2036.
How much will be the bladder cancer immunotherapy market growth between 2026 and 2036?
The bladder cancer immunotherapy market is expected to grow at a 10.0% CAGR between 2026 and 2036.
What are the key product types in the bladder cancer immunotherapy market?
The key product types in bladder cancer immunotherapy market are checkpoint inhibitor therapies (pd-1/pd-l1/ctla-4), intravesical immunotherapies (bcg & next-gen) and advanced immunotherapies (adc + io, vaccines, cell therapies).
Which indication segment to contribute significant share in the bladder cancer immunotherapy market in 2026?
In terms of indication, advanced/metastatic urothelial carcinoma segment to command 48.3% share in the bladder cancer immunotherapy market in 2026.