Lung Cancer Diagnostics Market
Lung Cancer Diagnostics Market Size and Share Forecast Outlook 2025 to 2035
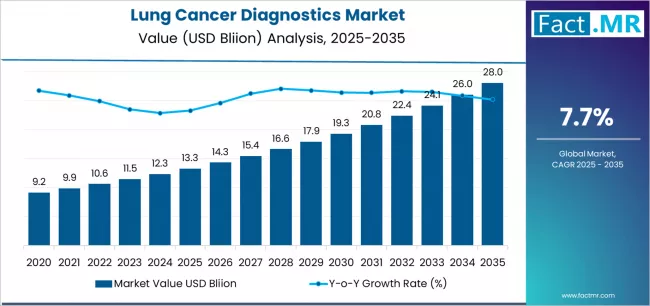
Lung cancer diagnostics market is projected to grow from USD 13.3 bliion in 2025 to USD 28.0 bliion by 2035, at a CAGR of 7.7%. Non-small Cell Lung Cancer (NSCLC) will dominate with a 66.9% market share, while egfr mutation test will lead the test segment with a 38.9% share.
Lung Cancer Diagnostics Market Forecast and Outlook 2025 to 2035
The global lung cancer diagnostics market is projected to reach USD 28.0 billion by 2035, recording an absolute increase of USD 14.7 billion over the forecast period. The market is valued at USD 13.3 billion in 2025 and is set to rise at a CAGR of 7.7% during the assessment period. The scope for lung cancer diagnostics will expand 2.1 times during the same period, supported by increasing demand from molecular biomarker testing operations and expanding applications in sequencing platform development across both developed and emerging healthcare markets.
The increasing use of EGFR mutation analysis, ALK gene rearrangement detection, and liquid biopsy technology is creating more chances for new diagnostic tests and specialized assay designs. Rising healthcare expenditure in Asia Pacific countries, coupled with expanding precision oncology capacity through advanced molecular pathology laboratories and modern diagnostic infrastructure, further accelerate market penetration across diverse patient segments.
Quick Stats for Lung Cancer Diagnostics Market
- Lung Cancer Diagnostics Market Value (2025): USD 13.3 billion
- Lung Cancer Diagnostics Market Forecast Value (2035): USD 28.0 billion
- Lung Cancer Diagnostics Market Forecast CAGR: 7.7%
- Leading Test in Lung Cancer Diagnostics Market: EGFR Mutation test (38.9%)
- Key Growth Regions in Lung Cancer Diagnostics Market: Asia Pacific, North America, and Europe
- Top Players in Lung Cancer Diagnostics Market: F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific, Illumina Inc., Agilent Technologies, Qiagen, Abbott, Bio-Rad, Neogenomics Laboratories, bioMérieux, Myriad Genetics, Inc.
The growing global incidence of lung cancer, particularly non-small cell lung cancer in aging populations and high-risk groups, generates sustained requirements for high-performance diagnostic tests and biomarker analysis platforms. Technical advancements in EGFR mutation testing technologies demonstrating measurable improvements in detection sensitivity, turnaround time efficiency, and companion diagnostic capabilities reinforce physician confidence in molecular testing economics, while oncology sector trends toward personalized treatment selection expand addressable market opportunities beyond traditional histopathology applications into comprehensive genomic profiling and liquid biopsy supply chains.
Hospitals and specialized laboratories increasingly incorporate advanced lung cancer diagnostic technologies into clinical workflows, achieving superior treatment selection accuracy, improved patient stratification, and enhanced therapeutic outcome prediction, creating mainstream adoption channels that extend beyond academic cancer centers into comprehensive community oncology environments.
Fluctuating reimbursement policies for advanced molecular diagnostics and stringent regulatory requirements for companion diagnostic approvals may pose challenges to market expansion. Technical complexity in comprehensive genomic profiling for treatment-naive patients and tissue sample adequacy limitations in certain clinical scenarios influence test utilization, requiring laboratories to develop specialized testing protocols catering to specific sample requirements across different geographical markets.
Supply chain complexity during specialized reagent procurement and the technical requirements for sequencing workflows and bioinformatics analysis may limit accessibility among smaller diagnostic facilities in developing regions with limited infrastructure for advanced molecular pathology testing and comprehensive quality assurance systems.
Lung Cancer Diagnostics Market Year-over-Year Forecast 2025 to 2035
Between 2025 and 2030, the lung cancer diagnostics market is projected to expand from USD 13.3 billion to USD 19.3 billion, resulting in a value increase of USD 6.0 billion, which represents 40.8% of the total forecast growth for the decade. This phase is shaped by rising demand for companion diagnostic testing and targeted therapy biomarker identification, product innovation in liquid biopsy technologies and comprehensive genomic profiling panels, as well as expanding integration with precision oncology treatment pathways and immunotherapy biomarker platforms. Companies are establishing competitive positions through investment in regulatory approval programs, clinical validation studies, and strategic market expansion across hospital pathology departments, reference laboratory segments, and specialized molecular diagnostic facilities.
From 2030 to 2035, the market is forecast to grow from USD 19.3 billion to USD 28.0 billion, adding another USD 8.7 billion, which constitutes 59.2% of the ten-year expansion. This period will be characterized by the expansion of specialized diagnostic technologies, including minimal residual disease monitoring assays and early detection screening platforms tailored for high-risk populations, strategic collaborations between diagnostic manufacturers and pharmaceutical companies developing targeted therapies, and an enhanced focus on artificial intelligence integration and real-world evidence generation. The growing emphasis on early-stage detection programs and comprehensive molecular characterization will drive demand for advanced lung cancer diagnostic solutions across diverse clinical applications.
Lung Cancer Diagnostics Market Key Takeaways
| Metric | Value |
|---|---|
| Market Value (2025) | USD 13.3 billion |
| Market Forecast Value (2035) | USD 28.0 billion |
| Forecast CAGR (2025-2035) | 7.7% |
Why is the Lung Cancer Diagnostics Market Experiencing Steady Growth?
The lung cancer diagnostics market is expanding by enabling oncologists, pathologists, and diagnostic laboratories to access advanced molecular testing technologies that support treatment decisions while meeting clinical demand for accurate biomarker identification and patient stratification. Oncology specialists and molecular pathologists face increasing pressure to deliver precision-medicine approaches with proven clinical utility and actionable biomarker detection.
Molecular diagnostic assays now play a central role by enabling accurate mutation identification and predictive biomarker analysis, both of which are critical for targeted therapy selection and immunotherapy response prediction. These technologies have therefore become essential for maintaining competitive clinical positioning in lung cancer management, precision oncology, and companion diagnostics.
The oncology specialty's need for reliable biomarker testing and consistent analytical performance creates demand for diverse diagnostic platforms that can provide comprehensive molecular profiling, maintain predictable results across different specimen types, and ensure clinical validity without compromising turnaround time or testing economics.
Government initiatives promoting cancer screening infrastructure and precision medicine adoption drive utilization in hospital oncology departments, reference laboratories, and specialized molecular pathology facilities, where diagnostic test selection has a direct impact on treatment outcomes and healthcare resource allocation.
The pharmaceutical industry's growing focus on companion diagnostics for targeted therapy approval further expands market opportunities, with clinical evidence demonstrating measurable outcome improvements from biomarker-guided treatment selection, including enhanced response rates and improved survival outcomes.
Supply chain complexity during specialized reagent procurement and the technical requirements for next-generation sequencing implementation and bioinformatics expertise may limit accessibility among smaller laboratories and developing regions with limited infrastructure for advanced molecular diagnostic capabilities and comprehensive laboratory accreditation.
Segmental Analysis
The market is segmented by type, test, end use, and region. By type, the market is divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). NSCLC is further segmented into squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, while SCLC includes small-cell carcinoma and combined small-cell carcinoma.
By test, the market encompasses CA test, HER2 test, ALK test, angiogenesis inhibitors, EGFR mutation test, and KRAS mutation test. By end use, the market includes hospitals, laboratories, and others. Regionally, the market is divided into Asia Pacific, North America, Europe, Latin America, and Middle East & Africa.
Which Type Segment Dominates in the Lung Cancer Diagnostics Market?
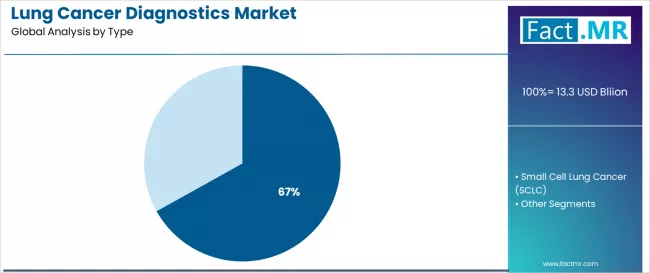
Non-small cell lung cancer (NSCLC) represents the dominant force in the lung cancer diagnostics market, capturing 66.9% of the total market share in 2025. This established type category encompasses diagnostic testing for adenocarcinoma, squamous cell carcinoma, and large cell carcinoma subtypes, including comprehensive molecular profiling that enables targeted therapy selection, immunotherapy biomarker assessment, and treatment response prediction across all NSCLC disease presentations.
The NSCLC segment's market leadership stems from its essential role in precision oncology, with NSCLC cases representing the majority of lung cancer diagnoses capable of benefiting from targeted therapy approaches while maintaining substantial testing volumes and diverse biomarker requirements across all molecular pathology practice environments. Within the NSCLC segment, adenocarcinoma testing accounts for the largest diagnostic volume share, driven by high mutation prevalence and established targeted therapy availability across global oncology centers.
The small cell lung cancer (SCLC) segment maintains approximately 33.1% market share, serving patients requiring diagnostic confirmation, staging assessment, and emerging biomarker evaluation for SCLC subtypes including small-cell carcinoma and combined small-cell carcinoma. These applications offer specialized diagnostic solutions for aggressive disease presentations and limited treatment-responsive populations while providing essential histopathological confirmation and evolving molecular characterization capabilities suitable for clinical trial enrollment and experimental therapy selection.
Key advantages driving the non-small cell lung cancer segment include:
- Established clinical evidence infrastructure with extensive biomarker validation that reduces testing uncertainty and ensures consistent guideline recommendations
- Critical therapeutic relevance enabling multiple targeted therapy options across different molecular alterations without significant treatment limitation
- Proven diagnostic utility, delivering actionable biomarker information while maintaining clinical standard-of-care integration across multiple oncology specialties
- Broad guideline acceptance enabling straightforward testing algorithms and multidisciplinary care coordination across comprehensive cancer centers and community practices
Which Lung Cancer Diagnostics Test is the Most Preferred?
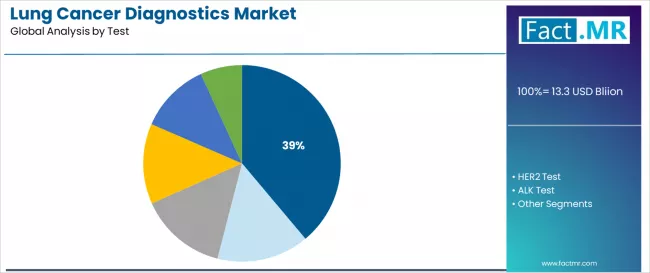
EGFR mutation test captures 38.9% of the lung cancer diagnostics market in 2025, driven by established targeted therapy availability and first-line treatment selection requirements in NSCLC patients, particularly adenocarcinoma histology. EGFR mutation testing demonstrates comprehensive clinical utility across treatment-naive patients, acquired resistance evaluation, and liquid biopsy monitoring applications, enabling precise tyrosine kinase inhibitor selection and optimized treatment outcomes across diverse patient populations.
Healthcare facilities implementing EGFR mutation testing benefit from established companion diagnostic platforms and extensive clinical guideline support across global oncology practice. ALK test maintains a 17.4% market share, serving NSCLC patients requiring ALK gene rearrangement detection for targeted therapy eligibility, representing critical biomarker assessment for younger patients and never-smokers with adenocarcinoma. ALK testing offers high-impact therapeutic decisions for ALK-positive patients with multiple FDA-approved inhibitor options and proven clinical benefit.
Additional test segments including KRAS mutation testing, HER2 testing, CA testing, and angiogenesis inhibitor-related biomarkers collectively address comprehensive molecular profiling requirements and emerging therapeutic target identification needs.
Which End Use Category Dominates in the Global Lung Cancer Diagnostics Market?

Hospitals account for the majority of all lung cancer diagnostics. In 2025, it accounts for 42.3% of all procedures. Hospitals provide academic medical centers with comprehensive molecular pathology laboratories, tertiary cancer centers with integrated precision oncology programs, community hospitals with established pathology departments, and specialized oncology hospitals providing multidisciplinary lung cancer care.
Hospital infrastructure provide support for complex diagnostic workflows, offer critical resources including specimen processing capabilities, molecular testing platforms, pathologist expertise, and integrated electronic health record systems essential for optimal clinical decision-making. Academic medical centers account for substantial testing volumes, supported by complex case referrals and established centers of excellence implementing comprehensive genomic profiling technologies.
The laboratories end use segment maintains significant market share, driven by reference laboratories providing specialized molecular testing services, commercial diagnostic laboratories offering comprehensive biomarker panels, and specialized molecular pathology laboratories focused on oncology diagnostics.
Laboratory settings offer centralized testing expertise, high-volume operational efficiency, and comprehensive test menu capabilities suitable for community oncology practice support and regional healthcare network integration. The others segment serves point-of-care testing applications, physician office laboratories, and emerging diagnostic settings implementing rapid testing technologies.
What are the Drivers, Restraints, and Key Trends of the Lung Cancer Diagnostics Market?
Increasing lung cancer incidence and aging population demographics create growing demand for diagnostic testing, with global lung cancer diagnoses expanding by 3-5% annually in major healthcare markets worldwide, requiring comprehensive molecular pathology infrastructure.
Expanding targeted therapy approvals and companion diagnostic requirements drive increased utilization of biomarker testing, with pharmaceutical companies launching multiple targeted agents requiring specific molecular diagnostic confirmation by 2030.
Technological advancements in next-generation sequencing and liquid biopsy platforms enable more effective and efficient comprehensive genomic profiling that expands detectable biomarkers while improving specimen utilization and turnaround time capabilities.
Market restraints include variable reimbursement policies for comprehensive genomic profiling that can limit test accessibility, particularly in regions where diagnostic reimbursement remains insufficient relative to testing costs or coverage policies favor single-gene testing approaches.
Technical complexity and interpretation challenges pose another significant challenge, as comprehensive molecular profiling generates complex genomic data requiring specialized bioinformatics analysis and clinical interpretation expertise, potentially causing increased operational costs and result reporting delays.
Specimen adequacy limitations and tissue availability constraints create additional diagnostic challenges for comprehensive testing, demanding ongoing innovation in low-input sequencing technologies and liquid biopsy validation.
Key trends indicate accelerated market penetration in Asia Pacific healthcare systems, particularly China and India, where cancer care infrastructure development and precision oncology adoption drive comprehensive molecular diagnostic implementation.
Technology integration trends toward artificial intelligence-based imaging analysis with radiomics integration, minimal residual disease monitoring enabling treatment response assessment, and multi-cancer early detection platforms incorporating lung cancer screening enable optimized diagnostic approaches that address clinical unmet needs and improve patient outcomes.
The industry could face disruption if significant advances in universal cancer vaccines or major shifts toward empiric immunotherapy approaches reduce reliance on comprehensive molecular biomarker testing.
Analysis of the Lung Cancer Diagnostics Market by Key Countries
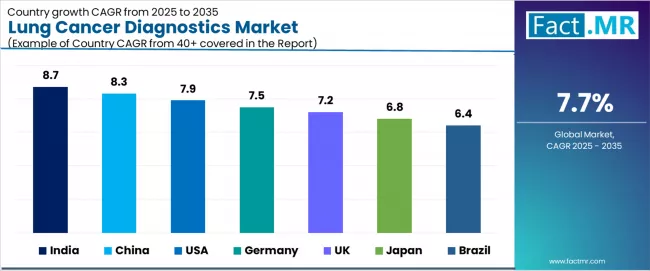
| Country | CAGR (2025 to 2035) |
|---|---|
| India | 8.7% |
| China | 8.3% |
| USA | 7.9% |
| Germany | 7.5% |
| UK | 7.2% |
| Japan | 6.8% |
| Brazil | 6.4% |
The lung cancer diagnostics market continues to record steady global expansion. India is projected to progress at a CAGR of 8.7% through 2035, supported by rapid investment in private oncology diagnostic networks and expanding access to biomarker testing. China is estimated to rise at an 8.3% CAGR, influenced by its large patient population and rising use of NGS panels and CT-based screening pathways.
The USA is expected to register 7.9% growth, reflecting broad adoption of AI-enabled diagnostic systems and advanced biomarker assays across major cancer centers. Germany is forecast to post 7.5% growth, with sustained penetration of molecular testing and well-structured national screening programs.
The UK is likely to grow at 7.2%, driven by NHS-supported improvements in early detection and standardized screening coverage. Japan is projected at 6.8% growth, aligned with its aging population and extensive use of molecular imaging across oncology departments. Brazil is anticipated to grow at 6.4% as healthcare providers continue the gradual modernization of cancer diagnostic infrastructure and expand access to clinical testing services.
India Leads Global Market Expansion
India demonstrates the strongest growth potential in the lung cancer diagnostics market with a CAGR of 8.7% through 2035. The country's leadership position stems from fast growth in private oncology diagnostic chains, expanding molecular pathology capabilities, and increasing awareness of precision oncology approaches enabling mainstream biomarker testing adoption.
Growth is concentrated in major metropolitan regions, including Delhi NCR, Mumbai, Bangalore, and Chennai, where corporate hospital networks and specialized oncology centers are implementing advanced lung cancer diagnostic programs for enhanced treatment selection.
Distribution channels through private diagnostic chains, hospital pathology laboratories, and reference laboratory networks expand access across urban cancer centers and emerging regional oncology facilities. The country's expanding cancer care infrastructure provides policy support for diagnostic development, including growing health insurance coverage for molecular testing.
Key market factors:
- Clinical demand concentrated in metropolitan tertiary cancer centers and private oncology hospitals with comprehensive molecular pathology programs
- Infrastructure development through private healthcare expansion and specialty oncology center growth
- Comprehensive diagnostic ecosystem, including growing molecular pathology expertise with international training partnerships
- Technology integration featuring next-generation sequencing platforms, liquid biopsy capabilities, and digital pathology systems
China Emerges as Testing Powerhouse
In major healthcare regions including Beijing, Shanghai, Guangzhou, and Shenzhen, the deployment and utilization of lung cancer diagnostics is accelerating across tertiary hospitals and specialized oncology centers, driven by large patient base and rapid growth in NGS and CT screening programs. The market demonstrates strong growth momentum with a CAGR of 8.3% through 2035, linked to massive cancer burden, integrated diagnostic infrastructure development, and sustained government support for precision medicine initiatives.
Chinese hospitals and laboratories are implementing advanced molecular testing platforms and comprehensive genomic profiling to enhance treatment decision-making while meeting growing oncologist demand in expanding urban cancer centers and aging population demographics. The country's Healthy China 2030 initiative creates persistent demand for cancer diagnostic capabilities, while increasing emphasis on early detection programs drives adoption of advanced screening and molecular testing technologies.
Key development areas:
- Tertiary hospitals and specialized cancer centers leading lung cancer diagnostics with comprehensive molecular pathology programs
- Integrated healthcare networks providing coordinated diagnostic services with extensive regional laboratory infrastructure
- Strategic partnerships between Chinese diagnostic companies and international technology providers are expanding platform access
- Integration of national cancer registries and comprehensive quality assurance protocols
USA Shows Innovation Leadership
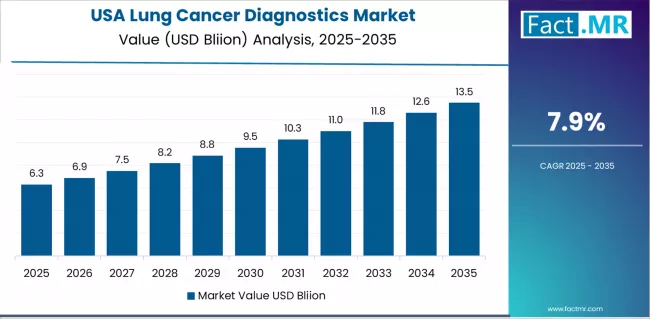
USA's market expansion is driven by high adoption of AI-based and biomarker diagnostics, including computational pathology integration and machine learning-enhanced interpretation systems, and continuous innovation in companion diagnostic development across academic medical centers. The country demonstrates strong growth potential with a CAGR of 7.9% through 2035, supported by established precision oncology infrastructure, comprehensive insurance coverage for molecular testing, and ongoing clinical research advancing biomarker validation.
American healthcare facilities face implementation challenges related to test utilization management and cost-effectiveness demonstration, requiring evidence generation supporting clinical utility and outcomes improvement. However, robust pharmaceutical pipeline activity and strong laboratory medicine capabilities create compelling adoption pathways for advanced lung cancer diagnostic technologies, particularly in comprehensive cancer centers where innovation and clinical trial activity have direct impacts on practice evolution and guideline development.
Key market characteristics:
- Academic cancer centers and comprehensive cancer care networks showing leadership in advanced diagnostic adoption and biomarker research
- Regional expansion trends focused on community oncology access improvement and molecular testing democratization
- Future projections indicate continued emphasis on liquid biopsy validation and minimal residual disease monitoring implementation
- Growing focus on value-based oncology care models and comprehensive diagnostic-therapeutic integration
Germany Demonstrates Testing Excellence
The market for lung cancer diagnostics in Germany leads in molecular testing infrastructure based on strong molecular testing penetration and screening programs integration for enhanced diagnostic capabilities. The country shows strong potential with a CAGR of 7.5% through 2035, driven by comprehensive healthcare system support, robust pathology infrastructure, and the expansion of specialized molecular diagnostic facilities in major university hospitals, including centers in North Rhine-Westphalia, Bavaria, Baden-Württemberg, and Berlin.
German laboratories are adopting advanced lung cancer diagnostic technologies including comprehensive genomic profiling and liquid biopsy applications, particularly in academic centers with sophisticated molecular pathology programs and quality assurance systems demanding rigorous validation standards. Technology deployment channels through university pathology departments and regional laboratory networks expand coverage across academic medicine and community oncology practices.
Leading market segments:
- University hospital pathology departments implementing comprehensive molecular diagnostic programs and quality-assured testing workflows
- Strategic partnerships between diagnostic manufacturers and clinical laboratories, achieving technology validation and guideline integration
- Collaborative research networks between German institutions and international partners are expanding biomarker discovery
- Focus on guideline-concordant testing implementation and comprehensive quality management systems
UK Emphasizes Structured Access
The market in UK demonstrates comprehensive healthcare system integration characterized by NHS-driven structured screening expansion and national molecular testing programs for enhanced population coverage. The country shows steady growth momentum with a CAGR of 7.2% through 2035, driven by centralized healthcare delivery and national cancer diagnostic initiatives across regional cancer centers.
The UK's emphasis on equitable access and evidence-based implementation creates requirements for validated lung cancer diagnostic technologies that support comprehensive national screening programs and standardized molecular testing pathways. The market benefits from strong NHS laboratory networks and national genomic medicine services, creating coordinated diagnostic ecosystems that prioritize clinical utility and population health impact. Regional molecular pathology hubs showcase advanced testing programs where standardized protocols achieve consistent diagnostic quality through integrated quality assurance.
Key market characteristics:
- NHS trusts and regional molecular pathology laboratories driving standardized testing adoption with emphasis on equitable access
- National program coordination enabling comprehensive screening implementation with centralized quality oversight
- Technology collaboration between NHS laboratories and diagnostic manufacturers is expanding validated testing platforms
- Emphasis on health technology assessment and cost-effectiveness evaluation methodologies
Japan Emphasizes Aging Population Care
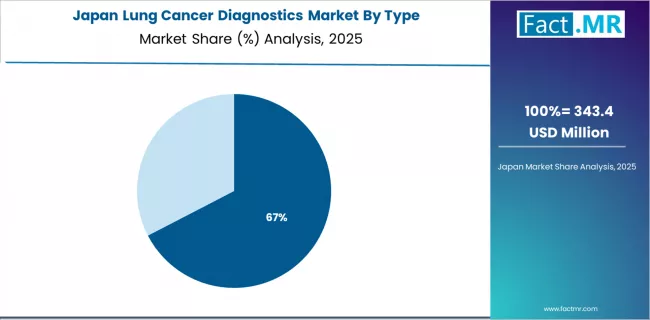
The Japanese market demonstrates sophisticated diagnostic delivery characterized by aging population and widespread molecular imaging use for enhanced lung cancer detection and characterization. The country shows steady growth momentum with a CAGR of 6.8% through 2035, driven by advanced healthcare infrastructure and comprehensive cancer screening programs in established medical centers. Japan's emphasis on thorough diagnostic evaluation and quality care creates requirements for validated lung cancer diagnostic technologies that support comprehensive workup protocols and treatment planning optimization.
The market benefits from strong relationships between pharmaceutical companies and clinical laboratories, creating comprehensive companion diagnostic ecosystems that prioritize biomarker testing quality and regulatory compliance. Cancer centers in major metropolitan regions showcase advanced molecular pathology programs where standardized testing achieves superior analytical performance through rigorous quality management.
Key market characteristics:
- University hospitals and specialized cancer centers driving molecular testing adoption with emphasis on analytical excellence
- National health insurance coverage enabling comprehensive biomarker testing access with standardized reimbursement frameworks
- Technology collaboration between Japanese laboratories and international diagnostic manufacturers is expanding platform implementation
- Emphasis on companion diagnostic validation and regulatory compliance optimization
Brazil Demonstrates Infrastructure Development
The market in Brazil experiences momentum based on gradual modernization of cancer diagnostic infrastructure and expanding molecular pathology capabilities for enhanced oncology care. The country demonstrates steady potential with a CAGR of 6.4% through 2035, driven by healthcare system evolution, growing private laboratory sector, and expansion of cancer diagnostic services in major urban centers, including São Paulo, Rio de Janeiro, Brasília, and Belo Horizonte.
Brazilian facilities are adopting molecular diagnostic technologies for lung cancer biomarker testing and precision oncology implementation, particularly in regions with expanding tertiary care infrastructure and specialty oncology center development demanding comprehensive molecular pathology capabilities. Technology deployment channels through private laboratory networks, academic pathology departments, and specialty cancer institutes expand coverage across urban oncology care platforms.
Leading market segments:
- Private reference laboratories and academic cancer centers implementing molecular diagnostic programs and biomarker testing services
- Strategic partnerships with international diagnostic suppliers, achieving technology transfer and technical training support
- Collaborative development between Brazilian institutions and global laboratories are expanding local expertise
- Focus on access improvement initiatives and molecular testing infrastructure development
Europe Market Split by Country
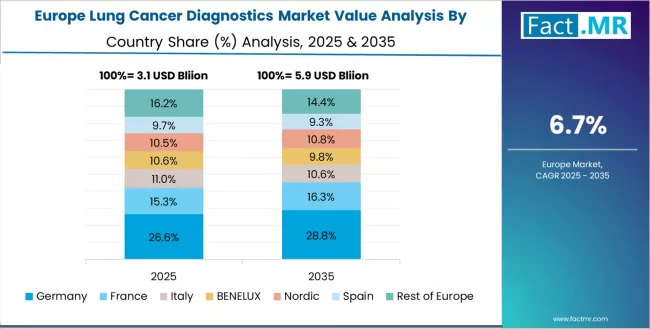

The lung cancer diagnostics market in Europe is projected to expand steadily from its 2025 base through 2035. Germany is expected to lead with an estimated 28.4% share in 2025, supported by advanced molecular pathology capacity, structured cancer screening programs, and strong clinical research output across major university hospitals.
France follows with a 21.7% share in 2025, reflecting its established oncology networks and academic pathology leadership. The UK holds an estimated 18.9% share in 2025 through NHS molecular pathology initiatives and the rollout of the national genomic medicine service. Italy accounts for a 15.2% share, driven by consistent investment in cancer diagnostics and expanding adoption of molecular testing. The Rest of Europe collectively represents a 15.8% share in 2025 as wider adoption of precision oncology continues across developing healthcare systems.
By 2035, Germany is projected to retain a leading 27.6% share. France is expected to maintain a solid 21.2% share, supported by continued academic pathology strength. The UK is anticipated to hold 19.3%, aligned with sustained national program delivery. Italy is projected at 15.4%, while the Rest of Europe increases to 16.5%, reflecting broadening diagnostic penetration across diverse healthcare settings and rising adoption of precision oncology and molecular testing across the region.
Competitive Landscape of the Lung Cancer Diagnostics Market
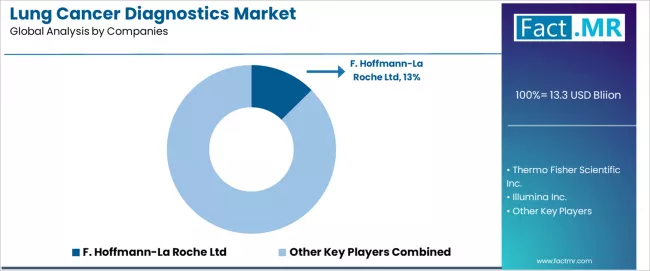
The lung cancer diagnostics market features approximately 15-20 meaningful players with moderate concentration, where the leading companies control substantial market presence through established diagnostic platforms and extensive clinical validation across oncology and pathology sectors.
Market leaders include F. Hoffmann-La Roche Ltd, which maintains competitive advantages through comprehensive companion diagnostic portfolios, global commercial infrastructure, and deep expertise in the oncology diagnostics and targeted therapy development sectors, creating strong relationships among oncologists and pathologists. F. Hoffmann-La Roche Ltd commands a 12.6% market share through integrated diagnostic-therapeutic platforms and strategic positioning in global precision oncology markets.
Challengers encompass Thermo Fisher Scientific, Illumina Inc., and Agilent Technologies, which compete through innovative sequencing platforms and comprehensive molecular testing solutions in key oncology markets. Specialty diagnostic providers, including Qiagen, Abbott Laboratories, and Bio-Rad, focus on specific testing technologies or biomarker segments, offering differentiated capabilities in PCR-based testing, immunoassay platforms, and quality control solutions. Additional meaningful players include Neogenomics Laboratories, bioMérieux, and Myriad Genetics, Inc., which compete through specialized laboratory services and proprietary testing platforms.
Regional players and emerging diagnostic developers create competitive pressure through liquid biopsy innovations and AI-enhanced interpretation systems, particularly in high-growth markets including China and India, where local presence provides advantages in market access and healthcare system integration.
Market dynamics favor companies that combine validated diagnostic technologies with comprehensive clinical evidence that addresses the complete testing pathway from specimen collection through result interpretation and clinical reporting. Strategic investments in artificial intelligence integration and liquid biopsy platform development enable market differentiation and clinical adoption across diverse healthcare settings.
Global Lung Cancer Diagnostics Market - Stakeholder Contribution Framework
Lung cancer diagnostic technologies represent a critical precision medicine tool that enables oncologists, pathologists, and cancer centers to achieve accurate biomarker identification and optimal treatment selection without invasive tissue acquisition in many cases, typically providing actionable molecular information and therapeutic guidance while supporting diverse patient populations.
With the market projected to grow from USD 13.3 billion in 2025 to USD 28.0 billion by 2035 at a 7.7% CAGR, these technologies offer compelling advantages in targeted therapy selection, immunotherapy biomarker assessment, and comprehensive molecular profiling. They have become essential for EGFR mutation testing (the dominant test type), hospital pathology programs (the largest testing setting), and oncology practices that require validated biomarker analysis. Scaling clinical adoption and market development requires coordinated action across healthcare policy, clinical guidelines, diagnostic manufacturers, pathology organizations, and clinical research institutions.
How Governments Could Spur Local Production and Adoption?
- Cancer Diagnostic Infrastructure Programs: Include advanced molecular pathology capabilities in national cancer control strategies, providing targeted funding for next-generation sequencing platforms in regions with comprehensive cancer centers and supporting laboratory accreditation through quality infrastructure investment and technical training grants.
- Reimbursement Policy Development: Implement evidence-based coverage frameworks for molecular biomarker testing, provide appropriate reimbursement for comprehensive genomic profiling reflecting testing complexity, and establish companion diagnostic pathways that recognize clinical utility and treatment decision impact.
- National Screening & Early Detection Programs: Create comprehensive lung cancer screening initiatives incorporating low-dose CT imaging and biomarker testing, provide funding for high-risk population identification, and establish coordinated diagnostic pathways that support early-stage detection and treatment optimization.
- Skills Development & Training: Fund fellowship training programs for molecular pathology specialists and genetic counselors. Invest in bioinformatics education and international training partnerships that build expertise in comprehensive genomic profiling interpretation and precision oncology implementation.
How Industry Bodies Could Support Market Development?
- Clinical Guidelines & Testing Standards: Define evidence-based biomarker testing algorithms for lung cancer management across different disease stages, establish quality standards for molecular pathology laboratories and analytical performance, and create proficiency testing programs that healthcare systems can implement for quality assurance.
- Market Education & Evidence Dissemination: Lead professional education initiatives that demonstrate diagnostic testing capabilities, emphasizing clinical utility, treatment decision impact, and outcome improvement compared to empiric treatment approaches and conventional diagnostic methods.
- Registry Development & Outcomes Research: Develop comprehensive databases documenting real-world testing utilization and clinical outcomes correlation, establish benchmarking protocols for laboratory performance comparison, and create collaborative research networks that advance biomarker validation and clinical evidence generation.
How Manufacturers and Technology Players Could Strengthen the Ecosystem?
- Diagnostic Technology Development: Develop next-generation testing platforms with enhanced sensitivity, broader biomarker coverage, and specimen-flexible capabilities that optimize diagnostic yield while improving turnaround time and cost-effectiveness across diverse clinical scenarios.
- Clinical Support Platforms: Provide comprehensive pathologist education programs that integrate assay validation support, interpretation guidance, quality assurance resources, and clinical decision support tools, enabling laboratories to optimize testing quality and maximize clinical impact.
- Evidence Generation Partnerships: Offer collaborative research programs with leading cancer centers, including companion diagnostic validation studies, real-world evidence generation, and health economic evaluations that support evidence-based testing adoption and informed healthcare policy development across diverse healthcare systems.
Key Players in the Lung Cancer Diagnostics Market
- F. Hoffmann-La Roche Ltd
- Thermo Fisher Scientific Inc.
- Illumina Inc.
- Agilent Technologies Inc.
- QIAGEN N.V.
- Abbott Laboratories
- Bio-Rad Laboratories Inc.
- NeoGenomics Laboratories Inc.
- bioMérieux S.A.
- Myriad Genetics, Inc.
Scope of the Report
| Items | Values |
|---|---|
| Quantitative Units | USD 13.3 Billion |
| Type | Non-small Cell Lung Cancer (NSCLC), Small Cell Lung Cancer (SCLC) |
| Test | CA Test, HER2 Test, ALK Test, Angiogenesis Inhibitors, EGFR Mutation Test, KRAS Mutation Test |
| End Use | Hospitals, Laboratories, Others |
| Regions Covered | Asia Pacific, North America, Europe, Latin America, Middle East & Africa |
| Country Covered | India, China, USA, Germany, UK, Japan, Brazil, and 40+ countries |
| Key Companies Profiled | F. Hoffmann-La Roche Ltd, Thermo Fisher Scientific, Illumina Inc., Agilent Technologies, Qiagen, Abbott, Bio-Rad, Neogenomics Laboratories, bioMérieux, Myriad Genetics, Inc. |
| Additional Attributes | Dollar sales by type, test, and end use categories, regional diagnostic adoption trends across Asia Pacific, North America, and Europe, competitive landscape with diagnostic manufacturers and laboratory service providers, clinical performance specifications and analytical requirements, integration with hospital pathology departments and reference laboratory operations. |
Lung Cancer Diagnostics Market by Segments
-
Type :
- Non-small Cell Lung Cancer (NSCLC)
- Squamous Cell Carcinoma
- Adenocarcinoma
- Large Cell Carcinoma
- Small Cell Lung Cancer (SCLC)
- Small-cell Carcinoma
- Combined Small-cell Carcinoma
- Non-small Cell Lung Cancer (NSCLC)
-
Test :
- CA Test
- HER2 Test
- ALK Test
- Angiogenesis Inhibitors
- EGFR Mutation Test
- KRAS Mutation Test
-
End Use :
- Hospitals
- Laboratories
- Others
-
Region :
- Asia Pacific
- China
- Japan
- South Korea
- India
- Australia & New Zealand
- ASEAN
- Rest of Asia Pacific
- North America
- USA
- Canada
- Mexico
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Rest of Europe
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkey
- South Africa
- Rest of Middle East & Africa
- Asia Pacific
Table of Content
- Executive Summary
- Global Market Outlook
- Demand to side Trends
- Supply to side Trends
- Technology Roadmap Analysis
- Analysis and Recommendations
- Market Overview
- Market Coverage / Taxonomy
- Market Definition / Scope / Limitations
- Market Background
- Market Dynamics
- Drivers
- Restraints
- Opportunity
- Trends
- Scenario Forecast
- Demand in Optimistic Scenario
- Demand in Likely Scenario
- Demand in Conservative Scenario
- Opportunity Map Analysis
- Product Life Cycle Analysis
- Supply Chain Analysis
- Investment Feasibility Matrix
- Value Chain Analysis
- PESTLE and Porter’s Analysis
- Regulatory Landscape
- Regional Parent Market Outlook
- Production and Consumption Statistics
- Import and Export Statistics
- Market Dynamics
- Global Market Analysis 2020 to 2024 and Forecast, 2025 to 2035
- Historical Market Size Value (USD Million) Analysis, 2020 to 2024
- Current and Future Market Size Value (USD Million) Projections, 2025 to 2035
- Y to o to Y Growth Trend Analysis
- Absolute $ Opportunity Analysis
- Global Market Pricing Analysis 2020 to 2024 and Forecast 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Type
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Type, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Type, 2025 to 2035
- Non-small Cell Lung Cancer (NSCLC)
- Small Cell Lung Cancer (SCLC)
- Y to o to Y Growth Trend Analysis By Type, 2020 to 2024
- Absolute $ Opportunity Analysis By Type, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Test
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By Test, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By Test, 2025 to 2035
- EGFR Mutation Test
- HER2 Test
- ALK Test
- Angiogenesis Inhibitors
- CA Test
- KRAS Mutation Test
- Y to o to Y Growth Trend Analysis By Test, 2020 to 2024
- Absolute $ Opportunity Analysis By Test, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By End Use
- Introduction / Key Findings
- Historical Market Size Value (USD Million) Analysis By End Use, 2020 to 2024
- Current and Future Market Size Value (USD Million) Analysis and Forecast By End Use, 2025 to 2035
- Hospitals
- Laboratories
- Others
- Y to o to Y Growth Trend Analysis By End Use, 2020 to 2024
- Absolute $ Opportunity Analysis By End Use, 2025 to 2035
- Global Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Region
- Introduction
- Historical Market Size Value (USD Million) Analysis By Region, 2020 to 2024
- Current Market Size Value (USD Million) Analysis and Forecast By Region, 2025 to 2035
- North America
- Latin America
- Western Europe
- Eastern Europe
- East Asia
- South Asia and Pacific
- Middle East & Africa
- Market Attractiveness Analysis By Region
- North America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- USA
- Canada
- Mexico
- By Type
- By Test
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Test
- By End Use
- Key Takeaways
- Latin America Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Brazil
- Chile
- Rest of Latin America
- By Type
- By Test
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Test
- By End Use
- Key Takeaways
- Western Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Germany
- UK
- Italy
- Spain
- France
- Nordic
- BENELUX
- Rest of Western Europe
- By Type
- By Test
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Test
- By End Use
- Key Takeaways
- Eastern Europe Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Russia
- Poland
- Hungary
- Balkan & Baltic
- Rest of Eastern Europe
- By Type
- By Test
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Test
- By End Use
- Key Takeaways
- East Asia Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- China
- Japan
- South Korea
- By Type
- By Test
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Test
- By End Use
- Key Takeaways
- South Asia and Pacific Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- India
- ASEAN
- Australia & New Zealand
- Rest of South Asia and Pacific
- By Type
- By Test
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Test
- By End Use
- Key Takeaways
- Middle East & Africa Market Analysis 2020 to 2024 and Forecast 2025 to 2035, By Country
- Historical Market Size Value (USD Million) Trend Analysis By Market Taxonomy, 2020 to 2024
- Market Size Value (USD Million) Forecast By Market Taxonomy, 2025 to 2035
- By Country
- Kingdom of Saudi Arabia
- Other GCC Countries
- Turkiye
- South Africa
- Other African Union
- Rest of Middle East & Africa
- By Type
- By Test
- By End Use
- By Country
- Market Attractiveness Analysis
- By Country
- By Type
- By Test
- By End Use
- Key Takeaways
- Key Countries Market Analysis
- USA
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Canada
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Mexico
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Brazil
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Chile
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Germany
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- UK
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Italy
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Spain
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- France
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- India
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- ASEAN
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Australia & New Zealand
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- China
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Japan
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- South Korea
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Russia
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Poland
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Hungary
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Kingdom of Saudi Arabia
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- Turkiye
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- South Africa
- Pricing Analysis
- Market Share Analysis, 2024
- By Type
- By Test
- By End Use
- USA
- Market Structure Analysis
- Competition Dashboard
- Competition Benchmarking
- Market Share Analysis of Top Players
- By Regional
- By Type
- By Test
- By End Use
- Competition Analysis
- Competition Deep Dive
- F. Hoffmann-La Roche Ltd
- Overview
- Product Portfolio
- Profitability by Market Segments (Product/Age /Sales Channel/Region)
- Sales Footprint
- Strategy Overview
- Marketing Strategy
- Product Strategy
- Channel Strategy
- Thermo Fisher Scientific Inc.
- Illumina Inc.
- Agilent Technologies Inc.
- QIAGEN N.V.
- Abbott Laboratories
- Bio-Rad Laboratories Inc.
- NeoGenomics Laboratories Inc.
- bioMérieux S.A.
- Myriad Genetics, Inc.
- F. Hoffmann-La Roche Ltd
- Competition Deep Dive
- Assumptions & Acronyms Used
- Research Methodology
List Of Table
- Table 1: Global Market Value (USD Million) Forecast by Region, 2020 to 2035
- Table 2: Global Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 3: Global Market Value (USD Million) Forecast by Test, 2020 to 2035
- Table 4: Global Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 5: North America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 6: North America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 7: North America Market Value (USD Million) Forecast by Test, 2020 to 2035
- Table 8: North America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 9: Latin America Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 10: Latin America Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 11: Latin America Market Value (USD Million) Forecast by Test, 2020 to 2035
- Table 12: Latin America Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 13: Western Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 14: Western Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 15: Western Europe Market Value (USD Million) Forecast by Test, 2020 to 2035
- Table 16: Western Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 17: Eastern Europe Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 18: Eastern Europe Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 19: Eastern Europe Market Value (USD Million) Forecast by Test, 2020 to 2035
- Table 20: Eastern Europe Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 21: East Asia Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 22: East Asia Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 23: East Asia Market Value (USD Million) Forecast by Test, 2020 to 2035
- Table 24: East Asia Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 25: South Asia and Pacific Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 26: South Asia and Pacific Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 27: South Asia and Pacific Market Value (USD Million) Forecast by Test, 2020 to 2035
- Table 28: South Asia and Pacific Market Value (USD Million) Forecast by End Use, 2020 to 2035
- Table 29: Middle East & Africa Market Value (USD Million) Forecast by Country, 2020 to 2035
- Table 30: Middle East & Africa Market Value (USD Million) Forecast by Type, 2020 to 2035
- Table 31: Middle East & Africa Market Value (USD Million) Forecast by Test, 2020 to 2035
- Table 32: Middle East & Africa Market Value (USD Million) Forecast by End Use, 2020 to 2035
List Of Figures
- Figure 1: Global Market Pricing Analysis
- Figure 2: Global Market Value (USD Million) Forecast 2020 to 2035
- Figure 3: Global Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 4: Global Market Y to o to Y Growth Comparison by Type, 2025-2035
- Figure 5: Global Market Attractiveness Analysis by Type
- Figure 6: Global Market Value Share and BPS Analysis by Test, 2025 and 2035
- Figure 7: Global Market Y to o to Y Growth Comparison by Test, 2025-2035
- Figure 8: Global Market Attractiveness Analysis by Test
- Figure 9: Global Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 10: Global Market Y to o to Y Growth Comparison by End Use, 2025-2035
- Figure 11: Global Market Attractiveness Analysis by End Use
- Figure 12: Global Market Value (USD Million) Share and BPS Analysis by Region, 2025 and 2035
- Figure 13: Global Market Y to o to Y Growth Comparison by Region, 2025-2035
- Figure 14: Global Market Attractiveness Analysis by Region
- Figure 15: North America Market Incremental Dollar Opportunity, 2025-2035
- Figure 16: Latin America Market Incremental Dollar Opportunity, 2025-2035
- Figure 17: Western Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 18: Eastern Europe Market Incremental Dollar Opportunity, 2025-2035
- Figure 19: East Asia Market Incremental Dollar Opportunity, 2025-2035
- Figure 20: South Asia and Pacific Market Incremental Dollar Opportunity, 2025-2035
- Figure 21: Middle East & Africa Market Incremental Dollar Opportunity, 2025-2035
- Figure 22: North America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 23: North America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 24: North America Market Y to o to Y Growth Comparison by Type, 2025-2035
- Figure 25: North America Market Attractiveness Analysis by Type
- Figure 26: North America Market Value Share and BPS Analysis by Test, 2025 and 2035
- Figure 27: North America Market Y to o to Y Growth Comparison by Test, 2025-2035
- Figure 28: North America Market Attractiveness Analysis by Test
- Figure 29: North America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 30: North America Market Y to o to Y Growth Comparison by End Use, 2025-2035
- Figure 31: North America Market Attractiveness Analysis by End Use
- Figure 32: Latin America Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 33: Latin America Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 34: Latin America Market Y to o to Y Growth Comparison by Type, 2025-2035
- Figure 35: Latin America Market Attractiveness Analysis by Type
- Figure 36: Latin America Market Value Share and BPS Analysis by Test, 2025 and 2035
- Figure 37: Latin America Market Y to o to Y Growth Comparison by Test, 2025-2035
- Figure 38: Latin America Market Attractiveness Analysis by Test
- Figure 39: Latin America Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 40: Latin America Market Y to o to Y Growth Comparison by End Use, 2025-2035
- Figure 41: Latin America Market Attractiveness Analysis by End Use
- Figure 42: Western Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 43: Western Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 44: Western Europe Market Y to o to Y Growth Comparison by Type, 2025-2035
- Figure 45: Western Europe Market Attractiveness Analysis by Type
- Figure 46: Western Europe Market Value Share and BPS Analysis by Test, 2025 and 2035
- Figure 47: Western Europe Market Y to o to Y Growth Comparison by Test, 2025-2035
- Figure 48: Western Europe Market Attractiveness Analysis by Test
- Figure 49: Western Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 50: Western Europe Market Y to o to Y Growth Comparison by End Use, 2025-2035
- Figure 51: Western Europe Market Attractiveness Analysis by End Use
- Figure 52: Eastern Europe Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 53: Eastern Europe Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 54: Eastern Europe Market Y to o to Y Growth Comparison by Type, 2025-2035
- Figure 55: Eastern Europe Market Attractiveness Analysis by Type
- Figure 56: Eastern Europe Market Value Share and BPS Analysis by Test, 2025 and 2035
- Figure 57: Eastern Europe Market Y to o to Y Growth Comparison by Test, 2025-2035
- Figure 58: Eastern Europe Market Attractiveness Analysis by Test
- Figure 59: Eastern Europe Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 60: Eastern Europe Market Y to o to Y Growth Comparison by End Use, 2025-2035
- Figure 61: Eastern Europe Market Attractiveness Analysis by End Use
- Figure 62: East Asia Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 63: East Asia Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 64: East Asia Market Y to o to Y Growth Comparison by Type, 2025-2035
- Figure 65: East Asia Market Attractiveness Analysis by Type
- Figure 66: East Asia Market Value Share and BPS Analysis by Test, 2025 and 2035
- Figure 67: East Asia Market Y to o to Y Growth Comparison by Test, 2025-2035
- Figure 68: East Asia Market Attractiveness Analysis by Test
- Figure 69: East Asia Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 70: East Asia Market Y to o to Y Growth Comparison by End Use, 2025-2035
- Figure 71: East Asia Market Attractiveness Analysis by End Use
- Figure 72: South Asia and Pacific Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 73: South Asia and Pacific Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 74: South Asia and Pacific Market Y to o to Y Growth Comparison by Type, 2025-2035
- Figure 75: South Asia and Pacific Market Attractiveness Analysis by Type
- Figure 76: South Asia and Pacific Market Value Share and BPS Analysis by Test, 2025 and 2035
- Figure 77: South Asia and Pacific Market Y to o to Y Growth Comparison by Test, 2025-2035
- Figure 78: South Asia and Pacific Market Attractiveness Analysis by Test
- Figure 79: South Asia and Pacific Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 80: South Asia and Pacific Market Y to o to Y Growth Comparison by End Use, 2025-2035
- Figure 81: South Asia and Pacific Market Attractiveness Analysis by End Use
- Figure 82: Middle East & Africa Market Value Share and BPS Analysis by Country, 2025 and 2035
- Figure 83: Middle East & Africa Market Value Share and BPS Analysis by Type, 2025 and 2035
- Figure 84: Middle East & Africa Market Y to o to Y Growth Comparison by Type, 2025-2035
- Figure 85: Middle East & Africa Market Attractiveness Analysis by Type
- Figure 86: Middle East & Africa Market Value Share and BPS Analysis by Test, 2025 and 2035
- Figure 87: Middle East & Africa Market Y to o to Y Growth Comparison by Test, 2025-2035
- Figure 88: Middle East & Africa Market Attractiveness Analysis by Test
- Figure 89: Middle East & Africa Market Value Share and BPS Analysis by End Use, 2025 and 2035
- Figure 90: Middle East & Africa Market Y to o to Y Growth Comparison by End Use, 2025-2035
- Figure 91: Middle East & Africa Market Attractiveness Analysis by End Use
- Figure 92: Global Market - Tier Structure Analysis
- Figure 93: Global Market - Company Share Analysis
- FAQs -
How big is the lung cancer diagnostics market in 2025?
The global lung cancer diagnostics market is estimated to be valued at USD 13.3 bliion in 2025.
What will be the size of lung cancer diagnostics market in 2035?
The market size for the lung cancer diagnostics market is projected to reach USD 28.0 bliion by 2035.
How much will be the lung cancer diagnostics market growth between 2025 and 2035?
The lung cancer diagnostics market is expected to grow at a 7.7% CAGR between 2025 and 2035.
What are the key product types in the lung cancer diagnostics market?
The key product types in lung cancer diagnostics market are non-small cell lung cancer (nsclc) and small cell lung cancer (sclc).
Which test segment to contribute significant share in the lung cancer diagnostics market in 2025?
In terms of test, egfr mutation test segment to command 38.9% share in the lung cancer diagnostics market in 2025.